Endoscopic approach for management of biliary strictures in liver transplant recipients: A systematic review and meta-analysis
Abstract
The most common biliary complication after liver transplantation is anastomotic stricture (AS) and it can occur isolated or in combination with other complications. Liver graft from a cadaveric donor or a living donor has an influence on the incidence of biliary strictures as well as on the response to endoscopic treatment. Endoscopic treatment using balloon dilation and insertion of biliary stents by endoscopic retrograde cholangiopancreatography (ERCP) is the initial approach to these complications.
Aim
The aim of this article is to compare different endoscopic techniques to treat post-liver transplantation biliary strictures.
Methods
The search was carried out on MEDLINE, EMBASE, Scielo-LILACS and Cochrane Library databases through June 2015. A total of 1100 articles were retrieved. Ten clinical trials were analyzed, and seven were included in the meta-analysis.
Conclusions
The endoscopic treatment of AS was equally effective when compared the use of fully covered self-expandable metal stents (FCSEMS) vs. plastic stents, but the use of FCSEMS was associated with a lower complication risk. The treatment of AS with balloon dilation or balloon dilation associated with plastic stents presented similar results. Deceased donor liver transplantation reduced the risk of biliary stenosis and the endoscopic treatment in these patients was more effective when compared with Living donor liver transplantation.
Introduction
Liver transplantation is the treatment for liver diseases that lead to glandular failure and may be performed with grafts from brain-dead or living donors. Most post-liver transplantation biliary complications such as anastomotic stricture (AS), non-anastomotic stricture (NAS) and biliary fistula may occur alone or in combination and potentially lead to graft loss, threatening the lives of recipients with great social harm.
Treatment of post-liver transplantation biliary strictures has diversified during the last few years, from surgical to endoscopic repair,1 with success rates of about 60% and 80% for living donor liver transplantation (LDLT) and deceased donor liver transplantation (DDLT), respectively.2 Among the minimally invasive procedures, dilation of the strictures and biliary stent insertion by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC) are emphasized. The main inconvenience of endoscopic therapeutic procedure is the progressive use of balloon dilations and insertion of one or more plastic stents with the need for multiple procedures that extend treatment for 12 or more months. The percutaneous transhepatic approach and surgical treatment are currently reserved for unsuccessful endoscopic treatment, or for multiple, intrahepatic, inaccessible strictures or Roux-en-Y anastomoses, in which case enteroscopy is the option to access the biliary tree. The rendezvous technique may also be an option whenever endoscopic access to the biliary stricture is not possible. Direct cholangioscopy, another advanced form of endoscopy, allows the direct visualization of the biliary-tree inner wall and may facilitate stent placement or stone extraction.
Despite the number of published studies that have evaluated the issue of ideal treatment for biliary strictures after liver transplantation, there are still many unanswered questions regarding the modality with best technical and clinic success rates, less morbidity, longer lasting effect, and lower social and economic costs. The aim of this study is to compare different endoscopic treatment techniques for post-liver transplantation biliary strictures by systematic review of the literature and meta-analysis.
Methods
We conducted a systematic literature review and meta-analysis of published clinical studies. The research protocol was submitted and approved by the Research Ethics Committee of the University of São Paulo Medical School (FCMUSP), São Paulo, Brazil, under the Institutional registry 304/13.
This meta-analysis was registered in PROSPERO—International Prospective Register of Systematic Review (www.crd.york.ac.uk/prospero/ ) under the number CRD42016033010.
Inclusion and exclusion criteria
Study types
Randomized, non-randomized and prospective, retrospective cohort studies were included for analysis. Case series, editorials, letters, reviews, studies with animals, and case reports were excluded. Only studies in English, French, Spanish or Portuguese were evaluated. There was no restriction concerning publication date.
Participant types
Patients who underwent endoscopic treatment for post-liver transplantation AS and NAS strictures with graft from a living or deceased donor were included in the present study. There was no restriction on age or gender. Studies with biliary strictures with unknown cause, malignant origin, post-surgery or non-hepatic transplant and number of patients lower than five were excluded from this analysis.
Intervention types
Interventions evaluated were ERCP with or without sphincterotomy, biliary stricture dilation with balloon and insertion of plastic or metal stents.
Studies involving biliary access by PTC were excluded.
Search strategy
Search and selection of the best available evidence for the endoscopic treatment of post-liver transplant biliary strictures were conducted by a structured search in the form of P.I.C.O., in which P is the group of patients under consideration or study population, I the intervention, exposition or thesis, C the comparison or control group, and O the outcome or expected result. The various terms of each group were combined by using the Boolean operator “OR” and groups were combined using the Boolean operator “AND”. In order to exclude one term, the Boolean operator “NOT” was used.
The search was performed using the databases MEDLINE/PubMed, Scielo-LILACS, EMBASE, and the Cochrane Library through June 2015 (Table 1 ).
| PICO—Post-liver transplantation stricture | |
|---|---|
| P | Patients with biliary stricture after liver transplantation |
| I | ERCP + (plastic or metallic) biliary stents with or without biliary dilation |
| C | Control group |
| O | Technical success, clinical success, adverse effects, recurrence |
- ERCP: endoscopic retrograde cholangiopancreatography.
Definitions
Technical success was defined by procedure success rates by ERCP with adequate opacification of the bile duct defined through fluoroscopic diagnosis, as well as adequately performing the proposed endoscopic therapy resulting in good therapeutic response with no need to change the procedure.
Clinical success was defined as improvement in jaundice and reduction of biliary stasis level markers.
Method safety was evaluated through the occurrence of complications related to sedation and bile duct endoscopic manipulation.
Recurrence was defined as recrudescence of biliary stricture signs and symptoms after initial clinical success.
The following terms were entered into the MEDLINE/PubMed (www.ncbi.nlm.nih.gov/pubmed ) database: (Post-liver transplantation OR Liver transplantation OR Liver transplant OR Hepatic Transplantation OR Liver Grafting) AND (Anastomosis, Surgical/adverse effects OR Constrictions, Pathologic OR Stricture* OR Stenose* OR Stenosis*) AND (Prosthesis Implantation/instrumentation* OR Stents* OR Cholangiopancreatography, Endoscopic Retrograde OR ERCP OR Cholangiography OR Endoscopic Retrograde Cholangiopancreatographies OR Endoscopy, Surgical OR Endoscopic Surgical Procedure OR Endoscopic Surgical Procedures OR Endoscopy) and a total of 1088 articles were retrieved.
A similar search strategy was used for EMBASE (www.embase.com ), Cochrane and LILACS (via BVS) databases. A manual search was also conducted through references of previously selected studies and published reviews.
Study selection
Two reviewers (DPSA and ELAA) independently searched databases to look for studies, using the search terms previously mentioned. They reviewed the title and abstract search with inclusion decisions for each study made independently based on the eligibility criteria. Any disagreement between reviewers was discussed with a third reviewer (EFSM) and agreement was reached by consensus.
Seven clinical trials, of which one was a randomized clinical trial and six were not randomized, and 32 cohort studies were included. The search strategy is summarized in Figure 1.
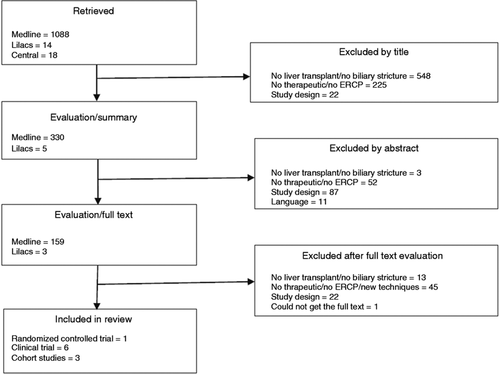
Study selection strategy.
ERCP: endoscopic retrograde cholangiopancreatography.
Data collection
Data were collected through standardized files for general study data collection. Studies were identified by surnames of their first author and publication year. Data on participants, performed interventions and observed clinical outcomes were collected.
Study quality evaluation
Selected articles were evaluated regarding their evidence strength according to the Oxford table. The single randomized clinical trial was evaluated using the Jadad Scale and was classified as adequate (score = 3) and those non-randomized were evaluated using the Newcastle-Ottawa Scale, considered adequate for analyzing texts with score equal to or higher than 6. The results are presented in Figure 2.
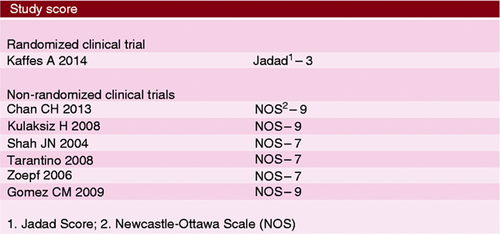
Score of studies according to the Jadad Scale for randomized clinical trials and the Newcastle-Ottawa Scale for non-randomized clinical trials.
Statistical analysis
Measures of effectiveness or damage of every intervention, expressed in absolute values, were analyzed through the difference of absolute risk and the ratio of number of patients presenting outcomes to the number of patients who underwent endoscopic treatment with a confidence interval (CI) of 95% and statistical significance of p < 0.05.
Statistical analysis was performed using the software programs RevMan 5 (Revision Manager 2008) and OpenMeta (Analyst).
Heterogeneity
Inconsistencies among clinical trials were evaluated through heterogeneity Chi-square (Chi2 ) test and quantified using the I 2 test. Values over 50% were considered substantial and representative of heterogeneity among studies.
Results
- According to donor liver graft (considering graft from brain-dead or living donors) analyses of stricture incidence, technical failure of endoscopic treatment, failure of stricture resolution, and stricture recurrence were conducted.
- According to the endoscopic therapy, comparing balloon biliary dilation alone (BD) vs. BD with biliary plastic stent insertion and comparing the use of biliary plastic stent vs. self-expandable metallic stent (SEMS), analyses related to treatment failure, recurrence and complications were performed.
Clinical outcomes related to transplantation with graft from a deceased liver donor vs. a living liver donor
Stricture incidence
Four studies evaluated the incidence of AS in DDLT vs. LDLT totaling 1285 patients.3-6 Stricture rate in the DDLT group was 13% (134 of 1143 patients) and 35% in the LDLT group (49 of 142 patients). Significant reduction of absolute risk of stricture incidence was found to be 16% in favor of the DDLT group compared to the LDLT group. (95% CI, −0.24 to −0.08; p = 0.0001; I 2 = 0%, Figure 3 ) through meta-analysis of selected studies.
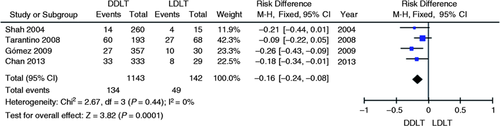
Meta-analysis and graph of four studies comparing the incidence of biliary anastomotic strictures in DDLT vs. LDLT. DDLT: deceased donor liver transplantation; LDLT: living donor liver transplantation.
Endoscopic treatment technical failure
Four studies evaluated the technical failure absolute risk of endoscopic treatment for anastomotic strictures in DDLT vs. LDLT.3-6 A significant reduction in the absolute risk of technical failure of the endoscopic treatment for post-liver transplantation AS in the DDLT group was found to be 23% (95% CI, −0.37 to −0.10; p = 0.0009; I 2 = 80%, Figure 4 ) through meta-analysis of studies.
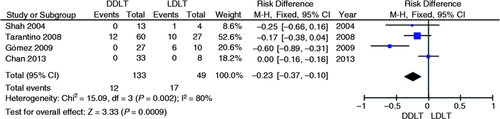
Meta-analysis and graph of four studies comparing the risk of technical failure of endoscopic treatment in DDLT vs. LDLT. DDLT: deceased donor liver transplantation; LDLT: living donor liver transplantation; CI: confidence interval.
Stricture resolution failure
Four studies evaluated the resolution failure absolute risk of AS with endoscopic treatment using BD and plastic stent comparing DDLT vs. LDLT.3-6 No significant increase or reduction in the absolute failure of resolution risk of AS was noted with endoscopic treatment between the DLDT vs. LDLT group (95% CI, −0.28 to 0.04; p = 0.15; I 2 = 75%, Figure 5 ) by the meta-analysis of the selected studies.
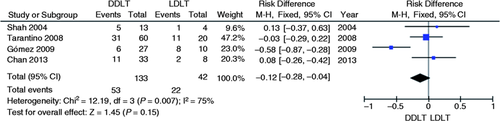
Meta-analysis and graph of four studies comparing resolution failure risk of anastomotic strictures with endoscopic treatment in DDLT vs. LDLT. DDLT: deceased donor liver transplantation; LDLT: living donor liver transplantation; CI: confidence interval.
Stenosis recurrence
Two studies evaluated the recurrence risk of AS between DDLT vs. LDLT.4, 6 The DDLT group was found to have lower absolute risk of stricture recurrence compared to the LDLT group (7% vs. 33%). A significant reduction in absolute recurrence risk of AS in the deceased donor group was found compared to the living donor group (95% CI, −0.48 to −0.02; p = 0.03; I 2 = 90%, Figure 6 ) through meta-analysis.
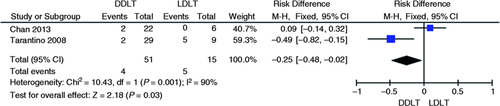
Meta-analysis and graph of two studies comparing recurrence risk of anastomotic strictures in DDLT vs. LDLT. DDLT: deceased donor liver transplantation; LDLT: living donor liver transplantation; CI: confidence interval.
Clinical outcomes related to the endoscopic treatment of biliary anastomotic strictures after liver transplantation: BD vs. BD plus plastic stent
Treatment failure
Two studies evaluated failure risk of the endoscopic treatment for post-liver transplant AS using dilation alone vs. dilation and insertion of plastic stents.7, 8 The incidence of failure in the initial treatment with BD alone was found to be lower than BD with endoprosthesis (BD + EP) (5% vs. 8%, respectively) by evaluation of the simple mean of studies. No decrease in the absolute failure risk of treatment either in favor of the dilation alone group or in favor of the dilation with plastic stents group was verified through meta-analysis (95% CI, −0.18 to 0.16; p = 0.89; I 2 = 0%, Figure 7 ).
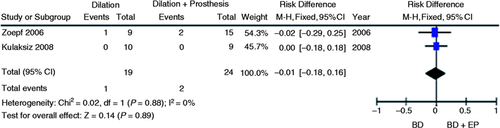
Meta-analysis and graph of two studies comparing failure risk of initial treatment of post-liver transplantation anastomotic strictures using BD vs. BD+EP. BD: balloon dilation; EP: endoprosthesis; CI: confidence interval.
Stricture recurrence
Two studies evaluated AS recurrence in post-liver transplantation patients after treatment with BD vs. BD + EP.7, 8 Incidence of stricture recurrence was noted to be higher in the exclusively BD group compared to the BD + EP group through the simple mean of studies (33% vs. 27%). However, no significant reduction of the absolute risk in favor of BD + EP treatment was found through meta-analysis (95% CI, −0.17 to 0.37; p = 0.45; I 2 = 64%, Figure 8 ).
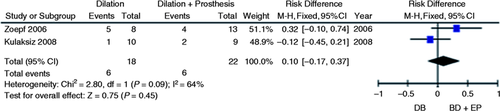
Meta-analysis and graph of two studies comparing stricture recurrence risk after initial treatment using BD vs. BD + EP. BD: balloon dilation; EP: endoprosthesis; CI: confidence interval.
Endoscopic treatment complications
Two studies evaluated the incidence of complications derived from procedure (pancreatitis and cholangitis) in the endoscopic treatment of post-transplant anastomotic strictures with dilation vs. dilation plus EP.7, 8 Simple mean of studies indicated the reduction of complication risk in favor of the dilation group compared to the dilation with EP group (11% vs. 19%). Reduction of complication risk in favor of the dilation group was verified, nonetheless this reduction was not significant after the meta-analysis was conducted (95% CI, −0.19 to 0.03; p = 0.17; I 2 = 70%, Figure 9 ).
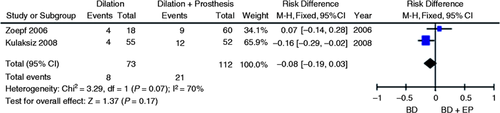
Meta-analysis and graph of two studies comparing complication risk of the endoscopic treatment for post-liver transplantation anastomotic strictures compared to the treatment with BD alone vs. BD + EP. BD: balloon dilation; EP: endoprosthesis; CI: confidence interval.
Plastic stent vs. SEMS
A single randomized clinical trial evaluated the endoscopic treatment for deceased donor post-liver transplantation AS comparing the use of a fully covered self-expandable metal stent (FCSEMS) vs. plastic stent.9 No increase or decrease in absolute risk failure of the initial endoscopic treatment (95% CI, −0.62 to 0.22; p = 0.35, Figure 10 ) and in the recurrence incidence (95% CI, −0.51 to 0.36; p = 0.74, Figure 11 ) were demonstrated by their results, as shown in the graphs with the outcomes from this study. A significant reduction of 40% in the absolute complication risk after endoscopic treatment was found in the metal stent group compared to the plastic stent group (95% CI, −0.76 to −0.04; p = 0.03, Figure 12 ) as shown in the following meta-analyses and graphs.
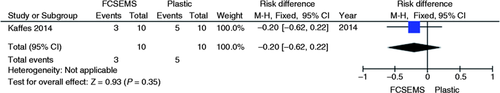
Meta-analysis and graph of failure risk of initial endoscopic treatment for post-liver transplant anastomotic stricture, comparing treatment with FCSEMS vs. plastic stent. FCSEMS: fully covered self-expandable metal stents; CI: confidence interval.
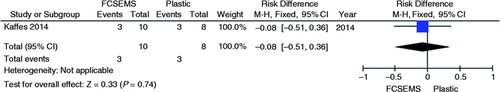
Meta-analysis and graph of recurrence incidence of post-liver transplant anastomotic stricture comparing treatment with FCSEMS vs. plastic stent. FCSEMS: fully covered self-expandable metal stents; CI: confidence interval.
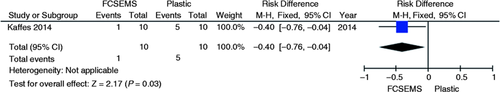
Meta-analysis and graph of complication incidence of post-liver transplant after endoscopic treatment of post-liver transplant anastomotic stricture comparing treatment with FCSEMS vs. plastic stent. FCSEMS: fully covered self-expandable metal stents; CI: confidence interval.
Prospective cohort studies
Nine prospective cohort studies (Tables2–5 ) or retrospective cohort studies (Tables 6 and 7 ) were retrieved. These studies were evaluated separately according to their design and type of intervention.
| Reference | Year | Study design | Patient | Reconstruction | Endoscopic intervention | N |
|---|---|---|---|---|---|---|
| Tarantino10 | 2015 | Prospective cohort | Refractory post-transplant biliary stenosis | DD or multiple anastomosis | ERCP + SEMS | 70 |
| Devière11 | 2014 | Prospective clinical trial | Benign biliary strictures (CP, OLT and CCY) | DD anastomosis | ERCP + FCSEMS | 42 OLT |
| Kahaleh12 | 2008 | Prospective cohort | BBS | NA | Sphincterotomy + PCSEMS | 79 |
- CP: chronic pancreatitis; OLT: orthotopic liver transplantation; CCY: cholecystectomy; BBS: benign biliary stricture; DD: duct-to-duct; NA: not available; ERCP: endoscopic retrograde cholangiopancreatography; SEMS: self-expandable metal stent; FCSEMS: fully covered self-expandable metal stent; PCSEMS: partially covered self-expandable metal stent.
| Reference | Stricture after LT | Mean age ± SD | Male, N (%) | Initial success, N (%) | Stricture resolution, N (%) | Recurrence, N (%) | Complications, N |
|---|---|---|---|---|---|---|---|
| Tarantino 201510 | 70 | 59.3 ± 8.7 | 48 (68.6) | 46 (65.7) | 28 (40.0) | 18 (39.0) | NA |
| Devière 201411 | 42 | 56.5 ± 11.4 | 35 (83.3) | NA | 28 (68.3) | 8 (28.5) | Cholangitis: 10 Abdominal pain: 4 Cholestasis: 1 Others: 2 |
| Kahaleh 200812 | 16 | 55 (21–93)a | 54 (68.3) | NA | 15 (93.7) | NA | NA |
- a Mean (range).
- LT: liver transplantation; SEMS: self-expandable metal stent; NA: not available.
| Reference | Year | Study design | Patient | Reconstruction | Endoscopic intervention | N |
|---|---|---|---|---|---|---|
| Wadhawan13 | 2013 | Prospective cohort | AS after OLT | NA | Sphincterotomy + BD + progressive stenting | 31 |
| Poley14 | 2013 | Prospective cohort | BC after LDLT | CDC or HJ | BD + plastic stent | 338 |
| Seo15 | 2009 | Prospective cohort | AS after LDLT | DD and HJ anastomosis | Sphincterotomy + BD + plastic stent | 239 |
| Holt16 | 2007 | Prospective cohort | AS after OLT | DD anastomosis | Sphincterotomy + BD + Plastic stent | 53 |
| Soejima17 | 2006 | Prospective cohort | BS after LDLT | DD or HJ anastomosis | Plastic stent | 182 |
| Graziadei18 | 2006 | Prospective cohort | BS after LT | CC anastomosis | Sphincterotomy + BD + plastic stent | 84 |
- AS: anastomotic stricture; OLT: orthotopic liver transplantation; LDLT: living donor liver transplantation; BC: biliary complication; BS: biliary stricture; LT: liver transplantation; HJ: hepatico-jejunal anastomosis; CDC: choledochocholedochostomy; DD: duct-to-duct anastomosis; CC: choledochocholedocho anastomosis; BD: balloon dilation; NA: not available.
| Reference | Stricture after LT treated with ERCP | Mean age ± SD | Male, N (%) | Technical success, N (%) | Stricture resolution, N (%) | Recurrence, N (%) | Complications, N |
|---|---|---|---|---|---|---|---|
| Wadhawan 201313 | 31 | 61 (28–75)a | 21 (67.7) | 31 (100) | 25 (80.6) | 6 (19.3) | Cholangitis: 12 Cholestasis: 11 Pancreatitis: 7 Pain: 3 |
| Poley 201314 | 35 | 48 (0.5–75)b | 278 (82.2) | 33 (75.0) | 40 (91.0)c | 7 (24.7) | Bleeding: 6d Pancreatitis: 1d |
| Seo 200915 | 68e | 49 ± 8.8 | 50 (73.5) | 15 (57.7) | 11 (73.3) | 4 (36.3) | Cholangitis: 1 |
| Holt 200716 | 53 | 48.5 (37–61)a | 21 (39.6) | 49 (92.4) | 34 (69.3) | 2 (5.0) | Pancreatitis: 2 Cholangitis: 1 Stent migration: 1 |
| Soejima 200617 | 27 | 52.6 ± 12.7 | 53 (50.0) | 24 (88.9) | 15 (62.5) | 0 | NA |
| Graziadei 200618 | AS: 65 | 54.2 | 46 (70.7) | NA | 50 (76.9) | NA | Leak: 2 Cholangitis: 2 Others: 1 |
| NAS: 19 | 51.0 | 15 (78.9) | NA | 0 | NA |
- a Median (range). b Mean (range). c ERCP + PTBD. d Leaks + strictures. e Anastomotic + non-anastomotic. LT: liver transplantation; ERCP: endoscopic retrograde cholangiopancreatography; AS: anastomotic stricture; NAS: non-anastomotic stricture; NA: not available.
| Reference | Year | Study design | Patient | Reconstruction | Endoscopic intervention | N |
|---|---|---|---|---|---|---|
| Faleschini19 | 2015 | Retrospective cohort | Biliary complications after OLT | DD anastomosis | Sphincterotomy + BD + plastic stent | 360 |
| Cai20 | 2015 | Retrospective cohort | BS after OLT | CC anastomosis | BD + plastic stent | 76 |
| Albert21 | 2013 | Retrospective cohort | AS after OLT | Side-to-side and Duct-to-duct anastomosis | BD ± plastic stent | 374 |
| Hsieh22 | 2013 | Retrospective cohort | AS after LDLT | DD anastomosis | BD ± plastic stent | 110 |
| Takeda23 | 2013 | Retrospective cohort | AS after LDLT | Duct-to-duct anastomosis | ERCP or PTCD + BD + plastic stent | 53 |
| Cai24 | 2012 | Retrospective cohort | AS and NAS after OLT | Choledocho-choledochostomy | Sphincterotomy + BD + plastic stent | 56 |
| Sanna25 | 2011 | Retrospective cohort | BC after OLT | CC-anastomosis | Sphincterotomy + BD + plastic stent | 94 |
| Kobayashi26 | 2011 | Retrospective cohort | AS after LDLT and OLT | DD anastomosis | BD + plastic stent | 12 |
| Kim27 | 2011 | Retrospective cohort | BS after LDLT | DD or HJ | Sphincterotomy ± BD + plastic stent | 147 |
| Chang28 | 2010 | Retrospective cohort | BS after LDLT | DD anastomosis | Dilation + stent + sphincterotomy | 113 |
| Tabibian29 | 2010 | Retrospective cohort | AS after OLT | Choledocho-choledochostomy | Dilation + plastic stent | 69 |
| Kato30 | 2009 | Retrospective cohort | BS after LDLT | DD and HJ anastomosis | BD + plastic stent + sphincterotomy | 141 |
| Lee31 | 2008 | Retrospective cohort | BS after LDLT or DDLT | CC or HJ anastomosis | Sphincterotomy + BD + plastic stent | 378 |
| Barriga32 | 2008 | Retrospective cohort | BS after OLT | CC or HJ anastomosis | Dilation + plastic stent | 25 |
| Elmi33 | 2007 | Retrospective cohort | AS after OLT | DD anastomosis | Stent ± BD ± sphincterotomy | 15 |
| Pasha34 | 2007 | Retrospective cohort | AS after DDLT | CC anastomosis | BD + plastic stent | 25 |
| Alazmi35 | 2006 | Retrospective cohort | Recurrence of AS after OLT | CC anastomosis | Sphincterotomy + dilation + stent | 24 |
| Verdonk36 | 2006 | Retrospective cohort | AS after OLT | DD or HJ anastomosis | BD + stent | 47 |
| Zoepf37 | 2005 | Retrospective cohort | BS after LDLT | DD or HJ anastomosis | Sphincterotomy + BD + plastic stent | 90 |
| Khuroo38 | 2005 | Retrospective cohort | BC after DDLT | DD or HJ anastomosis | BD + stent | 220 |
| Rerknimitr39 | 2002 | Retrospective cohort | BC after OLT | CC anastomosis | BD + stent | 367 |
| Mahajani40 | 2000 | Retrospective cohort | BS after | CC anastomosis | BD ± stent | 30 |
| Rizk41 | 1998 | Retrospective cohort | BS after OLT | DD or HJ anastomosis | Dilation + stent | 22 |
- BS: biliary stricture; BD: balloon dilation; OLT: orthotopic liver transplantation; LDLT: living donor liver transplantation; DDLT: deceased liver donor transplantation; AS: anastomotic stricture; NAS: non-anastomotic stricture; DD: duct-to-duct anastomosis; HJ: hepato-jejuno anastomosis; CC: choledochocholedocho anastomosis; ERCP: endoscopic retrograde cholangiography; PTCD: percutaneous transhepatic drainage.
| Reference | Stricture after LT treated with ERCP | Mean age ± SD | Male, N (%) | Technical success, N (%) | Stricture resolution, N (%) | Recurrence, N (%) | Complications, N (%) |
|---|---|---|---|---|---|---|---|
| Faleschini 201519 | 79 | 55 (48–60)a | 272 (75.6) | NA | 68 (86.0) | NA | Bleeding: 3 |
| Cai 201520 | AS: 48 | 57.33 ± 5.61 | 46 (60.5) | NA | 42 (87.5) | 8 (19.0) | Bleeding: 3 Pancreatitis: 7 |
| NAS: 28 | NA | 23 (82.1) | 8 (34.7) | ||||
| Albert 201321 | 47 | 49.8 | 254 (67.9) | NA | 45 (95.7) | 16 (34.0) | Cholangitis: 19 (9.5) Pancreatitis: 6 (3.0) Bleeding: 5 (2.5) Perforation: 2 (1.0) |
| Hsieh 201322 | 38 | NA | 23 (60.5) | 32 (84.2) | 30 (78.9) | 8 (21.1) | Cholangitis: 17 (9.1)b Occlusion: 12 (6.4)b Pancreatitis: 3 (1.6)b Others: 4 (2.1)b |
| Takeda 201323 | 11 | 49.9 (16–66)a | 15 (45.4) | 9 (81.8) | 2 (20.0) | 8 (80.0) | NA |
| Cai 201224 | AS: 38 | 59.9 ± 5.2 | 24 (63.2) | NA | 33 (86.8) | 6 (18.2) | 6 (15.8) |
| NAS: 18 | 57.1 ± 5.8 | 10 (55.6) | NA | 14 (77.8) | 7 (50.0) | 3 (16.7) | |
| Sanna 201125 | 44 | NA | 72 (76.6) | 136 (90.7)2 | 22 (64.7) | 0 | Bleeding: 8 (8.5) Pancreatitis: 6 (6.4) Fever: 1 (1.1) |
| Kobayashi 201126 | 12 | 42 (16–58)a | 6 (50%) | NA | 10 (83.3) | NA | Pancreatitis: 5 (12.5)b Cholangitis: 1 (2.5)b |
| Kim 201127 | 147 | 53.1 ± 8.6 | 115 (78.2) | 82 (55.8) | 52 (36.9) | 6 (11.5) | Cholangitis: 4 (0.8)b Pancreatitis: 26 (4.9)b Bleeding: 7 (1.3)b Death: 1 (0.2)b |
| Chang 201028 | 121 | 49.9 ± 8.2 | 89 (78.8) | 90 (79.6) | 30 (26.5) | NA | Cholangitis or liver abscess: 71 (19.5)b Pancreatitis: 30 (8.2)b |
| Tabibian 201029 | 69 | 52.5 (26–72)a | 45 (65.2) | 69 (100) | 65 (94.2) | 2 (3.1) | Pancreatitis: 2 (0.7)b Bacteremia: 2 (0.7)b |
| Kato 200930 | 41 | 55 (27–66)c | 26 (63.4) | 31 (75.6) | 28 (68.3) | 7 (25) | Cholangitis: 2 (4.9) Liver abscess: 1 (2.4) Biliary stone: 5 (12.2) |
| Lee 200831 | AS: 17 | 52.2 ± 1.7 | 22 (88.0) | NA | 15 (60.0) | NA | 6 (24.0)b |
| NAS: 8 | |||||||
| Barriga 200832 | 25 AS + NAS | 50.5 (19–72)c | 18 (72.0) | 19 (76.0) | 12 (48.0) | NA | Pancreatitis: 2 (3.3)b Cholangitis: 2 (3.3)b |
| Elmi 200733 | 15 | 52 ± 9.2 | 6 (40.0) | NA | 13 (86.7) | NA | Pancreatitis: 1 (1.9)b Perforation: 1 (1.9)b Cholangitis: 2 (3.8)b Bleeding: 1 (1.9)b Stent occlusion: 7 (13.2)b |
| Pasha 200734 | 25 | 46.7 ± 7.15 | 20 (80.0) | 22 (88.0) | 18 (82.0) | 4 (22.2) | Pancreatitis: 1 (0.9)b Fever:1 (0.9)b Hypoxia: 1 (0.9)b Migration: 2 (1.9)b |
| Alazmi 200635 | 143 | NA | NA | 143 (96.6) | 131 (91.6) | 24 (18.3) | NA |
| Verdonk 200636 | 46 | 46 (18–64)c | 24 (52.2) | 27 (75.0) | 24 (88.9) | 3 (11.1) | NA |
| Zoepf 200537 | 7 | 57 (45–66)c | 5 (71.4) | 7 (100) | 7 (100) | 1 (14.3) | Bleeding: 1 (4.0)b Cholangitis: 2 (8.2)b Pancreatitis: 3 (12.2)b |
| Khuroo 200538 | 15 | 45.1 ± 15.9 | 20 (80.0) | NA | 9 (60,0) | NA | NA |
| Rerknimitr 200239 | AS: 43 | 36.5 (9–68)c | 28 (51.0) | NA | 43 | NA | NA |
| NAS: 12 | 7 | ||||||
| Mahajani 200040 | 30 | 49.4 ± 11,9 | 12 (40.0) | NA | 19 (63.3) | 4 (21.0) | Cholangitis: 2 (6.7) Bleeding: 2 (6.7) Migration: 1 (3.3) |
| Rizk 199841 | AS: 10 | 40.7 | 8 (80.0) | NA | 7 (70.0) | NA | Bleeding: 1 (4.5) Pancreatitis: 1 (4.5) Cholangitis: 1 (4.5) |
| NAS: 12 | 46.3 | 8 (66.7) | NA | 2 (16.7) |
- a Median (range). b Procedure related. c Mean (range). LT: liver transplantation; ERCP: endoscopic retrograde cholangiography; AS: anastomotic stricture; NAS: non-anastomotic stricture; NA: not available.
Prospective cohort studies—SEMS
Three studies were retrieved,10-12 whose characteristics and data are summarized in Tables 2 and 3.
Resolution
Three prospective cohort studies evaluated stenosis resolution rate after liver transplant using metallic stents.10-12
Stricture resolution was achieved in 89 of 127 patients and the analysis of these studies revealed a 76.0% resolution rate (95% CI, 0.58 to 0.94, Figure 13 ). Heterogeneity (I 2 ) among studies was found to be 84.6% (p = 0.001).
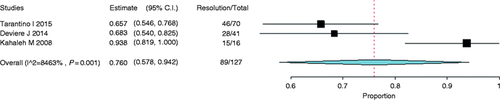
Forest plot of stricture resolution rate after liver transplant with metal stents. CI: confidence interval.
Recurrence
Two studies evaluated stricture recurrence.10, 11 Stricture recurrence was observed in 26 of 74 patients. A recurrence rate of 34.7% was demonstrated through the analysis of these studies (95% CI, 0.24 to 0.45, Figure 14 ). Heterogeneity (I 2 ) between studies was found to be 0% (p = 0.344).

Forest plot of stricture recurrence rate after treatment with metal stents. CI: confidence interval.
Prospective cohort studies—plastic stents
Six studies were retrieved,13-18 whose characteristics and data are detailed in Tables 4 and 5.
Resolution
Stricture resolution was reported in 164 of a total of 228 patients in six prospective cohort studies that evaluated the use of plastic stents.13-18 The analysis demonstrated a resolution rate of 72.7% (95% CI, 0.67 to 0.78, Figure 15 ). Heterogeneity (I 2 ) among studies was found to be 0% (p = 0.531).
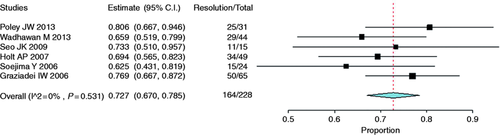
Forest plot of stricture resolution rate after liver transplantation with the use of plastic stents. CI: confidence interval.
Recurrence
Stricture recurrence was reported in 19 of a total of 130 patients. The analysis of five studies that reported recurrences revealed a recurrence rate of 12.7% (95% CI, 0.03 to 0.21, Figure 16 ). Heterogeneity (I 2 ) among studies was found to be 64.6% (p = 0.023).13-17
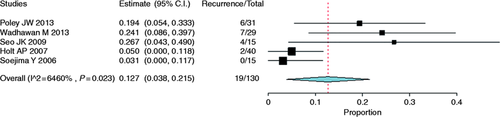
Forest plot of stricture recurrence rate after treatment with plastic stents. CI: confidence interval.
Retrospective cohort studies—plastic stents
Twenty-three retrospective cohort studies19-41 that evaluated the use of plastic stents to treat biliary strictures after liver transplants were retrieved and their characteristics and data are presented in Tables 6 and 7.
Clinical outcomes related to the endoscopic treatment of post-liver transplant strictures with plastic stents
Resolution
Stricture resolution using plastic stents was reported in 23 retrospective cohort studies,19-41 encompassing a total of 1112 patients. The analysis revealed a resolution rate of 70.6% (95% CI, 0.61 to 0.80, Figure 17 ). Heterogeneity (I 2 ) among studies was found to be 95.0% (p < 0.001).
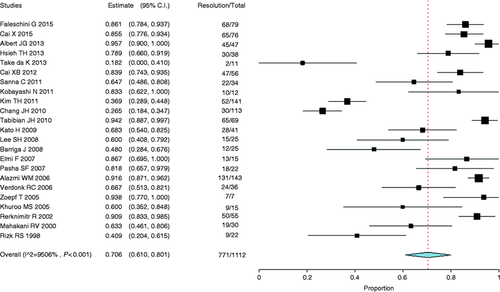
Forest plot of stricture resolution rate after liver transplantation with the use of plastic stents. CI: confidence interval.
Recurrence
Stricture recurrence after the treatment with plastic stents was reported in 14 studies20-25, 27, 29, 30, 34-37, 40 and 114 patients from a total of 576 presented recurrence. The analysis of 14 studies demonstrated a recurrence rate of 20.9% (95% CI, 0.14 to 0.28, Figure 18 ). Heterogeneity (I 2 ) among studies was found to be 85.4% (p < 0.001).
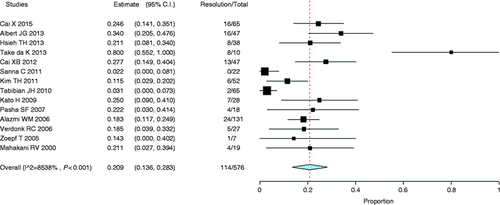
Forest plot of stricture recurrence rate after treatment with plastic stents. CI: confidence interval.
Discussion
Research topic
Biliary complications are common after liver transplantation and constitute a therapeutic challenge to endoscopists. They are considered the “Achilles heel” of liver transplantation as the major cause of morbidity and graft loss. Biliary complication frequency varies across the published series from 5% to 20% of transplants with brain-dead liver donors42-46 and from 20% to 34% of transplants with living liver donors3, 43, 47, 48 encompassing biliary fistulas and strictures, as well as formation of biliary stones, casts and sludge.
The etiology of post-liver transplantation complications is multifactorial and includes vascular, technical and immunologic components that determine their type, frequency, location, progression time, and treatment response. Biliary strictures are one of the most frequent complications after liver transplantation and are divided into AS and NAS. AS account for 4%–9% of post-liver transplantation complications in the contemporary literature,16, 36, 49 whereas NAS for 1%–19% of complications.50-52
Endoscopic intervention is currently the gold standard for post-liver transplantation AS treatment, but treatment strategy for this complication remains uncertain. Repeated endoscopic interventions, including radial dilation with balloon catheters with or without plastic stents for a prolonged period of up to 24 months, are in general necessary to achieve sustained clinical success.
Efficacy of plastic stents is limited to permeability loss in the short term and need for repeated procedures approximately every three months. The plastic stent exchange interval was about three months in most studies and the mean number of stents inserted per session was between two and three.53 The mean number of dilations varied from 2.5 to 5.5 per patients in the studies that compare BD vs. BD+ plastic stents for AS after orthotopic liver transplantation (OLT).7, 8
Some authors imply the time of stricture occurrence after transplant, suggesting that early AS (less than one month) is related to edema and probably easily resolved, with less stenting time.14 Current evidence of BD + multiple plastic stents in the management of AS after OLT suggest that shorter stent durations (four to six months) is associated with better outcomes for early AS, and later AS was treated with longer stent duration of at least 12 months.14, 16, 20-22, 25, 29, 34, 35, 39 But some studies didn’t confirm this evidence, showing that there is still uncertainty and few data to suggest the onset of AS being a predictor of successful endoscopic intervention.9
However, the literature reports that a number of patient-related factors, such as donor type, anatomy, SEMS as primary therapy or after BD and/or plastic stents could affect the resolution rate of the AS in OLT and thereafter, the time of stent permanence. When SEMS is used to treat these afflictions, the different types of the SEMS are also a limiting factor to compare the studies. SEMS duration of at least three months appeared to result in higher stricture resolution rates.53
The risk of bleeding, cholangitis, pancreatitis, biliary fistula, biliary and duodenal perforation, and stent migration is cumulative with successive endoscopic procedures. The use of covered SEMS to treat these lesions, despite their elevated initial cost, may reduce treatment time and number of procedures, since they provide long-lasting biliary drainage and, most of the time, only two procedures are performed: one for stent insertion and another for removal, therefore there is no need for frequent replacement. For many years SEMS were indicated exclusively as definitive treatment (palliation of unresectable bile duct tumors), but this paradigm has been reevaluated regarding benign pathologies for which partially or fully covered metal stents are used with the possibility of removal.
Kahaleh et al.12 reported in a study published in 2008 that the use of SEMS for benign biliary strictures is possible, but rather controversial because of factors such as: generally small caliber of bile ducts; annular strictures preventing adequate stent anchorage and favoring migration (14%) and Bismuth III and IV benign strictures that, for anatomic reasons, would hinder bile duct drainage contralaterally. The use of such stents in post-liver transplantation strictures was found to produce better results due to the difference in bile duct caliber with more space for stent placement, but caution is highly recommended because of the high risk of perforation.
The systematic literature review conducted by Kao et al.53 compared the use of multiple plastic stents vs. SEMS to treat post-liver transplantation biliary AS and they did not identify any randomized controlled study, but only small case series. Stricture resolution rate with plastic stents was 94%–100% for 12 months or more of stent permanence and with SEMS, 80%–95% for more than three months of stent permanence. Metal stent migration rate was 16%. Given the small number of patients in these studies, it was not clear whether FCSEMS or longer stent durations were predictors of stent migration rates. The use of metal stents for AS management after liver transplantation has not yet been found to present an advantage over plastic stents, although it seems to be a promising procedure.
Similarly, no controlled, randomized clinical trial was found through the systematic review conducted by Nacif et al.54 concerning outcomes from endoscopic AS treatment after liver transplantation, but only prospective and retrospective comparative studies. Studies comparing endoscopic with percutaneous access were included and similar clinical success rates for both groups were found, with longer treatment time in the percutaneous access group. No difference in success and complication rates was verified in the comparison between BD alone and BD plus plastic stents.
There is a large variability of studies regarding the endoscopic treatment of post-liver transplantation strictures, with conflicting results at times. The present research was motivated by this objective question in order to address this issue.
Analyzed clinical outcomes
Clinical outcomes related to graft used for liver transplantation (DD vs. LD)
Four clinical trials3-6 were selected to compare grafts from DDLT and LDLT.
Tarantino et al.4 showed the results and follow-up in a cohort with 261 patients with complications after liver transplantation from DDLT (193 patients) and LDLT (68 patients). Complications were found to occur more often in LDLT, but complication indexes were also found to be high in DDLT probably because it included donors with expanded criteria. In this study, 46.1% of LDLT were performed using organs from marginal donors and the biliary complication rate was found to be 41.6% in this subgroup compared to 33.6% in the subgroup with organs from non-marginal donors, although this difference was not statistically significant.
The possibility of forecasting the increase in biliary complications in adult LDLT was analyzed in the study conducted by Shah et al.3 The frequency of biliary fistulas (53% LDLT vs. 12% DDLT, p = 0.001) and biliary strictures (27% LDLT vs. 5% DDLT, p = 0.01) diagnosed by ERCP was found to be four times higher in LDLT than in DDLT. Contributing factors to higher biliary complication rate in LDLT were the need for multiple biliary anastomoses and the smaller diameter of biliary anastomosis. Additionally, vascular anastomoses with smaller diameters may have contributed to the increase of ischemic complications and stenosis in this group of patients. Success rates of endoscopic treatment of biliary complications in LDLT and DDLT were found to be similar in this study, so ERCP has to be the initial option for managing biliary complications in post-liver transplant patients with duct-duct anastomosis.
In a total of 362 patients who underwent liver transplantation, AS was found in 27.6% of LDLT and 9.9% in DDLT (p = 0.01) in a study by Chan et al.,6 whereas no difference between both groups was verified comparing mean time for stenosis development, endoscopic treatment response, mean time for stenosis resolution, or number of ERCP procedures to achieve resolution.
Biliary complications were observed to be significantly more frequent after LDLT compared to DDLT (33.3% vs. 9.5%, p = 0.001) in a study by Gómez et al.5 The endoscopic treatment of these complications was successful in only a minority of LDLT, compared to the good results from DDLT. The low endoscopic treatment success rate in LDLT was likely to be attributed to biliary anatomy, which is a challenge to endoscopists.
Clinical outcomes related to endoscopic treatment types for post-liver transplantation biliary strictures
BD alone vs. BD plus BP
Two comparative studies7, 8 were identified when comparing these two types of endoscopic treatment for post-liver transplantation biliary strictures.
Zoepf et al.7 conducted a retrospective analysis comprising 25 patients with post-liver transplantation AS comparing patients treated with stricture BD alone to dilation associated with the insertion of an increasing number of BP. In general, endoscopic treatment was successful in 22 of 25 patients (88%). BD primary success was found to be 89%, but recurrence was shown in 62%. BD + BP association was initially successful in 87% and recurrence was observed in 31% of patients. All recurrences were retreated with success by BD + BP. Complication rate was low with 7.3% of bacterial cholangitis, 9% of mild pancreatitis and 8% of mild bleeding after sphincterotomies. The high efficacy and safety of endoscopic treatment for biliary AS after liver transplantation and high recurrence rate with BD alone treatment were demonstrated by these data.
A prospective, randomized clinical trial comparing BD to BD + BP in the treatment of biliary strictures after liver transplantation was conducted by Kulaksiz et al.8 Because of difficulties in randomizing some patients, this study was transformed into a prospective comparative study because, in some cases, patients were allocated in a determined group at the discretion of the endoscopist. In AS patients treated with BD, initial technical success rate, as well as primary clinical success, was 100%, whereas in the BD + BP group, initial clinical success rate as 93% (one recurrence). Regardless of intervention type, sustained clinical success rate over three months in both groups was 100%, but complication rate was higher in the stent group. Incidence of stricture recurrence was higher in the BD group compared to the BD + BP group by the simple mean of studies (33% vs. 27%, respectively); however, there was no significant reduction in the absolute risk of stricture recurrence in favor of BD + BP treatment, according to the meta-analysis. Perhaps this analysis was hindered by the short-term study follow-up (17 to 19 months).
Plastic stent vs. SEMS
A single randomized clinical trial comparing the use of metal stent vs. plastic endoprosthesis for AS endoscopic treatment after liver transplantation was identified.9 Its results presented similar absolute risk rates of failure in initial endoscopic treatment (95% CI, −0.62 to 0.22; p = 0.35) and of stricture recurrence (95% CI, −0.51 to 0.36; p = 0.74) with a 40% reduction in absolute risk of complications after endoscopic treatment in the metal stent group compared to the plastic stent group (95% CI, −0.76 to −0.04; p = 0.03). No stent migration was reported in the study conducted by Kaffes et al.9 A complication rate reduction might be related to the prosthesis design, which is short, with central waist and a large loop for its removal inside the duodenum. Stent migration is usually one of the limiting factors for its use and is reported in 9%–45% of cases.55-60 Despite its small number of patients, the mean number of procedures was found to be two in the metal stent arm compared to 4.5 in the plastic stent arm (p = 0.0001). Mean treatment time, number of biliary dilations and prostheses, as well as number of hospital stay days for complications were also observed to be lower in the metal stent group, which may imply a reduction in hospital costs. In this study, the use of a metal stent for treating biliary strictures after liver transplantation reduced the number of ERCPs necessary for stricture resolution with less complication and resulted in a cost-effective procedure.
The analysis of prospective cohort studies using metal stents in AS treatment presented a resolution rate of 76% and recurrence rate of 34.7%. Those studies evaluating plastic stents presented a resolution rate of 72% and recurrence rate of 12.7%.
The analysis of retrospective cohort studies on stricture treatment after liver transplantation using plastic stents presented a resolution rate of 70.6% and recurrence rate of 20.9%.
Clinical practice implications
The present review establishes that liver transplantation with graft from deceased donor is associated with less risk of biliary complications compared to transplantation with a graft from a living donor. Since the frequency in the use of organs from living donors and from donors with expanded criteria has increased, all caution is mandatory to prevent the complications analyzed in this study.
Regarding the endoscopic treatment of biliary AS with BD alone or BD associated with plastic stent insertion, both methods were found to present similar effectiveness rates and no index of stricture resolution or of recurrence were found to be statistically significant between groups.
As for the comparison of the use of FCSEMS or plastic stents for treating post-liver transplantation biliary strictures, only one randomized clinical trial with a small number of patients with similar results in relation to treatment failure risk or stricture recurrence was published to date, showing a lower complication rate in the metal stent group. This study also indicates that the use of metal stents, despite their high initial cost, leads to a global cost reduction because of the lower number of procedures during the treatment period.
Research implications
Biliary complications after liver transplantation challenge surgeons, clinicians and endoscopists as a problem of difficult management in immunosuppressed patients. Endoscopic treatment is a safe and long-lasting alternative, but the selection of the ideal treatment method needs to be based on data with better methodological power and higher levels of evidence.
In order to achieve significant results to conduct a systematic review and meta-analysis with relevant clinical impact, well-designed clinical trials with adequate selection of patients are necessary. In addition, more thoroughly analyzed outcomes and adequate sample size comparing the three types of endoscopic treatment for post-liver transplantation biliary strictures, i.e. BD alone or associated with the insertion of plastic or metal stents, are necessary.
Conclusion
The use of graft from deceased donors for liver transplantation reduced the absolute risk of AS incidence, technical failure of endoscopic treatment and recurrence of strictures after endoscopic treatment.
The comparative analysis between the endoscopic procedures of BD alone and BD associated with plastic stent insertion to treat AS after liver transplantation has shown that both are similar regarding the outcomes of treatment failure, stricture recurrence and complications.
No statistically significant differences related to treatment failure risk or stricture recurrence were found by the only prospective, randomized study; however, a lower complication rate in the metal stent group was found.
Acknowledgment
The authors thank Dr Luca Bernardo for statistical analysis and technical support.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.




