Isolation, Characterization, and Application of Bacteriophage for Salmonella Control in Broiler Chickens
Abstract
The present study explores alternatives to antibiotics for poultry farms. The aims of this study were to isolate and characterize bacteriophages for selection of the appropriate phage, reduce Salmonella in the gastrointestinal tract of broiler chickens, and observe gut microbiota alterations after bacteriophage treatments. In this study, bacteriophages were isolated from two broiler chicken farms, two poultry processing plants, a goat farm, and a pig farm in the central region of Thailand. Out of the 33 samples analyzed, 25 (75.5%) tested positive for the presence of Salmonella bacteriophages. Among the 63 isolates examined, SEpBS-1 was selected for its ability to infect five Salmonella serovars: S. Enteritidis, S. Hadar, S. Typhimurium, S. Dublin, and S. Poona. Thermal stability test of phages showed that phages were stable at −6.5°C–50°C for 30 min, and significantly decreased (p < 0.05) at 60°C, and drastically decreased at 70°C. Furthermore, pH stability test of phages showed that phages were stable at pH 5-9. Phage SEpBS-1 was stable in acidic conditions. Phage titers decreased with increased salinity. The morphological characterization of the phage using transmission electron microscopy (TEM) revealed icosahedral heads and thin, long, noncontractile, flexible tails. The phage SEpBS-1 was classified as a member of the Siphoviridae family. The growth curve of the bacteriophage revealed that phage SEpBS-1 for SE had a latent period of 2 h, burst time of 2–3.5 h, and burst size of 166 PFU/infected cell. Phage SEpBS-1 for S. Typhimurium had a latent period of 2.5 h, burst time of 2.5–4 h, and burst size of 973 PFU/infected cell. Studying the effects of phage SEpBS-1 against Salmonella infection in broiler chickens found that Salmonella counts were slightly increased at 7 and 14 days after phage treatment. However, there was no statistically significant difference between groups (p > 0.05). Salmonella counts decreased by 40% at 14 days, while the positive control found the highest number of Salmonella in ceca. The application of lytic bacteriophages in the biocontrol of foodborne pathogens presents a promising approach for targeting Salmonella. Bacteriophage therapy offers an effective alternative to antibiotics for pathogen control.
1. Introduction
Antimicrobial resistance (AMR) is a critical concern in both human and animal health, particularly in food-producing animals. The AMR crisis contributes to a rising global incidence of infectious diseases in human populations, many of which have become increasingly difficult to treat with existing antimicrobial agents [1]. The forecast of the World Health Organization (WHO) in case, no action is taken to handle AMR, and the economic loss in terms of global production between now and 2050 would be US$ 100 trillion. Furthermore, the indirect costs of drug-resistant infections from morbidity, disability, premature death, and reduced effective labor supply are expected to cause a decrease in the global economic output, with estimated losses US$ 3.4 trillion annually [2]. In 2013, the Centers for Disease Control (CDC) in the USA asserted that humanity is now in the “postantibiotic” era. In May 2014, the WHO stated that the AMR crisis is becoming disastrous [3]. One of the current issues may be the results of overuse or irrational use of antibiotics in various cases, primarily in antimicrobial treatment in agricultural practices, animal healthcare, and the food-producing system [4]. Alternatives to antibiotics to treat bacterial infections are now under consideration and practices. One of the possible replacement options for antibiotics is the therapeutic use of bacteriophages [5]. Bacteriophages or phages are a group of viruses widely distributed in nature, which can infect and damage bacterial cells [6]. Phage infections have been identified across multiple bacterial species, including those from different genera such as Salmonella spp. and Campylobacter spp. Notably, phages can be isolated from the intestines of poultry, especially from free-range chickens. During processing at slaughterhouses, phages present in the gut may contaminate poultry meat, leading to their frequent presence in foods consumed by humans. In a previous study, field trials with phage application against Campylobacter in commercial broiler houses suggested that phages can lead to a reduction of up to log10 3.29 cfu in Campylobacter load [7]. Phages have been revealed to reduce microorganisms on contact surfaces, carcasses, and chicken meat [8].
Controlling Salmonella contamination remains a significant challenge for the poultry industry. The application of lytic bacteriophages for the biocontrol of foodborne pathogens offers a promising approach to managing Salmonella contamination, a method deemed safe by the U.S. Food and Drug Administration [9]. The objectives of this study were to (1) isolate and (2) characterize bacteriophages to identify an effective phage for reducing Salmonella in the broiler gut and (3) to examine the effects of bacteriophage treatment on the gut microbiota.
2. Materials and Methods
2.1. Sample Collection
Samples of Salmonella phages for isolation were collected from broiler farms and poultry processing plants (20 samples), a goat farm (4 samples), and a pig farm (1 sample) in the central region of Thailand. The samples were distributed as follows: poultry processing plant environment (wastewater treatment pond and fishpond), poultry processing plants including wastewater after spraying in lairage, water from carcass washing after scalding, defeathering and offal washing, poultry farms (cloacal swab and boot swab), fecal samples from goat farm, and wastewater from pig farm. Amber bottles with a capacity of 100 mL were sterilized prior to fluid sample collection. Each bottle was filled to three-fourths of its volume with the fluid sample and securely sealed. A cloacal swab was taken by inserting a sterile swab into the cloaca. The pools of 10 swabs were placed into 0.1% (w/v) buffered peptone water (BPW). Boot swabs were performed by wearing boot swabs and walking around the poultry house. Swabs were then placed in a sterile plastic bag for transportation on ice to the laboratory. Sampling personnel wore gloves while collecting goat fecal samples, which were then placed in sterile plastic bags and transported on ice to the laboratory for analysis.
2.2. Preparation of Salmonella
To prepare Salmonella as a host for phage cultivation, the Salmonella culture on a nutrient agar (NA) slant was transferred into 10 mL of nutrient broth (NB) and incubated at 37°C for 24 h. One mL of Salmonella suspension was transferred to 9 mL of BPW and performed tenfold serial dilution. The number of Salmonella was counted on xylose lysine deoxycholate (XLD) using a spread plate with serological confirmation.
2.3. Isolation of Bacteriophages
The methods for phage isolation were adapted from Atterbury et al. [10]. In brief, samples were diluted to 1:9 (w/v) in NB, followed by the addition of 1.0 × 108 cfu/mL of each of five Salmonella serovars: Salmonella Enteritidis (SE) ATCC DMST 15676, S. Dublin (SD), S. Hadar (SH) ATCC 10634, S. Poona (SP), and S. Typhimurium (ST) ATCC 13311 and incubated at 37°C for 24 h. A 1 mL sample from each culture was subjected to centrifugation at 4000 rpm for 5 min at 4°C, to remove the host cells. The supernatant was filtered through a 0.45-μm pore-size membrane with a plastic syringe. The agar overlay was performed by pouring the mixture of 0.8% agar, 9 mL of NB, novobiocin 50 μL at the concentrate of 1 μg/mL, and 1.0 × 108 cfu/mL of each Salmonella serovars on NA. The 20 μL volumes of filtrate were spotted onto the surface of the upper layer of overlay and incubated at 37°C for 24 h before examination for plaques. Individual plaques were extracted from the overlay agar with a pipette and suspended in a mixture of NB 10 mL, novobiocin 10 μL, and 0.5 mL of specific Salmonella to phage at a concentration of 1.0 × 108 cfu/mL. These suspensions were incubated at 37°C for 4 h by using a shaking incubator. Subsequently, the suspensions were centrifuged at 4000 rpm for 15 min at 4°C, and the supernatants were filtrated through 0.45-μm pore-size membranes. The filtrates were serially diluted in saline magnesium buffer (SM buffer) (50 mM Tris-HCl [pH 7.5], 0.1 M NaCl, 8 mM MgSO4·7H2O, 0.01% gelatin). A 1 mL of each dilution was poured into the mixture (0.8% agar, 10 mL of NB, 1 mL of specific Salmonella to phage at concentration of 1.0 × 108 cfu/mL) before pouring on NA and incubated at 37°C for 24 h. The numbers of plaques were counted as plaque-forming unit (PFU) [11]. A plaque was collected and mixed with SM buffer before adding 20 μL of 2.5% (v/v) chloroform and left at room temperature for 30 min, then adding 40% glycerol (v/v) and storing at −80°C.
2.4. Characterization of Bacteriophages
2.4.1. Determination of Host Range
Host range of bacteriophages was determined by performing spot tests [11]. Isolates of Salmonella spp. (SE, SD, SH, SP, and ST), Escherichia coli, Pseudomonas aeruginosa, Micrococcus luteus, Staphylococcus aureus, Enterococcus faecalis, Listeria monocytogenes, Bacillus subtilis, and Pediococcus acidilactici) each were used in this study to determine the host range of the isolated phages. Each of the bacterial isolates was transferred into NB for incubation at 37°C for 24 h. An agar overlay was performed containing a bacterial concentration of 1.0 × 108 cfu/mL. The 10 μL of bacteriophage preparation (1010 PFU/mL) was spotted on the prepared overlay. The plates were observed for positive result by observing the appearance of lytic clear zones after incubation at 37°C for 24 h.
2.4.2. Heat and pH Susceptibility Tests
For the phages capable of infecting five Salmonella serovars, the concentration was adjusted to 1011 PFU/mL. The 1 mL of phage was mixed in NB (9 mL) and measured for heat susceptibility by treating the phage stock at −6.5°C, 2°C, 6°C, 37°C, 50°C, 60°C, and 70°C for 30 min. For pH stability, the 1 mL of phage was mixed in a series of tubes containing NB (9 mL) of different pHs at 3, 5, 7, and 9 (adjusted using NaOH or HCl) and incubated at 41°C for 30 min. Bacteriophage titers were determined using the plaque assay method and repeated three times.
2.4.3. Phage Stability Under Salinity Conditions
For the salinity test, the phage was adjusted to a concentration of 1011 PFU/mL. One milliliter of phage was added to 2% and 5% NaCl solutions and then incubated at 37°C. For the control group, 1 mL of phage was added to sterile distilled water. Bacteriophage titers were determined at 0, 2, 6, and 24 h.
2.4.4. The Growth of Salmonella at Different Bacteriophage Concentrations
The growth of Salmonella at different bacteriophage concentrations to determine the multiplicity of infection (MOI) was adapted from Dallal et al. [12]. SE and ST were multiplied in NB by incubation at 37°C and centrifugation at 150 rpm for 6 h. Bacterial concentrations were adjusted to 1.5 × 105 cfu/mL and then gently mixed with phages at concentration of 1.5 × 104, 1.5 × 105, and 1.5 × 106 PFU/mL, respectively. Bacterial growth was monitored at 0, 1, 2, 3, 4, 5, 6, 7, and 8 h and compared to the control group.
2.4.5. The Growth of Bacteriophage
To determine the growth of bacteriophage, a characteristic was performed according to Dallal et al. [12]. Phages were added to the NB containing SE and ST with MOI of 0.1. before incubation at 37°C and centrifugation at 100 rpm. During incubation and centrifugation, bacteriophage titers were determined using the plaque assay method every 30 min for 5.5 h. This procedure was repeated three times.
2.4.6. Bacteriophage Morphological Characterization by Using Transmission Electron Microscope (TEM)
The selected phages were studied using a TEM. Bacteriophage preparation was adapted from Hayat [13]. The phage solutions at a concentration of 1.0 × 108 PFU/mL were filtrated through 0.22-μm pore-size membranes for 1–3 h. The filtrates were centrifuged at 35,000 rpm for 2 h. The pellet was resuspended in phosphate buffer, and the suspension was transferred to a copper mesh grid. One drop of 2% uranyl acetate was then placed on the grid and allowed to stand for 2 min. The grid was placed onto a filter paper (Whatman No. 1) and allowed to dry for 3–5 min before observation under TEM (HT7700, Hitachi-High Tech, Tokyo, Japan) operating at 80 kV.
2.5. The Use of Bacteriophage in Experimental Broiler Chickens
One hundred and thirty-five broiler chicks aged one-day-old were segregated into three equal groups: The first group kept as a negative control, the second group (positive control) was infected with 500 μL (108 cfu/mL) of SE per chick at one day old, while the remaining group was infected with SE at one day old and then treated with phage at 7 days old. The phage cocktails (300 μL) were given to the infected chicks (7 days of age) at a concentration of 1011 PFU/mL. A total of 5 birds were randomly selected from each group, at 7, 14, and 21 days of age, to collect cecum, liver, and spleen for detection of phage, and cecum, liver, and spleen were collected from the same birds for Salmonella count. The animal experiments were conducted in accordance with ethical guidelines and received approval from the Chulalongkorn University Animal Care and Use Committee, under approval number 2073023.
2.6. Microbiota Analysis
A total of three birds were randomly selected from each group at 7 and 21 days of age to collect ceca and ilea for microbiota analysis. Genomic DNA was extracted and purified using the TIANamp Stool DNA Kit (TIANGEN, China) according to the manufacturer’s instructions. The sequences allowed for the amplification of the V4 region of 16S rRNA genes (adapted from [14]). The microbiota was analyzed by using next-generation sequencing (NGS) using MiSeq Reagent Kit and MiSeq System according to the manufacturer’s instructions (Illumina).
2.7. Morphometric Analysis
At 21 days of age, three birds were randomly selected from each group, and ileal samples were collected for morphometric analysis using TEM. The villus height, villus width, and crypt depth were measured to calculate the absorption area, as adapted from [15].
2.8. Statistical Analysis
Results are presented as mean ± SD (standard deviation). The characterization of phage and Salmonella count was analyzed using one-way ANOVA with 95% confidence intervals (p < 0.05) followed by Tukey post hoc test to compare means.
3. Results
3.1. Isolation of Bacteriophages
Salmonella phages were isolated from two poultry farms, two poultry processing plants, a goat farm, and a pig farm. Of the 33 samples analyzed, 25 (75.8%) were found to contain Salmonella bacteriophages. A total of 63 phage isolates were identified, including 17 ST phage isolates, 12 SE phage isolates, 7 SH phage isolates, 16 SD phage isolates, and 11 SP phage isolates. The phage SEpBS-1 was selected for further testing based on its characteristics.
3.2. Characteristics of the Phage
3.2.1. Determination of Host Range
The host ranges of the bacteriophage SEpBS-1 were determined using several types of bacteria. The bacteriophage revealed a broad host range against Salmonella serovars including SE, SH, ST, SD, and SP. The bacteriophage did not produce lytic plaques on Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Listeria monocytogenes, Micrococcus luteus, Pediococcus acidilactici, and Staphylococcus aureus.
3.2.2. The pH Stability, Heat Stability, and Phage Stability Under Salinity Condition
The results of the pH stability, heat stability, and phage stability under salinity condition are shown in Table 1. For the pH stability, the titers of phage SEpBS-1 ranged from 5.31 ± 0.17 to 10.90 ± 0.11 log10 PFU/mL. The phage SEpBS-1 showed the highest titer at pH 7. The phage titers at different pH were significantly different (p < 0.05). The test of heat stability revealed the titers of phage SEpBS-1 ranged from 4.82 ± 0.06 to 11.52 ± 0.24 log10 PFU/mL. The phage SEpBS-1 showed the lowest titer at 70°C. There was no significant difference in titers between −6.5°C and 37°C (p > 0.05), but the higher temperature (50°C–70°C) revealed the lower titers (p < 0.05). For the phage stability under salinity, the titers of phage SEpBS-1 ranged from 7.84 ± 0.06 to 9.48 ± 0.00 log10 PFU/mL in the control group, 8.31 ± 0.03 to 9.14 ± 0.06 log10 PFU/mL in the 2% NaCl group, and 7.18 ± 0.18 to 9.00 ± 0.44 log10 PFU/mL in the 5% NaCl group. At 24 h, the titer of phage in the 5% NaCl group was significantly decreased (p < 0.05).
| Parameters | Levels tested | Contact time (h) | Mean ± SD (log10 PFU/mL) |
|---|---|---|---|
| pH | 3 | 0.5 | 5.31 ± 0.17d |
| 5 | 0.5 | 10.46 ± 0.10b | |
| 7 | 0.5 | 10.90 ± 0.11a | |
| 9 | 0.5 | 8.97 ± 0.07c | |
| Temperature (°C) | −6.5 | 0.5 | 11.07 ± 0.23a |
| 2 | 0.5 | 11.52 ± 0.24a | |
| 37 | 0.5 | 11.46 ± 0.10a | |
| 50 | 0.5 | 9.72 ± 0.06b | |
| 60 | 0.5 | 9.03 ± 0.27b | |
| 70 | 0.5 | 4.82 ± 0.06c | |
| Salinity | 0% | 0 | 9.48 ± 0.00 |
| 2 | 8.87 ± 0.03 | ||
| 6 | 8.16 ± 0.01 | ||
| 24 | 7.84 ± 0.06 | ||
| 2% | 0 | 9.14 ± 0.06 | |
| 2 | 8.75 ± 0.07 | ||
| 6 | 8.41 ± 0.37 | ||
| 24 | 8.31 ± 0.03 | ||
| 5% | 0 | 9.00 ± 0.44a | |
| 2 | 8.62 ± 0.31a | ||
| 6 | 8.78 ± 0.11a | ||
| 24 | 7.18 ± 0.18b | ||
- Note: Different superscripts in the same row mean statistically significant (p < 0.05).
3.3. The Growth of Salmonella at Different Bacteriophage Concentrations
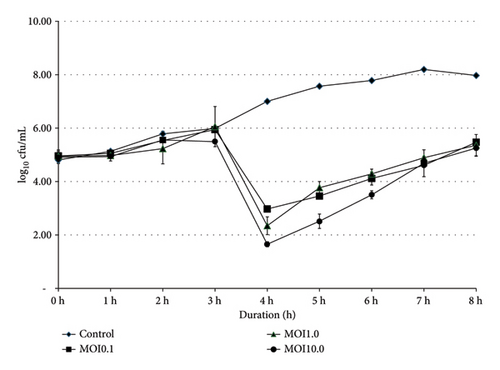
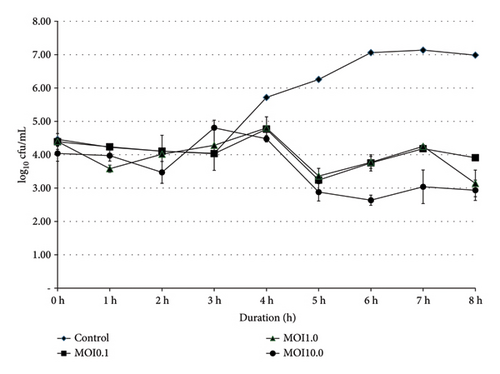
Bacterial growths of SE and ST were monitored at 0, 1, 2, 3, 4, 5, 6, 7, and 8 h, with varying titers of phage SEpBS-1 (104, 105, and 106 PFU/mL [MOI = 0.1, 1.0, 10]). The results of phage SEpBS-1 on SE were revealed by the bacterial counts shown in Figure 1(a). In the control group, bacterial counts had increased (3.17 log10 cfu/mL) after 8-h incubation. At MOI of 0.1, bacterial counts had decreased (1.98 log10 cfu/mL) after 4-h incubation and then increased (2.5 log10 cfu/mL) after 8-h incubation. At MOI of 1.0, bacterial counts decreased (2.6 log10 cfu/mL) after 4-h incubation and then increased (3.01 log10 cfu/mL) after 8-h incubation. At MOI of 10, bacterial counts decreased (3.25 log10 cfu/mL) after 4-h incubation and increased (3.6 log10 cfu/mL) after 8-h incubation.
 ), MOI of 0.1 (
), MOI of 0.1 ( ), MOI of 1.0 (
), MOI of 1.0 ( ), and MOI of 10 (
), and MOI of 10 ( ).
).
 ), MOI of 0.1 (
), MOI of 0.1 ( ), MOI of 1.0 (
), MOI of 1.0 ( ), and MOI of 10 (
), and MOI of 10 ( ).
).The results of phage SEpBS-1 on ST were revealed by the bacterial counts shown in Figure 1(b). In the control group, bacterial counts increased (2.53 log10 cfu/mL) after 8-h incubation. At MOI of 0.1, bacterial counts decreased (1.5 log10 cfu/mL) after 5-h incubation and increased (0.66 log10 cfu/mL) after 8-h incubation. At MOI of 1.0, bacterial counts decreased (1.03 log10 cfu/mL) after 5-h incubation and increased (0.23 log10 cfu/mL) after 8-h incubation. At MOI of 10, bacterial counts decreased (1.4 log10 cfu/mL) after 6-h incubation and increased (0.33 log10 cfu/mL) after 8-h incubation.
3.4. The Growth of Bacteriophage
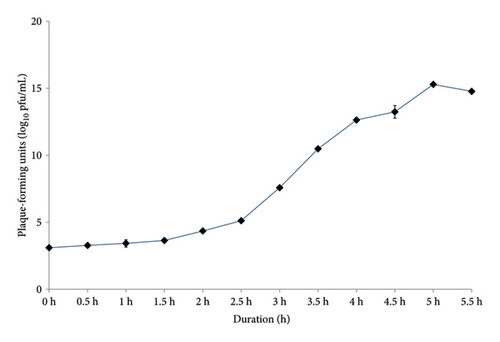
The latent period, burst time, and burst size of the phages were determined from a one-step growth curve, as shown in Figure 2. The latent period of phage SEpBS-1 for SE was calculated as 2 h. The burst time was 2–3.5 h, and the burst size was 166 PFU/infected cell. The latent period of phage SEpBS-1 for ST was calculated as 2.5 h. The burst time was 2.5–4 h, and the burst size was 973 PFU/infected cell.

3.5. Bacteriophage Morphological Characterization Using TEM
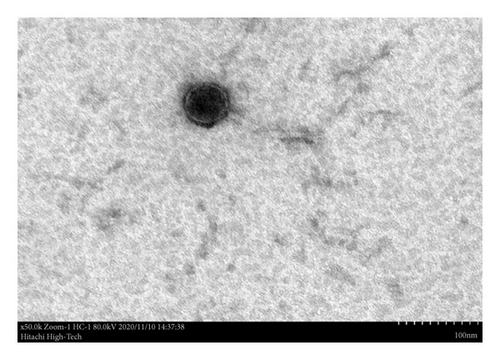
Ultrastructural analysis of phage SEpBS-1 revealed icosahedral heads approximately 56 nm in diameter and thin, long, noncontractile, flexible tails approximately 130 nm in length, as shown in Figure 3. Based on these characteristics, phage SEpBS-1 was classified as a member of the Siphoviridae family.
3.6. Salmonella Counts From the Liver, Spleen, and Cecum After Treatment With Phage
Salmonella counts from the liver, spleen, and cecum were performed before and after treatment with phage. The results are presented in Table 2. The Salmonella was not detected in the negative control. At 7 days postinoculation, Salmonella was detected in the spleen (0.4 ± 0.89 log10 cfu/g) and cecum (4.5 ± 0.62 log10 cfu/g) of the positive control and the spleen (0.49 ± 1.10 log10 cfu/g) of the treatment group. At 14 days postinoculation, Salmonella was detected in all selected organs (0.46 ± 1.02, 0.92 ± 1.26, and 3.40 ± 3.12 log10 cfu/g), while the treatment group showed Salmonella in the liver (0.49 ± 1.12 log10 cfu/g) and spleen (0.52 ± 1.16 log10 cfu/g). At 21 days postinoculation, Salmonella was detected in the spleen (0.58 ± 1.29 log10 cfu/g) and cecum (6.13 ± 0.23 log10 cfu/g) of the positive control and the cecum (1.21 ± 2.27 log10 cfu/g) of the treatment group. The Salmonella counts in the cecum were significantly higher in the positive control compared to the other groups (p < 0.05).
| Groups | Days postinoculation of Salmonella enteritidis | ||
|---|---|---|---|
| Day 7 | Day 14 | Day 21 | |
| Negative control | |||
| Salmonella counts (log10 cfu/g) | |||
| Liver | ND | ND | ND |
| Spleen | ND | ND | ND |
| Cecum | ND | ND | ND |
| %Positive | 0 | 0 | 0 |
| Positive control | |||
| Salmonella counts (log10 cfu/g) | |||
| Liver | ND | 0.46 ± 1.02A | ND |
| Spleen | 0.4 ± 0.89a,B | 0.92 ± 1.26a,A | 0.58 ± 1.29a,B |
| Cecum | 4.5 ± 0.62b,A | 3.40 ± 3.12b,A | 6.13 ± 0.23a,A |
| %Positive | 80 | 100 | 80 |
| Treatment | |||
| Salmonella counts (log10 cfu/g) | |||
| Liver | ND | 0.49 ± 1.12A | ND |
| Spleen | 0.49 ± 1.10a,B | 0.52 ± 1.16a,A | ND |
| Cecum | ND | ND | 1.21 ± 2.27B |
| %Positive | 80 | 100 | 40 |
- Note: ND = not detected by either direct plating or enrichment for Salmonella.
- a-bValues within a row with different superscripts are significantly different (p < 0.05).
- A-BValues within a column with different superscripts are significantly different (p < 0.05).
3.7. Microbiota Analysis
3.7.1. Gut Microbiota Diversity
A total of 2,502,311 sequences of the V4 region of the 16S rRNA gene were obtained from the broiler ceca at 7 and 21 days of age. Alpha diversity analysis, such as observed OTUs, Simpson, Chao 1, ACE, and PD whole tree, was used for consideration. Results of alpha diversity analysis did not show significant main effects of Salmonella challenge except Simpson (data not shown). At 14 days after treatment, the alpha diversity of microbiota in ceca showed no significant difference (data not shown). On the other hand, in beta diversity analysis (PCoA) (data not shown), the pattern of microbiota after Salmonella challenge was found that the treatment group at 7 days of age (D7Test) exhibited a tendency of separation between the positive control and the negative control. At 14 days after treatment, the treatment group at 21 days of age (D21Test) was tended to separate from the negative (D21Neg) and positive control (D21Pos) groups.
3.7.2. Diversity and Community Structure of Gut Microbiota During Chicken Development
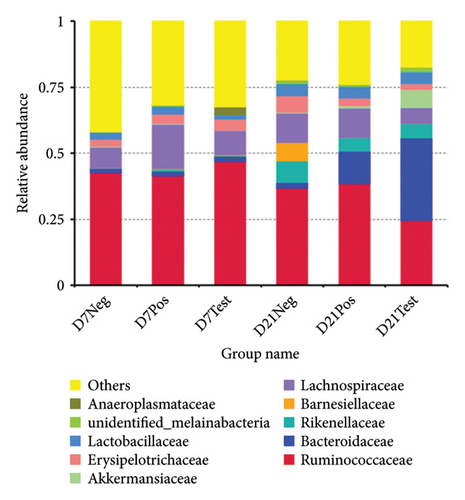
At the phylum level, the bacterial sequences from the treatment group at 21 days of age were composed predominantly of the phyla Firmicutes, Bacteroidetes, Verrucomicrobia, and Proteobacteria, and this group showed higher proportions of Bacteroidetes and Verrucomicrobia, compared to the other groups, as depicted in Figure 4. At the family level, the treatment group at 21 days of age consisted largely of Bacteroidaceae and Ruminococcaceae. Bacteroidaceae was the highest proportion. The proportion of Akkermansiaceae in this group was higher when compared to the other groups. At the genus level, gut microbiota of this group was composed of Bacteroides, Alistipes, unidentified Ruminococcaceae, Barnesiella, Akkermansia, Erysipelatoclostridium, Subdoligranulum, Lactobacillus, Faecalibacterium, and unidentified Lachnospiraceae. A higher abundance of Bacteroides and Akkermansia was observed when compared to other groups (Figure 5).

3.8. Morphometric Analysis
The villus height, villus width, and crypt depth of the ileum were measured to calculate the absorption area (Table 3). Villus surface area was significantly higher in the treatment group when compared to other groups (p < 0.05).
| Parameter | Groups of experiment | ||
|---|---|---|---|
| Negative control | Positive control | Treatment | |
| Ileal villus height (μm) | 183.33 ± 25.00b | 152.77 ± 63.05b | 327.77 ± 107.85a |
| Base width of the ileal villus (μm) | 40.00 ± 7.07a | 27.78 ± 4.41b | 37.78 ± 6.67a |
| Ileal crypt depth (μm) | 27.78 ± 4.01a | 14.44 ± 7.26b | 36.67 ± 10.00a |
| Villus surface area (mm2) | 0.024 ± 0.010b | 0.013 ± 0.005b | 0.040 ± 0.013a |
- Note: Values are means ± standard error. Mean values with different letters in the same row differ significantly (p < 0.05).
4. Discussions
S. enterica is classified into over 2600 serovars, which can cause disease in humans and different kinds of animals [16]. Additionally, S. enterica is known to exhibit resistance to a wide range of antibiotics [17, 18], including those from higher generations of antibiotic applications [19]. Contamination by Salmonella is a common issue in poultry meat processing. This microorganism can increase meat products during transportation and distribution, especially international transportation. Therefore, it is necessary to prevent and reduce microbial contamination before packaging, during transportation and distribution. The lytic bacteriophages are a safe and effective choice for control of Salmonella [9].
In this experiment, bacteriophages were isolated using the enrichment method, and one of the bacteriophages exhibited high lytic efficiency against various Salmonella isolates. The bacteriophages were named based on their specific hosts and the locations of their discovery, following the naming convention outlined by Jiang et al. [20]. One of the isolated bacteriophages, with suitable properties, was selected and named SEpBS-1 for further study.
Bacteriophages will target and kill specific strains of bacteria. They cannot replicate without the bacterial cell [21]. The characterization results showed that the phages are highly specific to SE, SH, ST, SD, and SP. The thermal stability test of phages showed that phages were stable at −6.5°C–50°C for 30 min, significantly decreased (p < 0.05) at 60°C, and maximally decreased at 70°C. The pH stability test showed that phages were stable at a pH of 5–9. Phage SEpBS-1 was more stable in the acidic solution than alkaline solution, according to Rahaman et al. [11]. The Salmonella phages which were isolated from a poultry farm environment were stable at 50°C and 60°C for 1 h. They survived at pH 4–9, but their titers decreased at pH 2–3. NaCl stability showed individual differences for individual phages in a study of NaCl tolerance of staphylococcal phages [22]. In this study, titers of phage SEpBS-1 decreased in response to increased salinity. The morphological characterization of phage SEpBS-1 using TEM revealed icosahedral heads with a diameter of 56 nm and thin, long, noncontractile, flexible tails measuring 130 nm in length. Based on these characteristics, phage SEpBS-1 was classified as a member of the Siphoviridae family.
The growth of Salmonella at different bacteriophage concentrations revealed that SEpBS-1 significantly decreased bacterial counts after 4 h incubation at all MOI on SE. SE counts were lowest at MOI of 10. The bacterial counts varied directly with time at every MOI. For phage SEpBS-1 on ST, bacterial counts were significantly decreased after 5-h incubation at all MOI and tended to increase with time except for MOI of 10, for which ST counts were 2.63–3.03 log10 PFU/mL. According to the one-step growth curve, phage SEpBS-1 on SE and ST had a latent period of 2 and 2.5 h, respectively. A previous study reported that some Salmonella phages had long latent periods [23]. The burst time of phage SEpBS-1 was 2–3.5 h. The phage SEpBS-1 had a shorter latent period and burst time on SE than on ST, but had a smaller burst size on SE than on ST. Both latent period and burst size depend on the type of bacteriophage [24], the host cell, culture media, pH, and temperature [25].
The test of the effect of phage SEpBS-1 against Salmonella infection in broilers found that Salmonella counts were slightly increased at 7 and 14 days after phage treatment. There was no statistically significant difference between groups (p > 0.05). Salmonella counts decreased by 40% at 14 days after phage treatment, while the positive control had the highest number of Salmonella in ceca. Bacteriophages may be efficient for Salmonella control. However, as chickens age, their immunity could respond to eliminate Salmonella. Adhikari and colleagues [26] studied the efficiency of bacteriophages against SE. The 0.2% of bacteriophages significantly reduced numbers of SE in the ceca when compared to the positive control. Furthermore, an increased expression of proinflammatory cytokine and chemokine genes was reported to be associated with increased resistance to SE, including higher levels of IL-1B, IL-6, IL-8, IL-18, and chemokine ligand-2 (CCLi2) in heterophils, monocyte-derived macrophages, the ceca, and cecal tonsil [27, 28]. The effect of a phage preparation can be mediated through two primary mechanisms. The first mechanism involves the direct killing of bacterial cells by bacteriophage virions during the lytic cycle. The second mechanism relies on inducing an immune response, either by the phage particles themselves or by other components of the phage preparation [29].
The morphology of the intestines reflects the health status of the poultry gut and its immune function. According to Sarrami et al. [30], the rapid development of intestinal integrity provides a suitable environment for bacteria to colonize and thrive. In our experiment, the treatment group showed significantly better parameters compared to the positive control group (p < 0.05), suggesting that phages can promote intestinal maturation. The ecology of the diverse microbiota in the poultry gut is closely linked to the stability of the gut microbiota. Bacteriophages can alter gut bacterial communities. Sequencing the V4 region of the 16S RNA characterized the bacterial communities in each broiler cecum. Beta diversity analysis (PCoA) can assess microbiota patterns. After treatment, the treatment group at 21 days of age (D21Test) showed a tendency to separate from both the negative (D21Neg) and positive control (D21Pos) groups. Alpha diversity analysis was used to characterize the bacterial communities. In line with the findings of Chang et al. [31], the Salmonella challenge in day-old chicks did not result in significant alterations to the bacterial communities. However, the Simpson index revealed a significant difference (p = 0.049), indicating a shift in community diversity.
The microbiota of broiler chickens has been estimated to surpass 900 bacterial species. The variation in microbiota composition may be explained by different host characteristics and environmental factors [31, 32]. In this study, Salmonella inoculation and bacteriophage treatment decreased the abundance of Firmicutes, while the abundance of Bacteroidetes, Verrucomicrobia, and Proteobacteria was increased.
The most pathogenic bacterial species, including Salmonella, Escherichia coli, and Shigella, are members of the phylum Proteobacteria. These bacteria have the ability to attach, colonize, and invade the intestinal epithelium, leading to disease in humans and animals. The increase of phylum Bacteroidetes and Verrucomicrobia may indicate a healthy gut in poultry. Carbohydrates are efficiently digested by members of the phylum Bacteroidetes [33]. Previous studies described substantially different Bacteroides population levels (107 to 1010 cfu/g) in ceca or feces of chickens at different ages (14–40 days of life) [34]. Bacteroides thetaiotaomicron, which is one of the most excellent fiber-digesting bacteria, is a member of the phylum Bacteroidetes. In this study, the highest average abundance of phylum Bacteroidetes was 226.33 and was significantly different (p < 0.05) among the groups. B. thetaiotaomicron can degrade dietary fiber by releasing outer membrane vesicles (OMVs) containing glucohydrolase. A previous study showed that B. thetaiotaomicron can ferment cello-polysaccharides to produce short-chain fatty acids (SCFAs) to provide energy for the host [35]. The highest average abundance of Akkermansia muciniphila (phylum Verrucomicrobia) was 7428.67, and abundances differed significantly (p < 0.05). A. muciniphila improves intestinal immunity and affects glucose metabolism and lipid metabolism which can suppress obesity [36]. Zhu et al. [37] found that A. muciniphila colonized in the intestine and then relieved intestinal mucosal damage in chicks caused by S. pullorum.
The results of this study on the efficiency of phage SEpBS-1 reinforce the concept of using bacteriophages as a biological control for Salmonella in the poultry industry. Phage SEpBS-1 effectively reduced Salmonella in the organs of birds 14 days after treatment. However, Salmonella was most frequently detected in the cecum of the treatment group. The use of bacteriophages provides a promising alternative to antibiotics. The application of lytic bacteriophages in the biological control of foodborne pathogens is an effective strategy for controlling Salmonella. The U.S. Food and Drug Administration has approved the use of bacteriophages on meat products as safe [9].
5. Conclusion
The findings of this study highlight the viability of bacteriophage SEpBS-1 as a potential antibiotic alternative for managing Salmonella in broiler chickens. Isolated from various animal farms and processing plants in Thailand, SEpBS-1 was able to infect multiple Salmonella serovars, indicating broad host specificity and robustness under various environmental conditions, including temperature and pH ranges commonly found in poultry environments. While in vivo tests showed a modest reduction in Salmonella counts in treated broilers, these reductions were not statistically significant, suggesting that while phage therapy is promising, further optimization in phage dosage, application frequency, or delivery methods may be necessary to achieve more pronounced results.
Overall, the findings underscore the potential of lytic bacteriophages as a biocontrol measure for Salmonella, especially as the poultry industry moves toward reducing antibiotic usage. Continued investigation into the impact of phage therapy on gut microbiota and resistance development will be key to establishing bacteriophages as a sustainable and effective approach in poultry production.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was financially supported by Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University (grant no. CU_GR_62_67_31_05).
Acknowledgments
We are grateful to the staff of the Avian Health Research Unit, Chulalongkorn University, Thailand, for all their support.
Open Research
Data Availability Statement
Data will be made available on reasonable requests.




