Molecular Prevalence and Identification of Zoonotic Plasmodium spp., Including Plasmodium knowlesi, Plasmodium cynomolgi, and Plasmodium inui, in Long-Tailed Macaques (Macaca fascicularis) of Southern Thailand
Abstract
Zoonotic malaria, caused by simian Plasmodium spp., poses a major public health challenge in Southeast Asia, including Thailand, where long-tailed macaques (Macaca fascicularis) serve as natural reservoirs. This study investigated the molecular prevalence and species identification of zoonotic simian Plasmodium spp. in macaques from four provinces in Southern Thailand: Phetchaburi, Satun, Phang Nga, and Surat Thani. A total of 310 blood samples were collected between May 2023 and June 2024 and analyzed using nested and seminested polymerase chain reaction (PCR) techniques targeting the 18S rRNA gene. Sequencing analyses confirmed the presence of zoonotic Plasmodium species. Overall, 11.3% (35/310; 95% CI: 7.9–15.3) of the macaques tested positive, with Plasmodium inui being the most prevalent species at 9.4% (29/310), followed by Plasmodium knowlesi and Plasmodium cynomolgi, each at 0.9% (3/310). The highest prevalence was observed in Surat Thani at 18% (18/100). These findings underscore the zoonotic potential of simian malaria and its geographic distribution in Southern Thailand, which may be associated with the significant increase in macaque populations and their expanding habitat overlap with human communities. In conclusion, this study highlights the major role of long-tailed macaques as reservoirs for zoonotic Plasmodium spp. Enhanced surveillance and community awareness are crucial for mitigating cross-species transmission and improving malaria control.
1. Introduction
Malaria is a life-threatening infectious disease caused by protozoan parasites of the Plasmodium genus, primarily transmitted through the bites of infected female Anopheles mosquitoes [1]. The disease remains endemic in tropical and subtropical regions, including Africa, Central and South America, Asia, and Oceania, where environmental conditions, such as temperature and rainfall, support favorable habitats for Anopheles vectors [2]. According to the World Health Organization report in 2024, a total of approximately 263 million cases of malaria were reported globally, with an estimated 597,000 malaria deaths worldwide in the year 2023 [3]. While malaria transmission has traditionally been less prevalent in urban areas, rapid urbanization and population growth have facilitated the movement of infected individuals, contributing to the spread of malaria pathogens across both urban and rural environments [2, 4]. The life cycle of Plasmodium spp. is complex, requiring both vertebrate and invertebrate (mosquitoes) hosts. In humans, the incubation period of the parasite typically ranges from 3 to 14 days, during which the infection remains asymptomatic. Once the parasites mature and invade red blood cells, affected individuals develop clinical symptoms, such as fever, chills, headache, muscle aches, and fatigue, which are characteristic of malaria [5].
Five Plasmodium species are known to cause malaria in humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi [6]. However, malaria is not restricted to humans. Various Plasmodium species also infect a wide range of animal hosts. Avian hosts harbor species, such as Plasmodium relictum, Plasmodium elongatum, Plasmodium circumflexum, Plasmodium matutinum, and Plasmodium vaughani [7–9], while reptilian hosts carry Plasmodium floridense and Plasmodium colombiense [6, 10, 11]. Rodent hosts are infected by species, including Plasmodium yoelii, Plasmodium berghei, Plasmodium chabaudi, and Plasmodium vinckei [12]. Notably, Plasmodium spp. infecting nonhuman primates pose a major zoonotic threat, as evidenced by documented cases of cross-species transmission, particularly from macaques to humans. Such instances complicated malaria control efforts in regions where human and wildlife populations coexist [13].
Nonhuman primates, particularly macaques, play an important role in zoonotic disease transmission. Several species of macaques are found in Thailand, consisting of the long-tailed macaque (Macaca fascicularis), pig-tailed macaque (Macaca nemestrina), stump-tailed macaque (Macaca arctoides), Assam macaque (Macaca assamensis), Indochinese rhesus macaque (Macaca mulatta), and northern pig-tailed macaque (Macaca leonina) [14, 15]. Frequently, these macaques inhabit areas near human settlements, leading to increased human–wildlife interactions. Such close contact has raised concerns regarding the transmission of zoonotic pathogens from macaques to humans. For example, studies have documented the presence of several potentially zoonotic pathogens in macaques in Thailand, including the herpes B virus, which has a high mortality rate in humans [16, 17], as well as Bartonella quintana [18], Giardia duodenalis and Cryptosporidium sp. [19], and Trichuris trichiura and Hymenolepis diminuta [20]. In addition, macaques serve as natural hosts for several Plasmodium species, consisting of P. knowlesi, Plasmodium cynomolgi, Plasmodium inui, Plasmodium coatneyi, and Plasmodium fieldi [14, 21]. The transmission of these parasites from macaques to humans, particularly in regions where macaques reside near residential areas, poses major public health concern. Several provinces in Thailand, such as Tak, Ranong, Yala, Narathiwat, Prachuap Khiri Khan, and Chanthaburi, have reported human infections with P. knowlesi and P. cynomolgi [22]. In addition, another study has reported cases of P. knowlesi infection in humans in Surat Thani province [23].
Despite growing evidence of zoonotic malaria in Thailand, there is still a lack of research on the transmission of Plasmodium species between humans and macaques. Although some studies have identified P. knowlesi and P. cynomolgi in macaque populations [24–26], there remains a pressing need for more comprehensive research on the prevalence and distribution of Plasmodium species in macaques across different areas of the country. Thus, the current study aimed to investigate the molecular prevalence of Plasmodium spp. infections in long-tailed macaques from Southern Thailand and to identify specific Plasmodium species using molecular techniques.
2. Materials and Methods
2.1. Ethical Approval
The protocols involving animal use in this research were approved by the Kasetsart University Institutional Animal Care and Use Committee (IACUC), Bangkok, Thailand, under the Ethical Review Board of the Office of the National Research Council of Thailand (NRCT) (Approval ID: ACKU67-VTN-001). This approval ensured the ethical conduct of the scientific research.
2.2. Study Period and Locations
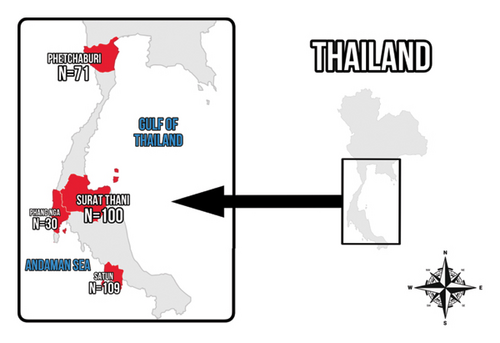
Blood samples were collected from May 2023 to June 2024 from long-tailed macaques in four provinces of Southern Thailand: Phetchaburi, Phang Nga, Surat Thani, and Satun (Figure 1). Laboratory analyses were conducted at the Faculty of Veterinary Technology, Kasetsart University, Bangkok, Thailand.
2.3. Sample Collection
The sample size was estimated using Epitools (https://epitools.ausvet.com.au) with an expected proportion of 0.29 based on another study in Thailand [14]. The precision of the estimate and the confidence level were set as 0.05% and 95%, respectively. In total, 310 blood samples were collected from free-ranging long-tailed macaques in the study areas, comprising 71 samples (37 males and 34 females) from Ban Laem district, Phetchaburi province; 109 samples (61 males and 48 females) from Mueang Satun district, Satun province; 30 samples (14 males 16 females) from Mueang Phang Nga district, Phang Nga province; and 100 samples (68 males and 32 females) from Phunphin district, Surat Thani province. The macaques were humanely captured and initially sedated with xylazine hydrochloride (0.5–2 mg/kg body weight), followed by anesthesia with tiletamine-zolazepam (2–5 mg/kg body weight) [14]. Both drugs were administered via intramuscular injection, in accordance with the approved protocol. Approximately 1 mL of blood was collected from the femoral vein of each individual into tubes containing ethylenediaminetetraacetic acid (EDTA). All procedures were performed by licensed veterinarians from the Department of National Parks, Wildlife and Plant Conservation. The samples were kept cool during transportation to the laboratory and aliquoted into 200 μL portions in 1.5 mL tubes for storage at −40°C prior to DNA extraction.
2.4. Molecular Analysis
DNA was extracted from 200 μL of blood using a QIAamp® DNA Blood Mini Kit (QIAGEN; Hilden, Germany), following the manufacturer’s instructions. Extracted DNA was stored at −40°C until further analysis. The detection of Plasmodium spp. was conducted using nested polymerase chain reaction (PCR) targeting the partial 18S rRNA gene. The genus-specific primers from Singh et al. [27] were used for the amplification. The primary PCR utilized the primers rPLU6 and rPLU5 under the following thermal cycling conditions: 95°C for 5 min; 35 cycles of 95°C for 30 s, 55°C for 60 s, and 72°C for 60 s; followed by a final extension at 72°C for 10 min. The secondary PCR used the primers rPLU3 and rPLU4 with the same thermal cycling profile as above. The expected amplicon sizes were 1100 bp for the primary PCR and 240 bp for the nested PCR. The 20 μL reaction mixture consisted of 10X buffer, 50 mM MgCl2, 10 mM dNTPs, 10 μM of each primer, and 5 U/μL of Taq polymerase (Invitrogen; Waltham, MA, USA).
Since the initial nested PCR method produced relatively low detection rates, a seminested PCR protocol, as reported by Imwong et al., was used to confirm the results [28]. This genus-specific primer set targeted the 18S rRNA gene of multiple simian Plasmodium species, which consisted of P. cynomolgi, P. knowlesi, P. inui, P. coatneyi, P. fieldi, P. chabaudi, P. berghei, P. brasilianum, P. simium, P. semiovale, P. fragile, P. vinckei, P. yoelii, and P. adleria. The primary PCR utilized the PlasmoM_N1F and PlasmoM_N1R primers under the following thermal cycling conditions: initial denaturation at 94°C for 3 min; 30 cycles of 94°C for 60 s, 53°C for 60 s, and 72°C for 60 s; followed by a final extension at 72°C for 10 min. Secondary PCR used the primers PlasmoM_N2F and PlasmoM_N1R with identical cycling conditions to those immediately above. The expected amplicon sizes ranged from 233 to 298 bp. The 20 μL reaction mixture was prepared using the same conditions as those described above for the nested PCR method. A summary of the primers used in the current study is provided in Table 1.
| Primer | Oligonucleotide sequence (5′–3′) | Target gene | Amplicon size (bp) |
|---|---|---|---|
| Genus-specific primers | |||
| rPLU6 | TTAAAATTGTTGCAGTTAAAACG | 18S rRNA | 1100 |
| rPLU5 | CCTGTTGTTGCCTTAAACTTC | ||
| rPLU3 | TTTTTATAAGGATAACTACGGAAAAGCTGT | 240 | |
| rPLU4 | TACCCGTCATAGCCATGTTAGGCCAATACC | ||
| Genus-specific primers | |||
| PlasmoM_N1F | ATGGCCGTTTTTAGTTCGTG | 18S rRNA | — |
| PlasmoM_N1R | TTGTGTTAGACACACATCGTTCC | ||
| PlasmoM_N2F | GTTAATTCCGATAACGAACGAGA | 233–298 | |
| PlasmoM_N1R | TTGTGTTAGACACACATCGTTCC | ||
2.5. DNA Sequencing Analysis
The PCR products were analyzed using 1.2% agarose gel electrophoresis with Tris-acetate-EDTA buffer and visualized under UV transillumination after nucleic acid staining with Thermo Scientific™ 6X DNA gel loading dye (Thermo Fisher Scientific; Waltham, MA, USA). Due to the similarity in amplicon sizes across different Plasmodium species, species-level identification was not performed based on gel electrophoresis results. Positive samples of the expected size for Plasmodium spp. were purified using a GenepHlow™ Gel/PCR Kit (Geneaid Biotech Ltd.; New Taipei City, Taiwan). Purified DNA was sequenced using the Sanger methods (ATGC Company Limited; Pathum Thani, Thailand).
2.6. Statistical Analysis
Statistical analyses were conducted using the STATA software Version 15.1 (Stata Corporation; Texas, USA). The odds ratio (OR) with the corresponding 95% confidence interval (95% CI) and p value were calculated to assess the associations between Plasmodium spp. infection and variable characteristics such as host sex and sampling location. A multivariable logistic regression model was used for the analysis. Statistical significance was defined as p < 0.05.
3. Results
From a total of 310 blood samples collected from long-tailed macaques in Southern Thailand, Plasmodium spp. infections were detected using nested and seminested PCR methods. Based on the analysis, 11.3% of the samples (35/310; 95% CI: 8.0–15.3) tested positive for Plasmodium spp. The identified species consisted of P. inui at 9.3% (29/310; 95% CI: 6.3–13.2), P. knowlesi at 0.9% (3/310; 95% CI: 0.2–2.8), and P. cynomolgi at 0.9% (3/310; 95% CI: 0.2–2.8). Focusing on the male macaques (n = 180), the prevalence of P. inui was 11.7% (21/180; 95% CI: 7.4–17.3), and that of P. cynomolgi was 1.7% (3/180; 95% CI: 0.3–4.8), with no detection of P. knowlesi. This resulted in an overall Plasmodium spp. prevalence of 13.3% (24/180; 95% CI: 8.7–19.2) among male macaques, which was higher than the prevalence observed in females. Among the female macaques (n = 130), the overall prevalence was 8.5% (11/130; 95% CI: 4.3–14.6), with P. inui detected in 6.1% (8/130; 95% CI: 2.7–11.8) and P. knowlesi in 2.3% (3/130; 95% CI: 0.5–6.6), while there were no cases of P. cynomolgi (Table 2).
| Variable | No. of animals | Positive N (%) | |||
|---|---|---|---|---|---|
| P. inui | P. knowlesi | P. cynomolgi | Total | ||
| Location by province | |||||
| Phetchaburi | 71 | 1 (1.4) | 0 | 0 | 1 (1.4) |
| Satun | 109 | 14 (12.8) | 0 | 1 (0.9) | 15 (13.8) |
| Phang Nga | 30 | 1 (3.3) | 0 | 0 | 1 (3.3) |
| Surat Thani | 100 | 13 (13) | 3 (3) | 2 (2) | 18 (18) |
| Sex | |||||
| Female | 130 | 8 (6.1) | 3 (2.3) | 0 | 11 (8.5) |
| Male | 180 | 21 (11.5) | 0 | 3 (1.7) | 24 (13.3) |
| Total | 310 | 29 (9.3) | 3 (0.9) | 3 (0.9) | 35 (11.3) |
The prevalence of Plasmodium spp. infections in the long-tailed macaques varied across the studied locations. The highest prevalence was observed in Phunphin district, Surat Thani province, at 18% (18/100; 95% CI: 11.0–26.9), with P. inui detected in 13 cases, P. cynomolgi in 2 cases, and P. knowlesi in 3 cases. Notably, Surat Thani was the only province where all three species were identified. The second highest prevalence was in Mueang Satun district, Satun province, at 13.8% (15/109; 95% CI: 7.9–21.7), consisting of 14 cases of P. inui and 1 case of P. cynomolgi. In Mueang Phang Nga district, Phang Nga province, the prevalence was 3.3% (1/30; 95% CI: 0.1–17.2), with P. inui being the sole species detected. Similarly, Ban Laem district, Phetchaburi province, had a prevalence of 1.4% (1/71; 95% CI: 0–7.6), also limited to P. inui. These findings indicated that P. inui was the only species present at all the studied locations. The nucleotide sequences of the 18S rRNA gene for all Plasmodium spp. detected in the long-tailed macaques in Thailand during this study were submitted to GenBank under the accession numbers PQ559856–PQ559867 and PQ678665–PQ678691 (Table 3).
| ID | Sex | Genus-specific primer | Genus-specific primer | ||
|---|---|---|---|---|---|
| Species | Accession no. | Species | Accession no. | ||
| Phetchaburi | |||||
| PBI 57 | F | — | — | Pi | PQ685654 |
| Satun | |||||
| STN 8 | M | Pi | PQ559857 | — | |
| STN 12 | M | Pi | PQ559858 | — | |
| STN 16 | M | Pi | PQ559859 | — | |
| STN 20 | F | Pi | PQ559860 | — | |
| STN 44 | M | — | — | Pi | PQ678686 |
| STN 46 | M | Pi | PQ559861 | Pi | PQ678687 |
| STN 47 | M | Pi | PQ559862 | Pi | PQ685653 |
| STN 67 | F | Pi | PQ559863 | — | |
| STN 68 | F | Pi | PQ559864 | Pi | PQ678688 |
| STN 69 | M | Pi | PQ559865 | — | |
| STN 71 | M | Pi | PQ559866 | — | |
| STN 82 | M | Pi | PQ559867 | Pi | PQ678689 |
| STN 86 | F | — | — | Pi | PQ678690 |
| STN 87 | F | — | — | Pi | PQ678691 |
| STN 89 | M | — | — | Pc | PQ678667 |
| Phang Nga | — | ||||
| PNA 20 | F | Pi | PQ559856 | Pi | PQ678672 |
| Surat Thani | — | ||||
| SNI 8 | M | — | — | Pi | PQ678673 |
| SNI 12 | M | — | — | Pi | PQ678674 |
| SNI 14 | M | — | — | Pi | PQ678675 |
| SNI 16 | M | — | — | Pi | PQ678676 |
| SNI 20 | M | — | — | Pi | PQ678677 |
| SNI 21 | M | — | — | Pi | PQ678678 |
| SNI 24 | M | — | — | Pi | PQ678679 |
| SNI 33 | F | — | — | Pk | PQ678669 |
| SNI 56 | F | — | — | Pk | PQ678670 |
| SNI 58 | M | — | — | Pi | PQ678680 |
| SNI 59 | M | — | — | Pc | PQ678665 |
| SNI 63 | F | — | — | Pi | PQ678681 |
| SNI 72 | F | — | — | Pk | PQ678671 |
| SNI 75 | M | — | — | Pc | PQ678666 |
| SNI 77 | M | — | — | Pi | PQ678682 |
| SNI 80 | M | — | — | Pi | PQ678683 |
| SNI 89 | M | — | — | Pi | PQ678684 |
| SNI 98 | M | — | — | Pi | PQ678685 |
- Abbreviations: F = female, M = male, Pc = Plasmodium cynomolgi, Pi = Plasmodium inui, and Pk = Plasmodium knowlesi.
Based on the nucleotide sequence analysis of the partial 18S rRNA sequences, P. inui displayed 99.48%–100% identity with sequences from P. inui found in M. fascicularis in Malaysia and wild macaques in Thailand (GenBank accession numbers: FJ619093, FJ619095, EU400391, and EU400397). P. knowlesi displayed 98.06% homology with P. knowlesi from M. fascicularis in Malaysia (KT852930), while P. cynomolgi displayed 98.53% identity with P. cynomolgi from Japan (AB2872989 and AB287290). The nucleotide sequences of the 18S rRNA gene for all Plasmodium spp. detected in the long-tailed macaques in Thailand during this study were submitted to GenBank under the accession numbers PQ559856–PQ559867 and PQ678665–PQ678691 (Table 3).
A comprehensive statistical analysis was conducted to evaluate the relationships between Plasmodium spp. infection and either the sex or geographical location of the long-tailed macaques. Based on the results, there was no significant association between Plasmodium spp. infection and the sex of the macaques after adjustment by location. In contrast, geographical location emerged as a critical determinant of infection risk. Specifically, the adjusted ORs revealed that macaques residing in Satun and Surat Thani provinces were 11.06 times and 14.61 times more likely, respectively, to be infected with Plasmodium spp. than macaques in Phetchaburi province after adjustment by sex (Table 4).
| Variable | No. of animals | % of positive samples (N) | 95% CI of proportion | OR | 95% CI of OR | p value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 130 | 8.5 (11/130) | 4.3–14.6 | 1 | Reference | |
| Male | 180 | 13.3 (24/180) | 8.7–19.2 | 1.43 | 0.66–3.10 | 0.367 |
| Location | ||||||
| Phetchaburi | 71 | 1.4 (1/71) | 0–7.6 | 1 | Reference | |
| Satun | 109 | 13.8 (15/109) | 7.9–21.7 | 11.06 | 1.43–85.80 | 0.021∗ |
| Phang Nga | 30 | 3.3 (1/30) | 0.1–17.2 | 2.46 | 0.15–40.77 | 0.529 |
| Surat Thani | 100 | 18 (18/100) | 11.0–26.9 | 14.61 | 1.90–112.57 | 0.010∗ |
| Total | 310 | 11.3 (35/310) | 8.0–15.3 |
- Abbreviations: CI = confidence interval, OR = odds ratio.
- ∗Denotes statistical significance (p < 0.05).
4. Discussion
The current study identified three species of simian Plasmodium spp. (P. inui, P. knowlesi, and P. cynomolgi) in wild long-tailed macaques from four provinces in Southern Thailand. To the best of our knowledge, this was the first report of these parasites in certain areas based on molecular methods, specifically P. inui, P. knowlesi, and P. cynomolgi in Surat Thani province, as well as P. inui in Phetchaburi province. These findings were consistent with other studies conducted in Malaysia, the Philippines, Vietnam, Myanmar, Cambodia, and Laos that reported these three Plasmodium spp. in M. fascicularis populations [29–32]. The detection of P. knowlesi in macaques in Surat Thani was particularly important, as it was consistent with previous reports of P. knowlesi infections in humans from the same province [23]. This finding suggested a potential zoonotic transmission of P. knowlesi from macaques to humans. Furthermore, Plasmodium spp. infections have been documented in long-tailed macaques in Thailand, including P. cynomolgi and P. inui in Saraburi and Ratchaburi provinces in Central Thailand [31]. In Southern Thailand, P. inui was identified in Ranong and Krabi provinces, while both P. inui and P. cynomolgi were reported in Narathiwat and Songkhla provinces [17, 26, 31].
In addition to these three species, other simian Plasmodium spp. have been detected in long-tailed macaques in Thailand, specifically P. coatneyi [31] and P. fieldi [26, 31]. Furthermore, in addition to long-tailed macaques, diverse Plasmodium species have been identified in other macaque species. For example, P. fieldi and P. coatneyi have been reported in stump-tailed macaques [14], while P. knowlesi was reported in pig-tailed macaques [24]. These findings suggested that the variety of macaque species in Thailand served as major reservoir hosts, potentially contributing to the transmission of Plasmodium spp. among macaques and, importantly, to humans.
The prevalence of Plasmodium infection in the current study was 11.3% (35/310), which was lower than that in other studies. For example, a prevalence of 13% (100/772) was reported in long-tailed macaques in Thailand in 2024 [31], 29% (23/93) in stump-tailed macaques in 2020 [14], and 64.1% (177/276) in long-tailed macaques across Southeast Asia, including Laos, Singapore, Cambodia, the Philippines, and Indonesia, in 2016 [32]. Among the Plasmodium species identified in the current study, P. inui was the most prevalent, accounting for 9.4% (29/310), followed by P. cynomolgi and P. knowlesi, each representing 0.9% (3/310). These findings aligned with other research conducted in Southern Thailand [26] and in various regions of Thailand, including the eastern, western, southern, and northern areas [14], that also identified P. inui as the dominant species. Similarly, studies from Malaysia in 2011 and 2015 highlighted the high prevalence and parasitemia rates of P. inui [33]. However, other research reported contrasting findings, with a higher prevalence of P. cynomolgi compared to P. inui [31, 32].
A key limitation of this study is the absence of blood film microscopy (BFMP), which is traditionally used alongside molecular methods for Plasmodium diagnosis. This was due to practical constraints during fieldwork, including limited time for immediate blood smear preparation and sample transport logistics [34]. However, microscopy-based diagnosis has its own challenges, as it requires a high level of expertise to accurately differentiate Plasmodium species. Misidentification can occur, particularly when morphologically similar species are present [35]. Additionally, microscopy has lower sensitivity than molecular methods, especially when parasite densities are low [35].
Despite the absence of microscopy, this study employed PCR-based detection and sequencing, which are highly specific and sensitive, allowing for accurate species identification [35]. While some studies have integrated both techniques [36], others have relied exclusively on molecular methods [14, 28]. Therefore, despite this limitation, our findings remain robust and contribute valuable insights into the prevalence of simian malaria in the study region. Future studies that integrate both techniques may further enhance diagnostic accuracy and comparative analysis. Another limitation of this study was the use of genus-specific primers (PlasmoM_N2F and PlasmoM_N1R), which were effective in detecting Plasmodium DNA but could not reliably distinguish between species based on amplicon size, particularly in cases of mixed infections. The absence of species-specific primers, such as those described by Lee et al. [37], restricted the ability to accurately and quickly identify individual Plasmodium species. Future research incorporating species-specific primers would improve detection accuracy and offer a clearer understanding of species diversity.
In the current study, the male macaques had a higher infection rate of 13.3% (24/180) compared to 8.5% (11/130) in female macaques. This finding was consistent with another study, which reported infection rates of 2.2% (10/446) in male long-tailed macaques and 2% (4/203) in females [17]. It remains unclear whether sex also affects parasite density as there was no significant association between Plasmodium spp. infection and the sex of macaques after adjusting for location. While long-tailed macaques are natural hosts, differences in infection prevalence between sexes may result from biological or behavioral factors, such as immune response, activity levels, or social interactions. However, there is no clear evidence suggesting mosquito preference for either sex. Notably, this study focused on detecting the presence of infection using PCR and did not quantify parasite density. Future research incorporating qPCR, microscopy, and studies on mosquito feeding behavior and host immunity could provide deeper insights into the role of sex in malaria transmission dynamics.
In Thailand, between 2007 and 2018, malaria cases caused by simian malaria parasites were confirmed in humans in Narathiwat, Chanthaburi, Ubon Ratchathani, Yala, and Tak provinces, with P. knowlesi accounting for 30 cases, P. cynomolgi for 21 cases, P. inui for 19 cases, and P. fieldi for 3 cases [38]. More recently, data from October 2021 to March 2022 indicated a significant rise in human malaria cases caused by P. knowlesi, with over 70 cases reported in Ranong, Songkhla, and Trat provinces [39]. In response, the Thai government and public health authorities implemented preventive measures, including public health advisories encouraging individuals in high-risk areas to avoid getting bitten by mosquitoes and to minimize contact with macaques [39, 40].
Although P. cynomolgi, P. inui, and P. fieldi have been less frequently reported in humans in Thailand, other studies have demonstrated their ability to infect humans [38, 41]. Notably, most patients with simian malaria exhibited mixed-species infections, accounting for 85.29% (58/68) of cases, with the majority coinfected with P. vivax [38]. These findings underscore the need for continued vigilance, not only against P. knowlesi but also in monitoring for the emergence of other simian malaria parasites that could pose a threat to public health.
Furthermore, studies have suggested that certain Anopheles species acted as vectors for simian malaria. For example, the Anopheles gambiae complex (a group of closely related species found in Africa) includes Anopheles gambiae s.s., Anopheles coluzzii, and Anopheles arabiensis that are considered the primary vectors of human malaria in the region [42, 43]. Similarly, Anopheles stephensi serves as a major vector in the Indian subcontinent and parts of the Middle East [44]. In the Amazon region, Anopheles darlingi was identified as the primary malaria vector [45]. In Southeast Asia, the Anopheles dirus complex consists of species that were major malaria vectors in countries such as Thailand and Myanmar [46]. However, there is still only limited research on the vectors responsible for transmitting simian malaria in Thailand. Consequently, further studies should focus on Anopheles species to identify which vectors are involved in transmitting each simian Plasmodium species and to assess regional variations in transmission patterns. A comprehensive understanding of mosquito vector diversity is crucial for evaluating malaria transmission risks, as different Anopheles species may vary in their ability to host and spread specific Plasmodium species. Additionally, investigating emerging mosquito species and their potential role in zoonotic transmission could provide valuable insights into the evolving epidemiology of malaria. The findings from the current research are expected to contribute to the development of public health strategies aimed at controlling malaria transmission among both human and macaque populations in Thailand.
5. Conclusion
This study confirmed the detection of three zoonotic Plasmodium species, namely, P. knowlesi, P. cynomolgi, and P. inui, in long-tailed macaques from four provinces in Southern Thailand. Among these, P. inui was the most prevalent. The identification of these parasites is of major public health importance in Thailand, particularly because P. knowlesi is capable of infecting humans and has been associated with both morbidity and mortality. Therefore, it is critical to mitigate the risk of further spread in Thailand by raising awareness about the dangers of these parasites among local communities and implementing effective strategies to control and prevent their transmission.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research supported by: Kasetsart University Research and Development Institute (KURDI), Bangkok, Thailand, under the Fundamental Fund ∗ FF(KU)39.67 and the Graduate School Fellowship Program at Kasetsart University.
Acknowledgments
We would like to express our sincere gratitude to the Kasetsart University Research and Development Institute (KURDI), Bangkok, Thailand, and the Graduate School Fellowship Program at Kasetsart University for their financial support. We also thank the Faculty of Veterinary Technology, Kasetsart University, Bangkok, Thailand, for providing laboratory facilities and technical support. We are especially grateful to the veterinarians from the Department of National Parks, Wildlife and Plant Conservation, Ministry of Natural Resources and Environment, for their invaluable assistance in sample collection.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




