Antioxidant and Anticancer Activities of Barleria longiflora L. f. From Siriya Kalvarayan Hills
Abstract
Barleria, the third largest genus of the Acanthaceae family, carries a distinct position due to cultural as well as economic significance. Its aerial parts including stem, flowers, leaves, and underground part roots have been used in ancient civilizations as ornament, food, and for religious activities since ages. The presence of diverse phytochemicals is accountable for diverse healthcare applications including analgesic, antioxidant, and antimicrobial. In current work, previous knowledge is exploited as the foundation for the evaluation of Barleria longiflora leaves from the Siriya Kalvarayan hills region against lung cancer. The crude ethanolic extract of leaves has shown significant anti-inflammatory and antioxidant activity. Leaf extract has considerable in vitro free radical scavenging activity in DPPH and ABTS assay, i.e., 62% and 64%, respectively, in comparison to L-ascorbic acid (100 g.mL−1). Leaf extract has also shown commendable cytotoxicity against A549 cells (IC50: 71.00 μL.mL−1). Gas chromatography-mass spectrometry identified around 38 phytochemicals including stigmasterol, resorcinol, and 3,4-anhydro-d-galactosan. In silico analysis identified good binding molecules through molecular docking studies, especially stigmasterol having significant Ki values against the cancer receptors such as PI3K, mTOR, and ERβ. The phytochemicals from Barleria longiflora have shown commendable antimicrobial and anticancer activities which is also supported with in silico analysis. The compounds responsible for anticancer activity can become major ingredient for drug formulation after trials.
1. Introduction
Plants have been used as medicine for centuries due to the lower frequency of side effects. Different traditional healthcare systems like Ayurveda in India [1], traditional Chinese medicines [2] and the East-Mediterranean region [3], Siddha, Unani, and Homeopathy [4] etc., have used plant-based formulations for health care, disease prophylaxis and treatment. Ancient literature like Vedas described numerous healthcare formulation including concoctions, fumes, infusions, tincture, decoctions, and teas [5, 6] prepared with plants and herbs due to the presence of abundant phytochemicals including amines, phenols [7], flavanols [8], and isoprene derivatives [9] that are accountable for their antioxidant, antibacterial, anticarcinogenic, anti-inflammatory, antidiabetic [10, 11], antitumor, antileprosy, and antiviral effects [12–15]. Currently, many commercial products and drugs are formulated using either natural phytochemicals or their derivatives.
In context to biodiversity, Indo-Himalayan region [16, 17] and Western Ghats’ forest and hills are among the richest natural resources regarding flora and fauna [18]. These plants carry a significant role in ancient human civilization, culture, religious beliefs, and ceremonies [19]. Barleria, one of the largest genera, belongs to Acanthaceae family distributed in Southeast Asia known for widespread pharmacological applications in curing inflammations, pain, leukemia, cancer, tumor, glycemia, amoeba, viral, and other microbial infection [12]. Barleria longiflora is one of the shrub species, widely distributed in “Western Ghats.” It is a tiny shrub with 1-2 m height with oppositely arranged pointed hairy leaves. The flower’s tube is white with a purplish color which is 8-9 cm long and narrow [20]. Various species of Barleria have shown conducive results for pharmacological properties [12]; however, B. longiflora has not been explored much for such analysis.
On the other hand, carcinogenesis incidents especially lung cancer cases have increased tremendously due to pollution and increased exposure to chemicals and radiation. World Cancer Research Fund International has identified lung cancer as the second most common cancer across the globe with more than 2.2 million cases alone in 2020. Hungary was the most affected country in 2020 with 10, 274 cases followed by Serbia, France, and New Caledonia [21]. Therefore, the current work was intended to examine the potential of B. longiflora as a potent source of bioactive phytochemicals with cytotoxic and inhibitory potential efficacy against lung cancer. The work combines the empirical and traditional knowledge with advanced statistical and in silico analysis for target specificity and ADMET profiling.
2. Materials and Methods
2.1. Collection of Plant Part (Leaves)
B. longiflora is prominently present in South Eastern Ghats of India. For the work, plant leaves were collected from Siriya Kalvarayan hills, part of South Eastern Ghats, Kallakurichi District (Tamil Nadu), India, in December-January. The collected sample specimen was identified at Department of Botany, Government Arts College, Nandanam, Chennai-600035. The specimen has been assigned the ID NO: GACNBOT101.
2.2. Extract Preparation
Phytochemical extraction from B. longiflora leaves was done with the Soxhlet apparatus using polar and nonpolar solvents, viz., aqueous, hexane, ethanol, acetone, methanol, and chloroform as per established protocol [22, 23].
2.3. Phytochemical Analysis
Quantitative analysis of phytoconstituents in the ethanolic leaf extract of B. longiflora was determined by different conventional methods. Chlorophyll and carotenoids were quantified by the Arnon method [24]. Total sugar and lipid content in extract were assessed by the DuBois method [25] and gravimetric method [26], respectively. Protein and amino acid concentration in extract was quantified by the “Lowry method” [27] and “ninhydrin assay” [28], respectively. Phenolics, flavonoids, and tannins were quantified by using the Folin–Ciocalteu method [29].
2.4. Antioxidant Activity of B. longiflora Leaf Extract
2.4.1. DPPH Assay
2.4.2. 2,2′-Azino-Bis(3-Ethyl-Benzothiazoline-6-Sulfonic Acid) (ABTS) Assay
The antioxidant efficacy of B. longiflora was tested by ABTS radical cation decolorization assay [31, 32]. For analysis ABTS (7 mM aqueous solution) and potassium persulfate (2.45 mM aqueous solution) mixed in equal amount and kept at room temperature in dark for 12–16 h.It generatesABTS radical cations (ABTSo+). ABTSo+ solution was diluted with ddH2O to attain OD of 0.700 at 734 nm. ABTSo+ fresh solution (3.995 mL) was mixed with 5 μL B. longiflora leaf extract and kept again for 30 min followed by absorbance recording at 734 nm. The scavenging potential was determined using equation (1).
2.5. Cytotoxic Activity of B. longiflora Leaf Extract
2.6. Anti-Inflammatory Activity
2.7. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The phytochemical constituents in leaf extract were identified using GC-MS (Perkin Elmer Clarus 500). Phytochemical profiling of leaf extracts of was carried out with GC-MS system equipped with Phenomenex Luna PFP analytical capillary column (30 m × 0.25 mm × 0.25 μm) and flame ionization detector. The analysis was conducted with carrier gas (He) flow rate of 1 mL.min−1, 10 μL injection volume injected at 280°C (injection port temperature), and stationary phase temperature ranging from 60°C to 300°C (10°C.min−1). Mass spectrum of extract was obtained with full scan mode (40–450 Daltons) and identification was done with the National Institute of Standards and Technology (NIST) library [36].
2.8. Docking Analysis
The structures of phytochemicals, identified by GC-MS, as well as receptor proteins for lung cancer, e.g., 5NGB (PI3K), 4JT6 (mammalian target of rapamycin (mTOR)), ERβ, and 3PP0 (human epidermal growth factor receptor-2 (HER-2)), were retrieved from the Protein Data Bank (PDB) and PubChem repositories for docking analysis using AutoDock Tools (ADT) [37, 38]. Different commercial inhibitors are used as control drugs for docking studies, e.g., taselisib and dactolisib for 3NG, rapamycin and ridaforolimus for 4JT6, toremifene for 3OS8, and neratinib and lapatinib for 3PP0.
3. Results and Discussion
The leaf extracts of B. longiflora contained sugar, catechin, flavonoids, triterpenoids, saponin, tannins, anthraquinone, amino acids, sterol, and carbohydrates, according to the results of phytochemical screening of the extracts (Table 1). The B. longiflora extracts in methanol, ethanol, and chloroform were particularly effective suppliers of various types of chemicals. This shows that these solvents’ strong polarity makes them useful for isolating biologically active molecules, and flavonoids were found in the leaf extracts of the plant in chloroform and acetone.
| Name of the test | Aqueous | Methanol | Ethanol | Acetone | Chloroform | Hexane |
|---|---|---|---|---|---|---|
| Triterpenoids | − | + | + | − | − | − |
| Sugar | + | + | + | + | + | + |
| Catechin | − | − | − | − | + | − |
| Flavonoids | − | − | + | + | + | − |
| Saponin | + | + | − | − | + | + |
| Tannins | + | + | − | − | − | − |
| Anthraquinone | − | − | + | − | + | − |
| Amino acid | − | − | − | − | − | − |
| Sterol | − | − | + | − | − | − |
Among all the solvents, ethanolic extract has shown higher potential bioactivity and was hence selected for further characterization. The leaf extracts of B. longiflora contained sugar, catechin, flavonoids, tannins, triterpenoids, anthraquinone, saponin, amino acids, and sterol (Table 2), which may be responsible for bioactivity.
| Test | Quantity (mg/g) |
|---|---|
| Chlorophyll a | 10.12 ± 0.4 |
| Chlorophyll b | 11.22 ± 1.1 |
| Total chlorophyll | 16.25 ± 0.1 |
| Total carotenoids | 4.22 ± 2.0 |
| Total sugar | 151 ± 0.3 |
| Total protein | 1.7 ± 2.1 |
| Total lipids | 121 ± 1.5 |
| Total free amino acids | 0.7 ± 0.4 |
| Total phenol | 79.51 ± 1.1 |
| Total tannin | 55.78 ± 0.2 |
| Total flavonoids | 162 ± 0.8 |
3.1. Antioxidant Activity Analysis of Plant Extract
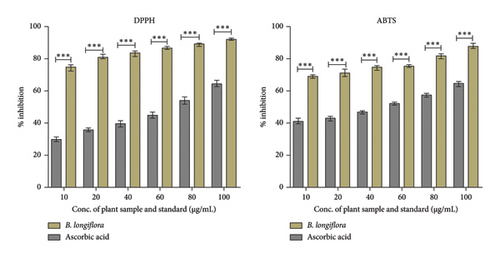
The majority of natural antioxidants originated from plants such as herbs, fruits, vegetable, and spices which contain mostly phenolic compounds, alkaloids, steroids, vitamins, and carotenoids. In the present investigation, ethanolic plant leaf extract of B. longiflora has shown considerable antioxidant properties, i.e., 30%–65% (DPPH assay) and 41%–63% (ABTS radical cation) at 10–100 μg.mL−1 concentration range in comparison to ascorbic acid (75%–95% in DPPH and 70%–90% in ABTS assay) (Figure 1).
3.2. Cytotoxicity of B. longiflora Leaf Extract Against A549 Cells
The anticancer or cytotoxic effect of B. longiflora extract against A549 cell line was investigated with ethanolic extract. Over the past 3 decades, cytotoxicity studies have been crucial in the development of new anticancer formulations. The cytotoxic action of B. longiflora extract demonstrated that plant extract inhibited the proliferation of cancer cells in dose and time-dependent manner (Figures 2(a), 2(b), and 2(c)).
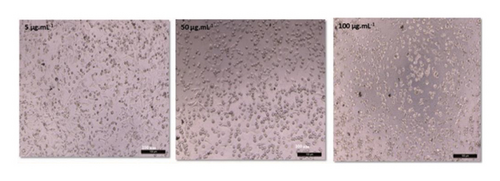
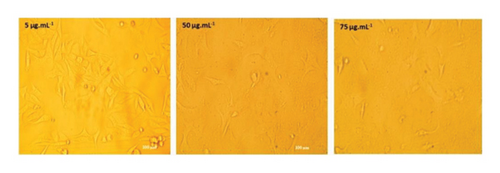
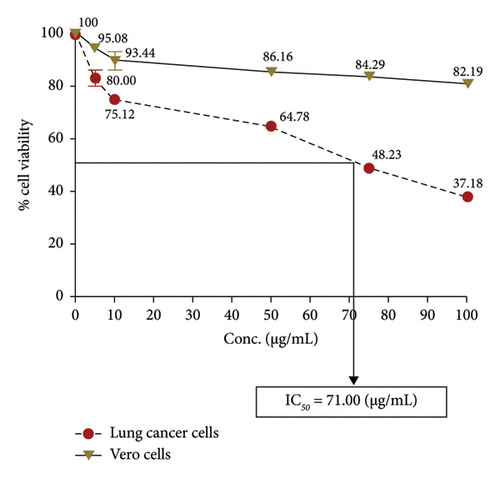
Compared to cancer cells, the cytotoxic study analyzed showed a considerable reduction in cell viability (A549). The percentage of cell viability of A549 revealed a clear declining trend in a dose-dependent manner. Similar patterns were seen with Vero cells at concentrations of 0–20 g.mL−1, with IC50 values for lung cancer cell lines less than 71.00 μL.mL−1 (Figure 2(d)). Consequently, an extract from B. longiflora may be utilized to treat cancer in people.
3.3. Anti-Inflammatory Analysis of B. longiflora Extract
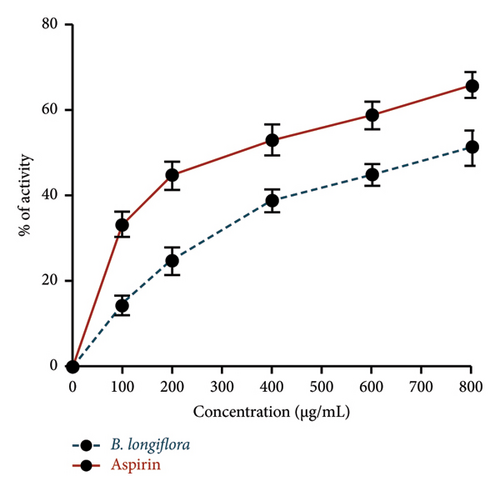
B. longiflora leaf extract, used in the current investigation, has shown anti-inflammatory activity as a clear declining trend in inflammatory markers was observed in a dose-dependent manner. Similar patterns were seen with aspirin cells at concentrations of 0–800 μg/mL (Figure 3).
3.4. GC-MS Analysis
The chemical profiling of B. longiflora leaf extract GC-MS has identified a total of 38 compounds (Table 3). Among the listed compounds, some compounds are critical for bioactivity of plant leaf extract while some are part of structural components. Among the identified compounds, 3 compounds were dominant with peak area, e.g., linoleic acid (16.84%), palmitic acid (11.69%), phthalic acid (8.09%), solanesol (4.13%), and palmitoleic acid (2.52%). In the current work as well, out of 38, only 7 were major compounds. Based on the available literature, all the compounds were examined for their bioactivity and used for docking with the selected receptors, which play a key role in lung cancer.
| Peak | RT | Area (%) | Compound name | Molecular weight (g/mol) | Molecular formula | Medicinal properties | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 5.31 | 0.39 | 1,2-Cyclopentanedione | 98.10 | C5H6O2 | No significant report found | — |
| 2 | 10.208 | 0.5 | 4H-Pyran-4-one | 144.12 | C6H8O4 | Antioxidant activity | [39] |
| 3 | 11.537 | 0.43 | Resorcinol | 110.11 | C6H6O2 | Cytotoxic and anti-inflammatory activity | [40] |
| 4 | 11.978 | 0.75 | 2,3-Dihydro-benzofuran | 110.11 | C6H6O2 | Antibacterial, antidermatophytic action, and antioxidant activity | [41] |
| 5 | 12.178 | 0.26 | 3,4-Anhydro-d-galactosan | 144.12 | C6H8O4 | No significant report found | — |
| 6 | 13.067 | 0.51 | 2H-Pyran-2-one, | 130.14 | C6H10O3 | No significant report found | — |
| 7 | 14.108 | 0.41 | 2-Methoxy-4-vinylphenol | 150.17 | C9H10O2 | Anticancer activity, anti-inflammatory | [42] |
| 8 | 16.916 | 0.69 | Indole-6-carboxaldehyde | 145.16 | C9H7NO | Anticancer activity | [43] |
| 9 | 17.089 | 0.32 | Phenol | 219.20 | C9H9N5O2 | No significant report found | — |
| 10 | 18.509 | 0.34 | Trichloroacetic acid, tridec-2-ynyl ester | 314.7 | C15H23Cl3O2 | No significant report found | — |
| 11 | 19.201 | 0.23 | Azacyclotridecan-2-one | 197.32 | C12H23NO | No significant report found | — |
| 12 | 19.386 | 0.54 | 3-tert-Butyl-4-hydroxyanisole | 180.24 | C11H16O2 | No significant report found | — |
| 13 | 19.455 | 0.45 | Benzoic acid | 168.15 | C8H8O4 | No significant report found | — |
| 14 | 19.918 | 0.28 | Diethyl phthalate | 222.24 | C2H14O4 | Antioxidant | [44, 45] |
| 15 | 20.779 | 2.0 | 1,3,4,5-Tetrahydroxycyclohexanecarboxylic acid | 192.17 | C7H2O6 | No significant report found | — |
| 16 | 22.753 | 0.47 | Coniferyl alcohol | 180.20 | C10H12O3 | Inhibits fungal growth | [46] |
| 17 | 23.147 | 0.68 | Myristic acid | 228.37 | C14H28O2 | No significant report found | — |
| 18 | 24.514 | 0.85 | Neophytadiene | 278.5 | C20H38 | Anti-inflammatory activity | [47] |
| 19 | 24.63 | 0.17 | 3,7,11,15-Tetramethyl-2-hexadecene | 280.5 | C20H40 | No significant report found | — |
| 20 | 25.247 | 0.27 | 3,7,11,15-Tetramethyl-2-hexadecen-1-OL | 296.5 | C20H40O | No significant report found | — |
| 21 | 25.999 | 0.24 | Methyl palmitate | 270.5 | C17H34O2 | Anti-inflammatory activity and anticancer effect | [48] |
| 22 | 26.263 | 2.52 | Palmitoleic acid | 254.41 | C16H13O2 | Anti-inflammatory activity | [49] |
| 23 | 26.646 | 11.69 | Palmitic acid | 256.42 | C16H32O2 | Anti-inflammatory activity, cytotoxic and apoptosis potential on breast cancer cells | [50] |
| 24 | 27.546 | 0.52 | Oleic acid | 282.5 | C18H34O2 | Antioxidant, anti-inflammatory activity, antimicrobial activity, and anticancer effect | [51] |
| 25 | 28.672 | 0.34 | Methyl linoleate | 294.5 | C18H34O2 | No significant report found | — |
| 26 | 28.767 | 0.5 | Linolenic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 352.5 | C21H36O4 | No significant report found | — |
| 27 | 29.365 | 16.84 | Linoleic acid | 280.4 | C18H32O2 | Anti-inflammatory activity | [52] |
| 28 | 34.562 | 1.65 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 330.5 | C19H38O4 | No significant report found | — |
| 29 | 34.675 | 0.88 | Dihydroselarene | — | — | No significant report found | — |
| 30 | 34.836 | 8.09 | Phthalic acid | 166.13 | C8H6O4 | Antimicrobial activity and antioxidant | [53] |
| 31 | 35.066 | 0.39 | Cholesterol | 386.7 | C27H46O | Antimicrobial activity, anticancer, and antioxidant effect | [54–56] |
| 32 | 36.792 | 0.59 | Linoleic acid propyl ester | 322.5 | C21H38O2 | No significant report found | — |
| 33 | 36.89 | 1.34 | cis,cis,cis-7,10,13-Hexadecatrienal | 234.38 | C16H26O | No significant report found | — |
| 34 | 37.161 | 0.65 | Glyceryl monostearate | 358.6 | C21H42O4 | Antimicrobial and anticancer activity | [57, 58] |
| 35 | 37.255 | 0.33 | Alpha-Kessyl acetate | 280.4 | C17H28O3 | No significant report found | — |
| 36 | 38.291 | 4.13 | Solanesol | 631.1 | C45H74O | Anti-inflammatory activity | [59] |
| 37 | 38.968 | 0.27 | Stigmasterol | 412.7 | C29H48O | Anticancer activity, anti-inflammatory activity, and antimicrobial activity | [60, 61] |
| 38 | 39.625 | 0.18 | 2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetraenyl)-cyclohexanol | 428.7 | C30H52O | No significant report found | — |
3.5. In Silico Docking Studies
The phytochemicals identified from Barleria longiflora leaf extract by GC-MS may be responsible for the bioactivity specifically anticancer activity but to get the type of interaction of phytochemicals with target receptors, docking analysis was conducted. Among 38 phytochemicals, only 8 have shown some affinity with the target receptors and hence were selected for further interaction studies (Figures 4(a), 4(b), 4(c), and 4(d) and Table 4).

| Protein targets | Compounds | Van der Waals/conventional hydrogen bond | Alkyl/pi-alkyl | Pi-sigma/pi-donor hydrogen bond/unfavorable donor-donor | Carbon hydrogen bond/covalent bond/unfavorable acceptor acceptor | Pi-pi T-shaped/pi-anion/pi-lone pair |
|---|---|---|---|---|---|---|
| A. 5NGB (PI3K) | Azacyclotridecan-2-one | Asp589, Met387, Arg389, Cys590, Gly593, Leu583, Phe587, Val618, Asp584, Phe585, Tyr621, Pro588, and Ser623 | — | — | Glu622 | — |
| Stigmasterol | Pro173, Cys815, Leu735, Gln795, Gln792, Gly814, Asp736, Asp642, Gln170, and 606 | Phe609, Leu816, Phe646, His650, and Met788 | — | — | — | |
| Neophytadiene | Arg389, Glu622, Ser623, Gly593, Asp626, Asp584, and Glu628 | Leu583, Pro588, Val618, Phe585, Tyr621, Met387, Cys590, Cys627, and Tyr | — | — | — | |
| Taselisib | Arg246, Glu248, Cys815, Lys642, Phe646, Gln795, Gln610, Gln792, Tyr261, Gln260, and Tyr813 | Phe609, Leu791, and Met788 | — | Leu735, Gln170, Ser738, Gly814, and Ser264 | Asp736 | |
| Dactolisib | Ser754, Asp897, Pro758, Lys779, Asp787, Thr833, Tyr813, Val828, and Phe908 | Met752, Ile825, Ile777, and Val827 | Ile910, Met900, and Trp760 | Asp911, Asp832, and Ser831 | — | |
| B. 4JT6 chain A (mTOR) | Stigmasterol | Asp2212, Leu2208, Asn2211, Glu2409, Glu2405, and Asn1898 | Pro2213, His2410, and Val2406 | — | — | — |
| Azacyclotridecan-2-one | Cys2243, Leu2354, Leu2185, Ile2237, Tyr2225, Gly2238, Val2240, and Met2345 | Ile2356 and Trp2239 | — | — | — | |
| 2-Methoxy-4-vinylphenol | Arg2348, Gly2351, Ser2307, Trp2304, Leu2303, and Arg2316 | Lys2306, Trp2313, Arg2317, Leu2346, and Tyr2320 | ||||
| Rapamycin | Ser2221, Lys2352, Ala2226, Asp2347, Ser2350, Ile2222, Asn2206, Gln2223, Pro2241, Val2240, Tyr2225, Trp2239, Gly2238, Phe2182, Pro2141, Thr2143, and Gly2142 | Phe2184 | — | Arg2224 | — | |
| Ridaforolimus | Arg2251, Arg2348, Trp2239, Thr2164, Gln2261, Ser2342, Asp2252, Ala2248, Asp2244, Thr2249, Leu2354, and Val2162 | Val2240, Met2345, Ile2237, Leu2185, and Ile2356, | — | Cys2243 and Ile2163 | — | |
| C. 3OS8 chain A (ER-beta) | Stigmasterol | Met357, His356, Glu353, Glu323, Ile386, Leu320, Gly390, Leu327, Lys449, Pro325, and Val446 | Arg394, Ile326, Trp393, Phe445, Pro324, and Leu387 | — | — | — |
| Azacyclotridecan-2-one | Phe445, Gly390, Arg394, Lys449, Ile326, Pro325, Ile386, Leu387, Pro325, Leu327, and Glu353 | Pro324 | — | — | — | |
| Neophytadiene | Thr347, Gly521, Phe404, Met343, Phe425, Arg394, and Glu353 | Val533, Ala350, Met388, Trp383, Leu391, Leu384, Leu346, Leu428, Ile424, Met421, and His524 | — | — | — | |
| Toremifene | Leu428, Phe425, Val422, Met388, Thr347, Val533, Trp383, Met342, Leu410, and Gly521 | Leu391, Leu346, Ala350, Leu384, Ile424, Leu525, Met343, and His524 | Phe404 and Met421 | Arg412 and Gly415 | — | |
| D. 3PP0 chain A (HER-2) | Stigmasterol | Asp845, Arg849, Asn850, Ala730, Leu866, Val884, Lys883, and Asp924 | Lys887, Lys921, Trp888, Pro922, Ala928, Ile886, and Pro885 | — | — | — |
| Neophytadiene | Asp863, Leu852, Thr862, Thr798, Ser783, Glu770, and Ile767 | Lys753, Ala751, Val734, Leu796, Leu785, Met774, Phe864, and Ala771 | — | — | — | |
| Azacyclotridecan-2-one | Phr759, Arg756, Arg868, Leu755, Ile767, Glu770, Leu866, and Glu766 | Phe731 and Ala763 | — | Gly865 | — | |
| Lapatinib | Leu869, Asp769, Leu870, Ile714, Leu790, Leu712, Ile788, Lys765, Glu766, Arg868, and Tyr877 | Leu768 | Tyr772 | — | — | |
| Neratinib | Leu790, Ile788, Ile714, Lys765, Tyr772, Leu768, Asp769, Leu869, Ala710, and Leu711 | Ala771 and Ala775 | Leu712 | — | — | |
Table 4 summarizes the interaction profile of selected phytochemicals and compares them with commercially available inhibitors available in the market, i.e., taselisib and dactolisib for 5NGB, rapamycin and ridaforolimus for 4JT6, toremifene for 3OS8, and neratinib and lapatinib for 3PP0. For 5NGB, dactolisib has the lowest binding energy along with an inhibitory constant of 0.004 μM. In comparison to commercial inhibitors, only one phytochemical “azacyclotridecan-2-one” has shown compatible results with a binding energy of −8.43 kcal/mol; however, the inhibitory constant was much higher (0.665 μM). Only one phytochemical “stigmasterol” has shown potential efficacy against the other three protein targets, i.e., 3PP0, 3OS8 chain A, and 4JT6 chain A. Against 4JT6 and 3OS8, stigmasterol has lower binding energy and lower inhibitory constant which suggest that the effective concentration of phytochemical is much lower in comparison to commercial inhibitors.
3.5.1. PI3K
PI3K has a crucial role in several cancers and is a downstream effector of receptor tyrosine kinases such as insulin receptor and HER-2, which transduce growth factor signaling [62]. PI3K governs phosphatidylinositol-3,4,5-triphosphate (PIP3) production and activates Akt (protein kinase B) and other kinases. This protein has a “p85” regulatory domain and “p110” catalytic domain. “PI3K-Akt-mTOR” pathway reigns cellular longevity, cellular proliferation through nutrient uptake (as well as anabolism) and finally, increases cell survival through apoptosis inhibition. The “PI3K/Akt/mTOR pathway” is commonly dysregulated in almost all human cancers, and hence the proteins of this pathway are prime targets of anticancer therapeutic regimes [63, 64].
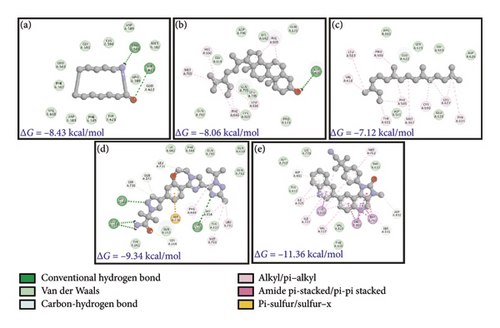
Often, inhibitors of this pathway decrease cellular proliferation and increase cell death. Since cancer cells achieve immortality, many therapeutic molecules are directed at the activation of apoptosis. In this work, some of the phytochemicals identified from B. longiflora extract with their favorable binding energies and low Ki values are compatible with control compounds such as TLB and DLB. As mentioned above, azacyclotridecan-2-one (ACT) and stigmasterol (SSL) have minimum binding energy among all phytochemicals (−8.43 and −8.06 kcal/mol, respectively) but higher than the commercial inhibitors like taselisib and dactolisib (−9.34 and −11.36 kcal/mol, respectively). In terms of inhibitory constant and effective dosage,the quantity of compounds is minimal in the case of dactolisib (0.004 μM) and taselisib (0.143 μM) which is much lower in comparison to 0.665 μM (azacyclotridecan-2-one) and 1.23 μM (stigmasterol).
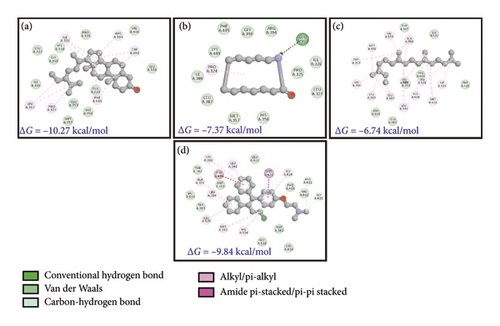
The kinase domain of human p110 is located between residues ∼696 and 1068. In PI3K p110α, the key residue involved in phosphoryl group transfer reaction is Lys802. Residues involved in substrate stabilization in PI3K which line the binding pocket are 941-KKKKFGYKRER-951 [65]. The drug XL765 was found to dock at the site of natural ligand LXX in the kinase domain of p110 and some common residues were found in interactions of both the inhibitor XL765 and the natural ligand [66]. In our analysis, we found that diverse kinds of interactions were proffered by PI3K in docking the plant compounds. The best interactions comprised of bonding and nonbonding interactions; in SSL association with PI3K, a hydrogen bond between ASP606 and PI3K p110 can be seen (Figures 5(a), 5(b), 5(c), 5(d), and 5(e)); also, other interactions such as pi-alkyl or alkyl (as well as nonbonded interactions such as van der Waals) are seen. In RCL binding, pi-stigma was observed. Strangely, the commercial compounds did not show high-affinity interactions with PI3K.
3.5.2. mTOR
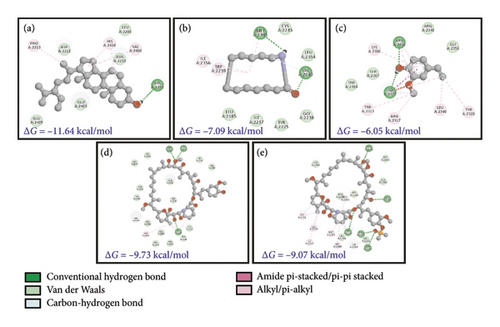
mTOR is a PI3K-related Ser/Thr kinase which is responsible for cell growth through direct or indirect phosphorylation of ∼800 proteins [67]. Since the protein had two distinct chains (A and C in PDB ID: 4JT6), docking was done separately for the two chains. Against the larger chain A, the plant compounds showed better ΔG values and were able to interact better with chain A, as evidenced by the favorable bonding (conventional H-bonding of SSL and Pi-alkyl bonding of RCL and several other forces such as van der Waals). Comparatively, the control compounds (RMN and RFL) had better dock scores (−9.07 to ∼−9.73) which are responsible for the existence of several carbon-hydrogen bonds with the protein (Figures 6(a), 6(b), 6(c), 6(d), and 6(e)). The predicted Ki values for the docked plant compounds were much lower than those of controls (Table 5).
| Protein targets | Ligands | Binding energy (kcal/mol) | Ligand efficiency | Inhib_constant (μM) | IE | VDE | EE |
|---|---|---|---|---|---|---|---|
| A. (PI3K) 5NGB | Azacyclotridecan-2-one | −8.43 | −0.6 | 0.665 | −8.43 | −8.41 | −0.01 |
| Stigmasterol | −8.06 | −0.27 | 1.23 | −9.85 | −9.74 | −0.11 | |
| Neophytadiene | −7.12 | −0.36 | 6.09 | −10.99 | −10.99 | 0.0 | |
| 2,3-Dihydro-benzofuran | −5.87 | −0.65 | 50.2 | −5.87 | −5.8 | −0.06 | |
| 2-Methoxy-4-vinylphenol | −5.67 | −0.52 | 70.22 | −6.56 | −6.54 | −0.02 | |
| 1,2-Cyclopentanedione | −4.84 | −0.69 | 283.22 | −4.84 | −4.66 | −0.18 | |
| Resorcinol | −4.45 | −0.56 | 548.65 | −5.04 | −4.92 | −0.12 | |
| 3,4-Anhydro-d-galactosan | −4.09 | −0.41 | 1000 | −4.39 | −4.36 | −0.03 | |
| Taselisib | −9.34 | −0.27 | 0.143 | −10.83 | −10.59 | −0.24 | |
| Dactolisib | −11.36 | −0.32 | 0.004 | −12.38 | −12.19 | −0.18 | |
| B. (mToR) 4JT6 | Stigmasterol | −11.64 | −0.39 | 0.002 | −13.43 | −13.34 | −0.09 |
| Azacyclotridecan-2-one | −7.09 | −0.51 | 6.3 | −7.09 | −7.03 | −0.06 | |
| 2-Methoxy-4-vinylphenol | −6.05 | −0.55 | 36.63 | −6.95 | −6.82 | −0.12 | |
| 2,3-Dihydro-benzofuran | −5.55 | −0.62 | 85.93 | −5.55 | −5.46 | −0.09 | |
| Neophytadiene | −5.35 | −0.27 | 120.45 | −9.22 | −9.23 | 0.0 | |
| Resorcinol | −4.61 | −0.58 | 418.0 | −5.21 | −4.78 | −0.42 | |
| 1,2-Cyclopentanedione | −4.54 | −0.65 | 468.58 | −4.54 | −4.46 | −0.08 | |
| 3,4-Anhydro-d-galactosan | −4.37 | −0.44 | 629.69 | −4.67 | −4.36 | −0.32 | |
| Rapamycin | −9.73 | −0.15 | 0.074 | −12.41 | −11.97 | −0.44 | |
| Ridaforolimus | −9.07 | −0.13 | 223.69 | −12.06 | −11.98 | −0.07 | |
| C. (ERβ) 3OS8 | Stigmasterol | −10.27 | −0.34 | 0.0029 | −12.06 | −12.07 | 0.01 |
| Azacyclotridecan-2-one | −7.37 | −0.53 | 3.96 | −7.37 | −7.29 | −0.08 | |
| Neophytadiene | −6.74 | −0.34 | 11.54 | −10.61 | −10.61 | 0.0 | |
| 2,3-Dihydro-benzofuran | −5.5 | −0.61 | 93.0 | −5.5 | −5.22 | −0.28 | |
| 2-Methoxy-4-vinylphenol | −5.4 | −0.49 | 111.02 | −6.29 | −5.89 | −0.4 | |
| Resorcinol | −4.48 | −0.56 | 524.29 | −5.07 | −4.78 | −0.29 | |
| 1,2-Cyclopentanedione | −4.47 | −0.64 | 532.41 | −4.47 | −4.0 | −0.46 | |
| 3,4-Anhydro-d-galactosan | −4.25 | −0.43 | 767.84 | −4.55 | −4.34 | −0.2 | |
| Toremifene | −9.84 | −0.34 | 0.061 | −12.53 | −12.42 | −0.11 | |
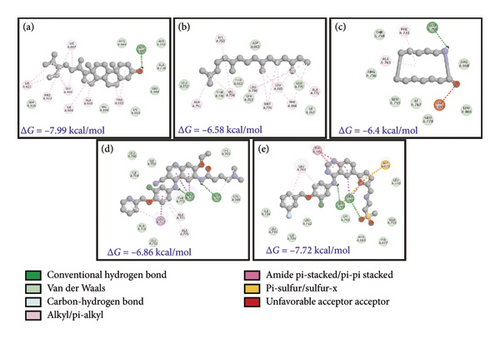
| D. (HER-2) 3PP0 | Stigmasterol | −7.99 | −0.27 | 1.39 | −9.78 | −9.62 | −0.16 |
| Neophytadiene | −6.58 | −0.33 | 15.13 | −10.45 | −10.46 | 0.0 | |
| Azacyclotridecan-2-one | −6.4 | −0.46 | 20.39 | −6.4 | −6.26 | −0.14 | |
| 2,3-Dihydro-benzofuran | −5.06 | −0.56 | 196.8 | −5.06 | −5.01 | −0.04 | |
| 1,2-Cyclopentanedione | −5.05 | −0.72 | 198.64 | −5.05 | −4.9 | −0.15 | |
| 2-Methoxy-4-vinylphenol | −5.2 | −0.47 | 153.22 | −6.1 | −6.07 | −0.03 | |
| Resorcinol | −4.37 | −0.55 | 627.91 | −4.97 | −4.69 | −0.29 | |
| 3,4-Anhydro-d-galactosan | −4.02 | −0.4 | 1130 | −4.32 | −3.92 | −0.4 | |
| Neratinib | −6.86 | −0.17 | 9.4 | −10.14 | −9.18 | −0.96 | |
| Lapatinib | −7.72 | −0.19 | 2.2 | −11.0 | −10.16 | −0.84 | |
Stigmasterol (SSL), Resorcinol (RCL), and 3,4-Anhydro-d-galactosan (ADG) have Ki values of 0.002 μM, 418.0μM, and 629.69 μM, respectively with mTOR. On the other hand, the smaller subunit (chain C) was found to interact with the control drugs RMN and RFL with binding scores (ΔG) of −9.73 and −9.07 kcal/mol, respectively (shown in red, Figures 6(d) and 6(e)), yielding predicted Ki values in both nanomolar and micromolar ranges. However, from literature survey, the kinase site of mTOR possesses two lobes, the KD N lobe and the KD C lobe, and the kinase domain of mTOR is located in chain A. Key residues of mTOR reported earlier for docking of substrates as well as inhibitors are located in the KD N lobe and the residue numbers are Asp2195, Asp2357, Tyr2225, Ile2163, Pro2169, Leu2185, Asn2343, Lys2187, Glu2190, Asp2357, and Asp2338 [68, 69]. Compared to the interactions between mTOR chain A and C, since the kinase activity of the protein is present in chain A, it is assumed that the docking results obtained for B. longiflora compounds with chain A could point to the ability of the compounds to inhibit the kinase activity of mTOR, and hence stigmasterol could serve as potential drugs for mTOR inhibition.
3.5.3. ERβ
Estrogen receptors α and β are responsible for binding to estrogen and triggering the expression of estrogen-responsive genes and both these proteins have 97% homology [70, 71]. Since ER is a nuclear receptor, it has a ligand binding domain which binds to estradiol (EDL) and then transactivates into the nucleus. Ligand-bound ER recognizes estrogen-response elements (EREs) and regulates protein kinase cascades, activation of eNOS through phosphorylation, and phosphorylation of other target proteins. Inside the nucleus, ER regulates transcription of genes responsible for endocrine, cardiovascular, and metabolic pathways, bone growth (and maturation), and skin health, apart from regulating the expression/development of secondary sexual characteristics [71]. In cardiomyocytes, ER signaling regulates vascular function and also the inflammatory response. Figures 7(a), 7(b), 7(c), and 7(d) show the binding of B. longiflora compounds to ERβ (3OS8) and the control compounds EDL as well as TMN. The phytochemicals were able to interact with target at two different pockets.
3.5.4. Human EpidermalGrowth Factor Receptor-2 (HER-2)
HER-2, an epidermal growth factor receptor, has tyrosine kinase activity. It exhibits autophosphorylation of tyrosine residues within the cytoplasmic domain of the receptors upon dimerization of the receptor which governs the signaling pathways responsible for cell proliferation and tumorigenesis. HER-2 mutation is mainly associated with 15%–30% of breast cancers followed by 10%–30% of gastric/gastroesophageal cancers and cancer associated with the bladder, colon, endometrium, ovary, and lung [72]. In lungs, HER-2 is linked with non-small-cell lung cancer (NSCLC). Three mechanisms have been suggested for NSCLC including 1%–4% gene mutation, 2%–5% gene amplification, and 2%–30% protein overexpression [73]. Docking studies showed that stigmasterol has an effective binding affinity toward HER-2. The binding energy of stigmasterol for HER-2 is almost close to lapatinib (−7.99 and −7.72 kcal.mol−1, respectively). The inhibitory constant also emphasizes on higher suitability of phytochemicals as a lower dose is required.
The interaction studies and participation of different residues were also analyzed (Figures 8(a), 8(b), 8(c), 8(d), and 8(e) and Table 5). It is clear from the analysis that van der Waals forces are crucial for the interaction between target receptors and molecules (drug and phytochemical). The analysis revealed stigmasterol as one of the potent candidates for drug formulation.
4. Discussion
Plants are the safest and richest sources for various biomolecules and bioactive compounds [74], which played a crucial role in food and healthcare [15] for ages in different ancient and tribal practices [75]. Besides common biomolecules like carbohydrates, proteins, and lipids, plants are also rich in phytochemicals like alkaloids, tannins, and polyphenols which are well reported for healthcare and promoting abilities [76].
Barleria longiflora is one such species that has not been explored fully, and only a few studies are available related to its significance. The presence of diverse phytochemicals in Barleria albostellata’s leaf and stem has been reported which contribute to its anti-inflammatory, analgesic, antihyperglycemic, antileukemic, antitumor, and antimicrobial activities [75]. Jothimuniyandi and Jayachitra [77] also reported the highest anti-inflammatory potential with ethanolic extract of B. longiflora leaf (56%) followed by methanol (48%) and chloroform (46%). The antioxidant activity of ethanolic extract was also maximum (83%) among all the solvents used including aqueous, chloroform, petroleum ether, methanol, ethanol, and ethyl acetate. Comparative evaluation of methanolic and aqueous extract of leaves for phytochemical analysis revealed that phytochemical diversity was higher in methanolic extract. Leaf extract contains alkaloids, saponins, tannins, flavonoids, and proteins [78].
Irregular inflammation and oxidative stress are also associated with other complications like cancer, and plant extract has shown promising results against both. Amarasiri et al. [79] reported that extracts with organic solvents have a high fraction of polyphenols and flavonoids in comparison to water and hence have higher antioxidant activity. Barleria extract also helped in countering the DOX-induced oxidative stress by modulating the activity of glutathione peroxidase and glutathione reductase enzymes. Among all solvents explored, hexane-based extract has improved glutathione peroxidase activity by 111% while water-based extract only has 55%. It has been suggested that treatment with the Barleria prionitis extracts suppressed TNF-α and IL-1β significantly. In the rat model, the anti-inflammatory potential and TNF-α, and IL-1β suppression was in the order of aqueous > butanol > ethyl acetate > hexane [79]. Panchal et al. [80] also evaluated the anticancer potential of Barleria prionitis against different cell lines including breast, lung, colon, and respective metastatic cells. The maximum inhibition against cancer cells reported was 76.97% against colon metastatic cells followed by 71% against breast cancer cells. In comparison, the anticancer potential was higher in current work (65%). Alkahtani et al. [81] reported viability of L929, MCF-7, and A549 cells by MTT assay against the methanolic extract of Barleria hochstetteri. The IC50 value for methanolic extract was 144.30 μg/mL (A549) and 219.67 μg/mL (MCF-7). For L929 cells, IC50 was much higher which suggested that methanol extract was less toxic against the noncancerous L929 cell line. It was suggested that extract activated caspase-3 to initiate apoptosis by inhibiting Bcl-2 protein.
In the current work, a total of 38 compounds have been detected including stigmasterol, resorcinol, and phenol derivatives as major population. Alkahtani et al. [81] also identified palmitic and linolenic acid derivatives and solanesol as major compounds in the methanolic extract of Barleria hochstetteri. GC-MS identified 9 major compounds from the extract. Gangaram et al. [75] also confirmed that decanoic acid and its derivate were one of the most prominent groups of phytochemicals in B. albostellata leaves and stem extract in different solvents. Alcoholic extract (methanol) has confirmed the presence of stigmasterol in methanolic extract of stem as well as leaves. It was also observed that phytochemical diversity increased in alcoholic extract in comparison to chloroform and hexane or other nonpolar as well as aqueous extract. Therefore, the activity was also much higher in similar extracts.
5. Conclusions
Plants are the biggest and most eco-friendly source of bioactive compounds with the least chances for side effects. Barleria is one of such genera carrying a significant role in traditional activities, food, and healthcare. Different species of Barleria have been explored for bioactivities and diverse phytochemicals; however, B. longiflora has only a few reports in records. In the present work, B. longiflora leaves from the Siriya Kalvarayan hills region were evaluated for antioxidant, anti-inflammatory, and anticancer (lung cancer). Ethanolic leaf extract has shown considerable scavenging activity against DPPHo and ABTSo+ that is compatible with previous literature. Docking studies suggested that some of the phytochemicals could be more effective in inhibiting lung cancer–associated receptors and may prove effective in finding safer and more effective drugs for cure.
Ethics Statement
The ethical clearance for anti-inflammatory analysis was taken from Saveetha Institute of Medical and Technical Sciences, Thandalam, Chennai, Tamil Nadu, India (Ref. No. 487/12/2024/Staff/SRB/SMCH).
Consent
Written informed consent was obtained from the subject and subject information sheet has been provided.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Panjatcharam Varadharasu: experimentation, Kandavel Dhandayuthapani: concept and project mentoring, Pushparaj Annadurai: in silico analysis and validation, Vishal Ahuja: review editing and project mentoring, Shikha Chauhan: writing, review, and editing, Kalpana Tilak: writing and draft editing, Balamurugan Sudarrajan: concept and project mentoring, GholamReza Abdi: review and draft editing and mentoring, and Deepak Sharma: experimentation and analysis. Panjatcharam Varadharasu and Kandavel Dhandayuthapani contributed equally as co-first authors.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data are disclosed in the manuscript. More information can be provided by the corresponding authors upon request.




