Integrative Network Pharmacology to Evaluate Bioactive Phytochemicals of Aesculus hippocastanum and Their Multitarget Mechanisms Against Chronic Venous Insufficiency
Abstract
Chronic venous insufficiency (CVI) affects the venous system of the lower limbs, leading to pain, edema, skin anomalies, ulceration, and extended venous hypertension. Aesculus hippocastanum seed extract has been traditionally used for CVI treatment. This study aims to elucidate its mechanism of action using in silico approaches. An integrated network pharmacology technique identified potential components for CVI treatment. From 105 chemical elements of A. hippocastanum collected via literature research, 12 active phytochemicals with notable drug-like properties and bioavailability were selected. These chemicals were linked to 213 putative target genes using the SwissTargetPrediction database. Comparison with CVI-related genes from GeneCards and OMIM databases narrowed these 147 crucial genes shared by A. hippocastanum and CVI pathophysiology. A compound-target network revealed interactions influencing CVI-related pathways. After that, protein–protein interaction analysis using STRING and Cytoscape identified key hub genes, including SRC and MAPK3, critical to CVI pathogenesis. GO and KEGG pathway analyses further highlighted biological functions and signaling pathways associated with A. hippocastanum’s anti-CVI effects. Moreover, virtual screening and molecular docking analysis demonstrated high binding affinities of alpha-spinasterol with MAPK3 (−9.3 kcal/mol) and kaempferol with SRC (−9.0 kcal/mol). Molecular dynamics simulation confirmed the binding capacity and stability of SRC–kaempferol and MAPK3–alpha-spinasterol. Ligands showed strong catalytic site binding, as evidenced by molecular mechanics–generalized born surface area (MM-GBSA) analysis. This comprehensive investigation highlights the potential therapeutic benefits of A. hippocastanum seed extract for CVI treatment and encourages further studies on its mechanism and clinical applications.
1. Introduction
Chronic venous insufficiency (CVI) is a prevalent yet often overlooked disease that significantly reduces a patient’s quality of life (QoL) and imposes a growing burden on healthcare systems. CVI encompasses reticular and varicose veins, telangiectasias, chronic venous hypertension, and other related venous pathologies [1]. Patients with CVI typically present with twisted and enlarged veins in their lower limbs [2, 3]. Risk factors associated with CVI include obesity, a history of venous disorders within a family, advanced age, pregnancy, leg injuries, and prolonged standing or reclining [2]. The condition primarily affects the venous system of the lower limbs, with venous hypertension arising from structural changes in the vein wall, venous valve insufficiency, and hereditary susceptibility [4, 5].
Clinically, CVI ranges from cosmetic concerns like spider veins to more severe symptoms, such as heaviness, pain, leg swelling, edema, itching, varicose veins, and throbbing sensations, which worsen with prolonged standing [6, 7]. Reports estimate that CVI affects up to 17% of men and 40% of women, with varicose veins being the most prevalent symptoms, particularly in individuals over 50 years of age [8, 9]. Pathophysiologically, CVI is often linked to the great saphenous vein (GSV) and, in some cases, the involvement of the perforator vein or small saphenous vein (SSV). The development of varicose veins has been linked to a continuous increase in venous wall stress or biomechanical stretch [6, 10]. Advances in endovascular therapy have demonstrated efficacy in restoring venous outflow and reducing venous blockages in patients with CVI, offering a minimally invasive alternative to traditional surgical treatments like vein ligation and stripping [11]. In recent years, endovascular therapy has demonstrated efficacy in eliminating blockages and restoring venous outflow in patients with CVI. Despite these developments, the precise etiology of CVI remains unclear, complicating the search for effective pharmaceutical interventions [12].
Emerging evidence highlights the role of inflammatory processes and molecular signaling in CVI pathogenesis. An increase in matrix metalloproteinases (MMPs) has been implicated in venous insufficiency, potentially mediated by inflammation-induced venular degeneration and endothelial dysfunction [6]. Additionally, research has identified SRC kinase and MAPK3 (ERK1) as key regulators in vascular remodeling and inflammation. Activation of the MAPK/ERK signaling cascade in response to mechanical stress promotes MMP expression, venous wall remodeling, and endothelial dysfunction, while SRC kinase exacerbates vascular inflammation. These findings suggest that targeting these pathways could provide novel therapeutic strategies for CVI.
CVI is prevalent in developed nations, with lifestyle factors and physical inactivity contributing to its high incidence. Up to 2%–3% of adults worldwide suffer from lower limb ulceration, with prevalence increasing in those over 65 years of age [13, 14]. The economic burden is substantial, with annual treatment costs ranging from $1–5 billion in the United States and exceeding $1 billion in the United Kingdom [15, 16]. To mitigate these costs, healthcare providers must emphasize prevention and early management, particularly for high-risk individuals [1].
Plant-derived products represent promising and cost-effective resources for managing CVI [17]. The development of uniform dosage forms, quality control procedures, and pharmacological evidence has facilitated the exposure and utilization of these products. Natural remedies derived from various plants contain hundreds of phytochemicals. Studies indicate that phytochemicals in certain herbaceous plants possess significant potential to inhibit cell proliferation, suppress carcinogenesis, and prevent tumor spread [18]. However, the mechanisms underlying their actions and the collaboration of their molecular processes remain unclear.
Aesculus hippocastanum L., generally referred to as horse chestnut or buckeyes, is a rapidly growing ornamental tree in the family of Hippocastanaceae, and it has a long history of use in traditional for treating vascular problems, including venous insufficiency, varicose veins, and hematomas [19]. In conventional medicine, isolates from A. hippocastanum are utilized to address conditions such as prostate gland enlargement, peripheral and vascular disorders, and hemorrhoids, as well as swelling, pain, and heaviness in the lower limbs [20–23]. Horse chestnut seeds are rich in active phytochemicals, including flavonoids (e.g., quercetin and kaempferol), which exhibit anti-inflammatory, antioxidant, and vasoprotective properties, as well as aescin, a saponin with potent anti-inflammatory-and-circulatory-benefits. Certain components found in chestnut seeds, such as flavonoids, coumarins, and simple phenolics, exhibit antioxidant action. This action may help alleviate oxidative stress during vascular inflammation and edema [24]. These components contribute to improved venous tone, reduced inflammation, and alleviated vascular symptoms, making horse chestnuts an attractive candidate for CVI treatment [25].
Network pharmacology, an interdisciplinary approach combining systems biology, computational science, and pharmacology, offers a holistic framework to investigate the multitarget mechanisms of plant-derived compounds [26]. Unlike traditional single-target drug development, this approach explores how various active ingredients interact with biological networks to produce therapeutic effects. [27]. Network pharmacology integrates big data analysis and artificial intelligence (AI) to identify drug–target interactions, signaling pathways, and potential protein–protein interaction (PPI) networks, enhancing drug discovery and development. It further examines the complex biological mechanisms underlying active phytochemicals and their associated signaling pathways in the development of drugs targeting multiple pathways. This approach is commonly employed to identify active pharmaceutical compounds and understand their full mechanism of action of these substances, thereby providing advanced technological and scientific support for innovative drug research, development, and clinical application [28].
The method focuses on identifying active ingredients, enhancing therapeutic effects, reducing toxicity, and linking active ingredients to disease genes to explore potential targets through the development of PPI networks and gene annotation studies [29–33]. The use of a multivariate target-recognition approach in network pharmacological research is advancing, enabling the prediction of key phytochemical-active ingredients and potential target proteins of herbal products. This methodology holds promise for elucidating the mechanisms through which traditional Chinese medicines (CHMs) provide combined therapeutic benefits for various ailments [34, 35].
In this study, network pharmacology and bioinformatics techniques were employed to investigate the molecular mechanisms underlying the efficacy of A. hippocastanum in treating CVI. The active phytochemicals, target proteins, and molecular pathways of A. hippocastanum seed extract were identified and analyzed as shown in Figure 1. The results were additionally supported through molecular docking and molecular dynamics simulation (MDS), providing valuable insights into its therapeutic potential for CVI.
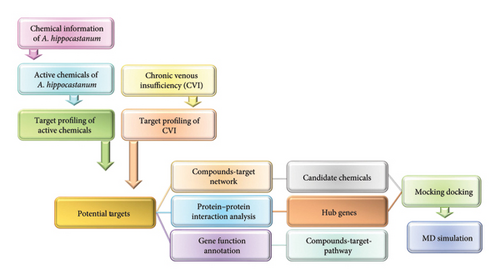
2. Materials and Methods
2.1. Screening of Chemical Components of A. hippocastanum
All chemical components of seed extracts of A. hippocastanum were gathered from published literature, Dr. Duke’s Phytochemical and Ethnobotanical database (https://phytochem.nal.usda.gov/phytochem/search) [36] and Indian Medicinal Plants, Phytochemistry, And Therapeutics (IMPPAT) (https://cb.imsc.res.in/imppat/) [37]. The names and CID/SID numbers of all identified compounds were used to search the PubChem database (https://www.pubchem.ncbi.nlm.nih.gov/) for their physiochemical characteristics and two-dimensional structure. The canonical SMILES for all components was retrieved from the PubChem database. The Traditional Chinese Medicine System Pharmacology (TCMSP) (https://tcmsp-e.com/tcmsp.php) [38] was employed to assess the pharmacokinetic properties of each compound, specifically oral bioavailability (OB) and drug likeness (DL) [39]. OB refers to the degree to which pharmacological compounds can be absorbed and become available in the bloodstream after oral administration, whereas DL reflects the similarity of a compound to known drugs, suggesting its potential as a drug candidate. Components meeting the DL threshold (greater than 0.18) and the OB threshold (greater than 0.30) were selected as active ingredients for further analysis [40].
2.2. Screening of Potential Targets of A. hippocastanum for CVI
The SwissTargetPrediction database (https://www.swisstargetprediction.ch/) [39] is a web service that provides precise predictions of target molecules’ similarity to bioactive compounds, based on their chemical properties [39]. In this study, SwissTargetPrediction was utilized to identify potential targets of selected active components using the canonical SMILES retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/). The species considered for this analysis was Homo sapiens. For subsequent analysis, targets with a combined score greater than zero were selected only. Duplicated genes were removed by aligning UniProt IDs using the UniProt Knowledgebase (https://www.uniprot.org/help/uniprotkb). To retrieve target genes and their variants associated with CVI, data were gathered from DisGeNET (https://www.disgenet.org/), GeneCards (https://www.genecards.org/) (which explore the connections between genes and diseases) [41], and Online Mendelian Inheritance in Man (OMIM; https://www.omim.org/) databases, which include over 15,500 gene entries with focus on gene–phenotype correlation [42]. The records obtained from these databases were combined to predict additional potential targets involved in disease pathways. A Venn diagram was generated to identify the common targets between CVI and A. hippocastanum [17].
2.3. Construction of Compound-Target Network
Cytoscape V3.9.1 (https://cytoscape.org/) was employed to illustrate a compound-target network, showcasing the interaction between the active compounds of A. hippocastanum and potential therapeutic genes [43]. In this network, the nodes represent the active constituents and potential targets, while their relationship is denoted by edges. The topological characteristics of the network were subsequently analyzed using the integrated network analyzer tool in Cytoscape. This analysis was conducted to explore the connections among protein targets, active components, and associated pathways [44].
2.4. PPI Analysis
The STRING database (https://string-db.org/) was used to examine the interactions between potential therapeutic targets and other human proteins [45, 46]. To generate a PPI network, cotargets were input into the STRING database, incorporating both physical and functional interactions. The targeted species was specified as “Homo sapiens,” with a confidence score threshold of 0.7. The isolated points within the network were eliminated. Cytoscape was utilized to visualize the PPI network and further optimized based on the degree of connectivity [47]. In this context, “degree” refers to the number of connections a node has within the network, indicating the significance and interactions of specific nodes. To identify highly connected nodes, the Cytoscape plug-in CytoHubba was employed, and nodes with the highest degrees were prioritized for further analysis. A higher degree indicates stronger relationships among targeted genes.
2.5. Gene Function Annotation
The DAVID database (https://david.ncifcrf.gov/) is a functional enrichment database that provides comprehensive gene annotation and pathway analysis retrieved from the KEGG database. This database was used for functional enrichment pathway analysis, with data retrieved from the KEGG database. This pathway analysis aimed to understand the functions and signaling pathways associated with potential target genes. Gene Ontology (GO) analysis was conducted and classified into three categories: cellular component (CC), biological process (BP), and molecular function (MF) [45]. The KEGG database was used to analyze enriched signaling pathways, and the top 10 annotations and pathways with p-value below 0.05 were selected. These pathways were visualized in Cytoscape V3.9.1 [43]. Additionally, bubble maps of GO annotations and KEGG pathways were created using the R-ggplot2 tool to provide comprehensive visual representation.
2.6. Construction of Compound-Target-Pathway Network
In network analysis, target identification was conducted to discover active components, ket targets, and their pathways [48]. The top 10 enriched pathways were selected from the DAVID database and used to construct a pathway-target network. This network was subsequently merged with the compound-target network to form the compound-target-pathway network. It has been observed that biological networks exhibit modular characteristics, with many effective drugs exerting therapeutic effects by modulating multiple proteins rather than targeting a single protein. This multitarget approach is more effective than single-target strategies in complex biological systems [48].
2.7. Molecular Docking
Molecular docking was employed to study the interactions between ligands and receptors, predicting binding modes and affinities. Molecular docking helps elucidate how ligands interact with their corresponding proteins and predict binding affinities. The 3D structures (SDF files) of active components were downloaded from the PubChem database [49], while hub gene structures (PDB files) were retrieved from the Protein Data Bank (RCSB) (https://www.rcsb.org/) [50]. Proteins were selected based on criteria such as complete 2D structure, lower resolution, and relevance to human protein. Chimera software was used to preprocess the protein structures by removing ligands, water molecules, and resolving structural issues. The molecular docking process was conducted using AutoDock Vina [51]. In this process, the active components acted as ligands, while the target proteins served as receptors. The docking process simulated ligand–receptor interactions and calculated binding affinities, expressed as binding energy values in kcal/mol. The docked complexes with the lowest binding energy values (most negative) were considered the most stable and biologically relevant.
To ensure accuracy, protein structures were optimized through 1000 iterations using Chimera, removing nonstandard residues to prevent steric clashes during docking. The finest docking results were visualized using Chimera X [52] and Discovery Studio [53], allowing for a detailed examination of ligand–receptor binding modes and interactions. This approach provided insights into the preferred interaction methods of ligands with proteins, as predicted by AutoDock Vina.
2.8. MDS
MDS was performed using the DESMOND program in the Schrodinger suite [54, 55]. To study the dynamic interactions of protein–ligand complexes, molecular dynamics simulations were conducted for 100 ns. Before optimization and minimization, complexes were preprocessed and minimized using the OPLS_2005 forcefield [56]. An orthorhombic box measuring 10 × 10 × 10 Å was used to incorporate the transferable intermolecular potential with three points (TIP3P) solvent model using the System Builder tool [55, 57]. By adding counter ions, the model was neutralized, and NaCl (0.15 M) salt was included to mimic the physiological state. The pressure and temperature conditions of the NPT ensemble were set to 1 atm and 300 K respectively. Furthermore, before initiating, the simulation complex was relaxed, and after every 50 ps, each trajectory was saved to analyze the simulation results.
3. Results
3.1. Screening of Chemical Components of A. hippocastanum
A comprehensive search identified 105 chemical compounds from the seed extract of A. hippocastanum [58]. After filtering and removing duplicates, 12 active phytochemicals based on pharmacokinetic criteria: OB ≥ 30% and DL ≥ 0.18 were selected as shown in Table 1. Bioavailability is the rate and extent to which an active component is absorbed into the bloodstream, with higher values indicating greater effectiveness [59]. Highly bioavailable compounds work better because they help the body absorb nutrients. DL quantifies the likelihood of a compound being orally administered as a drug, based on bioavailability and structural attributes, because DL is extensively employed in the first stages of drug development to exclude undesired molecules by utilizing the characteristics and attributes of current medications and drug candidates [60]. The physiochemical and pharmacokinetic characteristics of the 12 selected components are presented in Table 1. These components were deemed effective constituents for further analysis.
| Sr. no. | Chemical name | Mol. formula | Mol. weight (g/mol) | DL | OB % | 2D structure | CID |
|---|---|---|---|---|---|---|---|
| 01 | Alpha-spinasterol | C29H48O | 412.7 | 0.76 | 42.98 |  |
5,281,331 |
| 02 | Beta-sitosterol | C29H50O | 414.7 | 0.75 | 36.91 |  |
222,284 |
| 03 | Campesterol | C28H48O | 400.7 | 0.71 | 37.58 |  |
173,183 |
| 04 | Cholesterol | C27H46O | 386.7 | 0.68 | 37.87 |  |
5997 |
| 05 | Eucalyptol | C10H18O | 154.25 | 0.32 | 60.62 |  |
2758 |
| 06 | Fraxin | C16H18O10 | 370.31 | 0.42 | 36.76 |  |
5,273,568 |
| 07 | Kaempferol | C15H10O6 | 286.24 | 0.24 | 41.88 |  |
5,280,863 |
| 08 | Luteolol | C15H10O6 | 286.24 | 0.25 | 36.16 |  |
5,280,445 |
| 09 | Quercetin | C15H10O7 | 302.23 | 0.28 | 46.43 |  |
5,280,343 |
| 10 | Stigmast-4-en-3-one | C29H48O | 412.7 | 0.76 | 36.08 |  |
5,484,202 |
| 11 | Stigmasterol | C29H48O | 412.7 | 0.76 | 43.83 |  |
5,280,794 |
| 12 | Taraxerol | C30H50O | 426.7 | 0.77 | 38.4 |  |
92,097 |
3.2. Screening of Potential Targets of A. hippocastanum for CVI
The SwissTargetPrediction database was employed to determine the protein–protein targets for the active phytochemicals in A. hippocastanum. By using the SwissTargetPrediction database, 213 potential target genes for 12 active components of A. hippocastanum seed extract were identified.
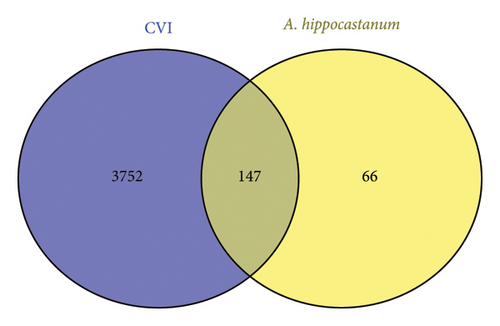
Additionally, 3752 genes that are associated with CVI were retrieved from GeneCards database and OMIM database. Afterwards, a Venn diagram was constructed to identify which genes in A. hippocastanum seed extract might have common targets with those in CVI. Venn diagrams are the most common and effective approach for identifying genes shared between diseases and bioactive compounds. As illustrated in Figure 2, the Venn diagram reveals three distinct sets of genes: 3752 genes uniquely associated with CVI, 66 genes uniquely identified as potential targets of A. hippocastanum’s active components, and 147 genes that are shared between the two datasets. Thus, the 147 shared genes are part of the 3752 CVI-associated genes and represent the overlap between the disease and drug-target datasets. These shared genes serve as critical reference points for further investigation into potential therapeutic targets [48, 61].
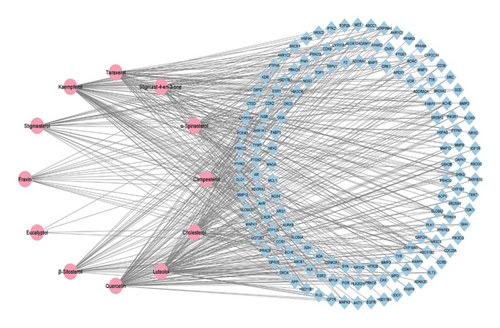
3.3. Construction of Compound-Target Network
Network analysis is commonly employed to investigate the mechanisms by which medicinal plants exert their therapeutic effects [62, 63]. In Cytoscape, a compound-target network was established to elucidate the relationships between active components and their putative targets. The resulting network revealed interactions between 12 active compounds and 147 target genes, forming 159 nodes and 458 edges. Network parameters included a density of 0.036, heterogeneity of 1.918, and centralization of 0.405 (Figure 3). The chemical compounds with degree values, that is, luteolol (69), quercetin (69), and kaempferol (68), were more densely interacted in the network and targeted multiple genes in the network. These higher degree nodes in the network may act as potential drugs (Table 2).
| Sr. no. | Chemical | Category | Degree |
|---|---|---|---|
| 01 | Luteolol | Flavonoid | 69 |
| 02 | Kaempferol | Flavonoid | 69 |
| 03 | Quercetin | Flavonoid | 68 |
| 04 | α-Spinasterol | Steroid | 43 |
| 05 | Taraxerol | Terpenoid | 41 |
| 06 | Cholesterol | Steroid | 39 |
| 07 | Stigmast-4-en-3-one | Steroid | 36 |
| 08 | β-Sitosterol | Steroid | 30 |
| 09 | Stigmasterol | Steroid | 27 |
| 10 | Fraxin | Hydroxycourmin | 17 |
| 11 | Campesterol | Steroid | 15 |
| 12 | Eucalyptol | Terpenoid | 4 |
3.4. PPI Analysis
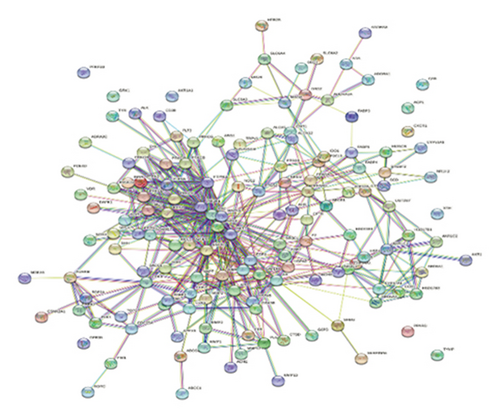
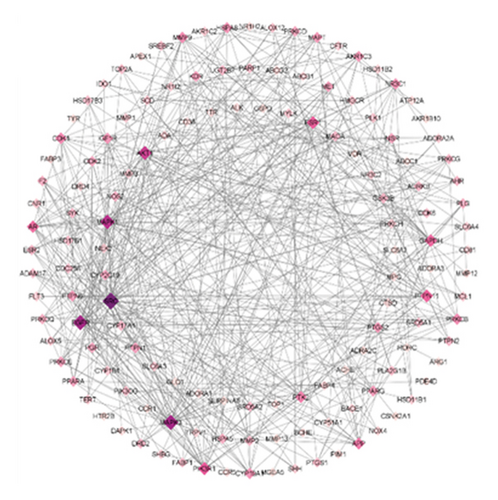
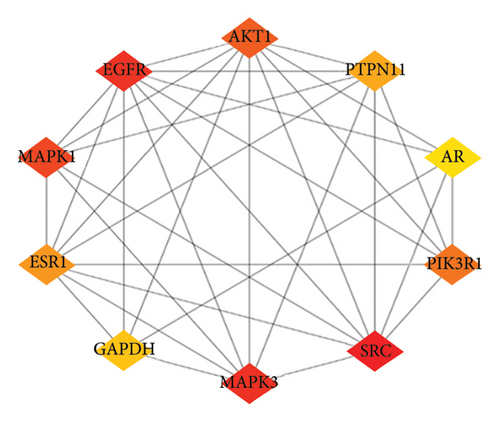
PPI networks are highly significant for understanding cellular functions and biological processes. The networks hold great significance due to their diversity, adaptability, and selectivity, highlighting critical nodes and interactions essential for system biology [62, 63]. Building upon the compound-target interactions identified in Section 3.3, we analyzed the PPI network to understand the relationships among the target proteins and constructed a PPI network of 147 nodes and 184 edges using the Cytoscape (Figure 4(a)). Our analysis discovered 10 disconnected points within the study which researchers eliminated through Cytoscape. Further based on the node degree, the network was optimized with nodes having higher degrees represented as darker and larger. Conversely, nodes with lower degrees are smaller and lighter in color. In addition, to identify the most influential nodes, we utilized two critical topological parameters: degree centrality and betweenness centrality. These metrics highlight the significance of action targets within the network [64], enabling the identification of key nodes. The optimization process emphasizes nodes with higher degrees, which play pivotal roles in the network (Figure 4(b)). Notably, hub genes such as SRC, MAPK3, EGFR, MAPK1, AKT1, PIK3R1, ESR1, PTPN11, GAPDH, and AR were identified as having higher degrees. The significance of these hub genes was further underscored by degree centrality and betweenness centrality metrics, which identified them as critical in the network’s structure (Figure 4(c)).
3.5. Gene Function Annotation
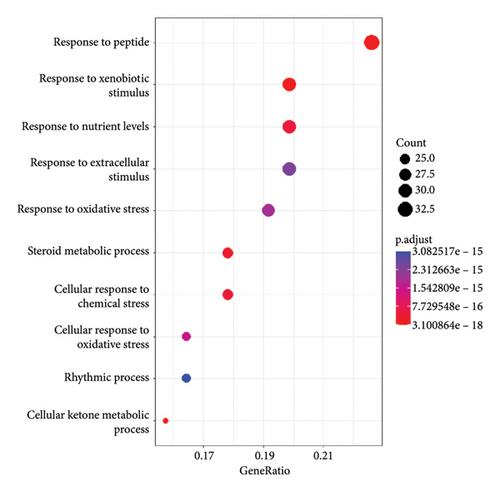
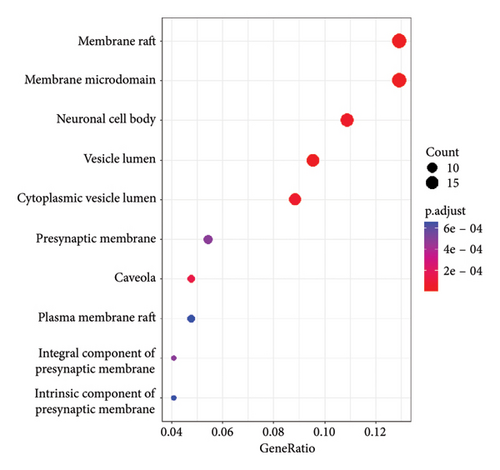
To illustrate the molecular mechanism of A. hippocastanum in the treatment of CVI, functional enrichment analyses were conducted using the GO and KEGG pathway databases. These analyses offered insight into the underlying cellular components (CCs), molecular functions (MFs), and biological processes (BPs) related to 147 identified target genes. KEGG pathway analysis revealed pathways associated with the 147 target genes. GO analysis revealed 67 CCs including critical elements such as the membrane raft, neural cell body, vesicle lumen, presynaptic membrane, and both integral and intrinsic components of the presynaptic membrane. These findings suggest the involvement of structural and functional subcellular elements in mediating the effects of A. hippocastanum. Moreover, 383 Biological Processes were identified, such as response to peptide xenobiotic stimuli, oxidative stress, and external stimuli. These processes underscore the therapeutic potential of A. hippocastanum in modulating oxidative and stress-related pathways, key contributions to the progression of CVI.
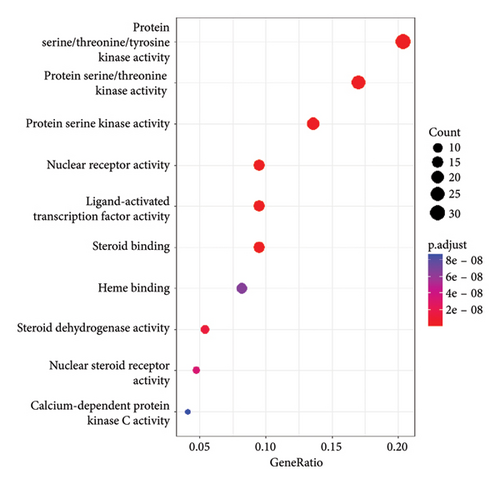
In addition, 141 MFs were discovered, highlighting nuclear receptor activity, protein serine/threonine/tyrosine kinase activity, steroid binding, and heme-binding activities. These functional annotations suggest A. hippocastanum’s active components interact with key molecular targets that regulate signaling cascades relevant to CVI pathology.
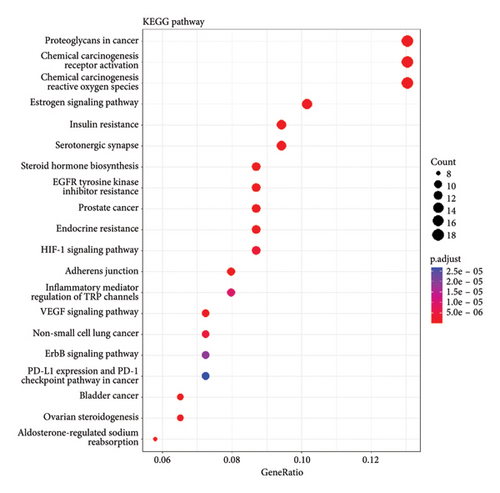
A KEGG pathway analysis detected 120 important pathways linked to anti-CVI targets. The analysis identified the VEGF signaling pathway and inflammatory response pathways also detecting oxidative stress response mechanisms among the top 20 pathways with p < 0.05 significance cutoff. The identified pathways demonstrate crucial functions during CVI development. A visual presentation shows the top 10 gene enrichment findings including (BPs, CCs, and MFs) and the top 20 KEGG pathways in Figure 5.
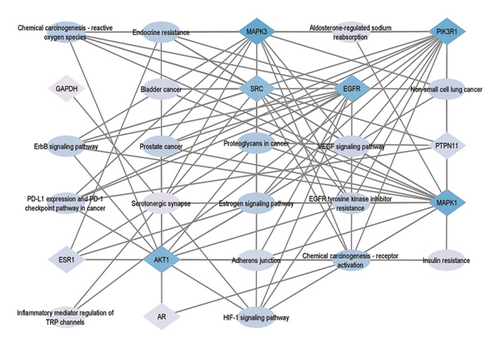
Additionally, an interaction network (Figure 6) was constructed using Cytoscape, illustrating the connectivity between hub genes and enriched pathways. This network highlights the intricate interplay between the identified targets and the pathways implicated in CVI, thereby demonstrating the therapeutic relevance of A. hippocastanum.
3.6. Compound-Target-Pathway Network
The compound-target networks and pathway-target networks were integrated to illustrate the intricate interactions among prospective candidate targets, bioactive chemical constituents, and disease mechanisms. Bioactive compounds were assessed for their interactions with multiple molecular targets, followed by pathway enrichment analysis to determine the association of these targets with specific biological pathways (Figure 7), highlighting their potential therapeutic significance.
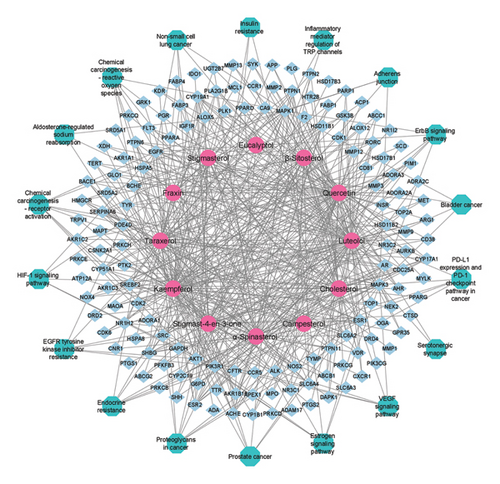
To construct this network, bioinformatics tools were utilized to identify interactions between active phytochemicals and genes implicated in CVI-related pathways. Network pharmacology served as a framework to select phytochemicals with drug-like properties and their interactions with key targets involved in disease mechanisms. By mapping these connections, the network reveals how individual compounds can affect multiple biological processes and pathways, providing insights into their potential roles in disease modulation. The network includes critical signaling pathways such as the inflammatory response pathway, signaling pathway of VEGF, cellular response to hypoxia, EGFR tyrosine kinase inhibitor resistance pathway, oxidative stress pathway, and signaling pathway of ErbB. These pathways are integral to progressing and managing CVI and related conditions. Each pathway is represented by nodes linked to specific targets, which in turn connect to active compounds, visualizing the multitarget effects of natural products (NPs).
3.7. Molecular Docking
Molecular docking is a powerful method employed in structural molecular biology and computer-aided drug design to develop new medicines [65, 66]. By integrating network pharmacology and molecular docking, the process of experimental validation and target identification is significantly expedited [67]. The molecular docking was utilized to identify potential active component targets that may reduce the risk of CVI. The targets identified using network pharmacology are classified as receptors. The targets identified through network pharmacology are classified as receptors, which are proteins that interact with specific bioactive compounds to mediate biological responses. These receptors play a key role in understanding the therapeutic potential of active components and their mechanisms of action.
The 12 active components extracted from the seed of A. hippocastanum were subjected to docking analysis with the 2 targets that is, SRC and MAPK3. Protein Data Bank (PDB) was used to retrieve the three-dimensional structures of target proteins which were optimized using Chimera software through 1000 iterations [68]. This step removed nonstandard residues to prevent steric clashes and ensure proper configurations during the docking process. The docking analysis correctly predicted substantial binding affinity between the binding pockets of two target proteins and active components, with binding scores ranging from −5.1 to −9.3 kcal/mol. The docking scores highlight the strong interactions of specific ligands, such as alpha-spinasterol, which showed the highest binding affinity with MAPK3 (−9.3 kcal/mol), and kaempferol, which strongly bound to SRC (−9.0 kcal/mol). These findings suggest that alpha-spinasterol and kaempferol could act as potential drugs targeting MAPK3 and SRC, respectively. The accuracy of all docked complexes was determined based on the model with the most negative binding energy, reflecting the strength and stability of the ligand–receptor interactions (Table 3).
| Chemical name | Binding affinity (kcal/mol) | |
|---|---|---|
| SRC | MAPK3 | |
| Alpha-spinasterol | −7.4 | −9.3 |
| Beta-sitosterol | −6.5 | −8.6 |
| Campesterol | −6.4 | −8.5 |
| Cholesterol | −7.2 | −8.3 |
| Eucalyptol | −5.1 | −5.5 |
| Fraxin | −5.8 | −8.1 |
| Kaempferol | −9 | −6.6 |
| Luteolol | −6.3 | −6.8 |
| Quercetin | −8.9 | −6.7 |
| Stigmast-4-en-3-one | −6.2 | −5.6 |
| Stigmasterol | −6.9 | −6.5 |
| Taraxerol | −8.2 | −9.1 |
Further, to better understand the molecular interactions, visual representations of the docked complexes were generated using Chimera X [52] and Discovery Studio [53] (Figure 8). To carry out target docking between core targets and active molecules [69], these tools allowed detailed examination of the binding pockets and interactions, emphasizing molecular docking importance to understand the relationship between bioactive compounds and their targets. The docking scores and visualizations support the hypothesis that SRC and MAPK3 are key therapeutic targets for CVI and that their interactions with alpha-spinasterol and kaempferol warrant further investigation.
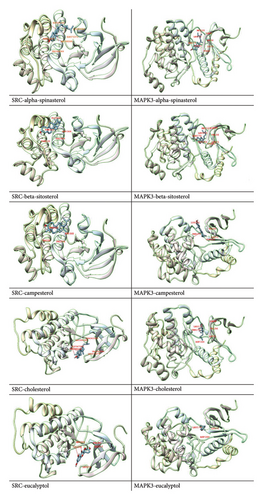
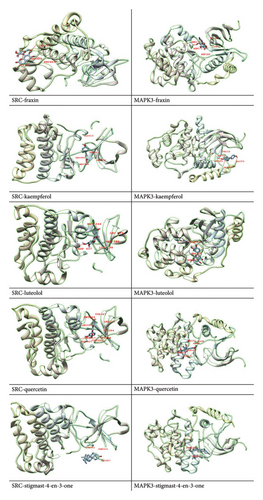
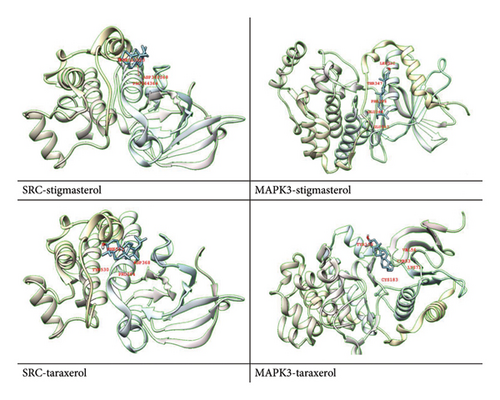
Drug development depends on an understanding of the chemical interactions between bioactive substances and target proteins. The binding energy values, expressed in kcal/mol, provide a quantitative measure of the interaction’s stability and strength. Lower (more negative) binding energy values indicate stronger and more stable interactions, suggesting the therapeutic potential of the compound. For example, the strong binding of alpha-spinasterol to MAPK3 (−9.3 kcal/mol) and kaempferol to SRC (−9.0 kcal/mol) underscores their potential as effective drug candidates. Furthermore, the examination of binding pockets between active components and core proteins lays the foundation for future investigations into the mechanism of action of these compounds against CVI.
To further validate these findings, the SRC and MAPK3 complexes were specifically analyzed in conjunction with alpha-spinasterol and kaempferol. The results indicate that these active constituents from A. hippocastanum seed extract exhibit stable binding to the target proteins, suggesting their potential as therapeutic agents against CVI. Furthermore, molecular docking analyses revealed strong binding affinities, providing robust evidence to support the methodological approach of the study. Understanding the interactions between these target proteins is pivotal for elucidating the active compounds’ mechanism of action in treating CVI. This research contributes to the increasing evidence that supports the role of these proteins as potential targets, facilitated by compounds derived from A. hippocastanum seed extract.
3.8. MDS
To investigate the interactions and dynamic behavior, MDSs were performed on the two selected protein–ligand complexes. The Desmond software was employed for simulation which is renowned for its precision in capturing MD. This platform enabled a comprehensive exploration of the dynamic interplay between ligands and their respective proteins.
3.8.1. Root Mean Square Deviation (RMSD)
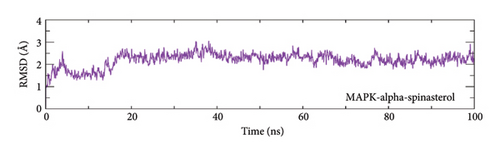
To evaluate the protein–ligand complexes stability, the RMSD of carbon alpha atoms in proteins and ligands was computed from the simulation trajectories [70]. The MAPK3–alpha-spinasterol complex reached equilibrium at 5 nanoseconds, as shown in Figure 9(a). The RMSD stabilized around 1.75 Å until 15 ns and then rose rapidly to around 2.5 Å by 20 ns. The RMSD reached stability around 2–2.5 Å after 20 ns and remained constant until the end of the run. The RMSD of SRC-kaempferol grew steadily to approximately 2 Å at 20 ns and remained within this range until 60 ns. Subsequently, to this point, the RMSD began to diverge and increased to ∼ 3 Å at 70 ns. The RMSD consistently fluctuated over the last phase and peaked at around 3.5 Å by the conclusion of the simulation (Figure 9(b)).
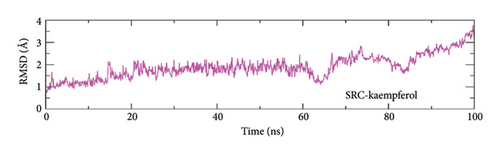
3.8.2. Root Mean Square Fluctuation (RMSF)
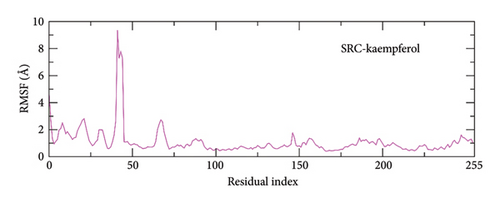
To analyze the protein’s flexibility during ligand interaction, the RMSF values were calculated [71]. The RMSF values for protein residues were predominantly stable, with fluctuations under 2 Å (Figure 10). The RMSF analysis showed that the protein residues maintained rigidity without significant variations throughout the simulation, indicating combined protein-ligand stability. The loop residues in the SRC-kaempferol complex peaked at approximately 9 Å indicating localized flexibility.

3.8.3. Protein–Ligand Contacts
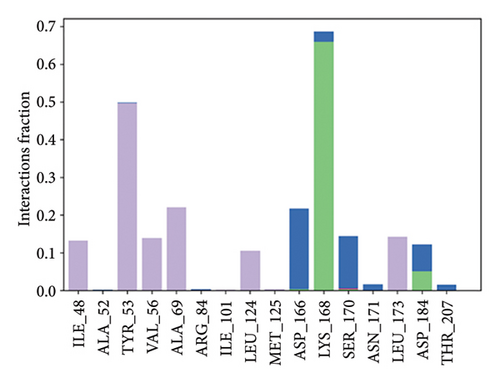
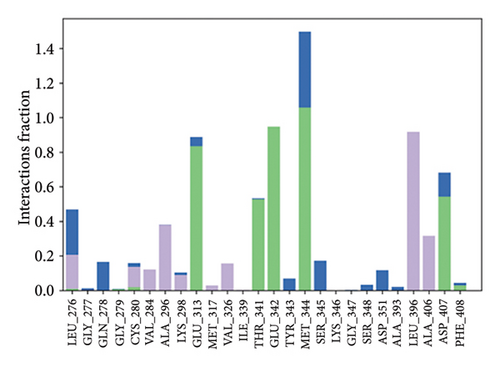
The primary protein–ligand interactions identified in the study were hydrogen bonds, hydrophobic interactions, and ionic bonds, as assessed by MDSs. For MAPK3-alpha-spinasterol complex, Asp166, Lys168, Ser170, and Asp184 contributed to hydrogen bonding (Figure 11(a)). For the SRC-kaempferol complex, Leu276, Cys280, Glu313, Thr341, Glu342, Met344, Asp407, and Phe408 were involved in hydrogen bonding (Figure 11(b)).
3.8.4. Principal Component Analysis (PCA)
To assess the protein’s dynamic behavior in both complexes, PCA was utilized. PCA is useful for determining the collective motions of MD trajectories. Both complexes’ PCA plots are displayed in Figures 12(a) and 12(b). The eigenvalue, which stands for hyperspace dynamics, was plotted against the proportion of variance. Against the proportion of variance, a plot of the eigenvalue was made, which represents hyperspace dynamics. The three PCs that were plotted encompassed all the significant variations. PC1 had the greatest variance of 24.8% among the MAPK-alpha-spinasterol complex components. Figure 12(a) shows that PC2 had a variability of 17% and PC3 of 10%. In contrast, PC1 showed the greatest variance (53.68%) in the SRC-kaempferol complex. Figure 12(b) shows 9.98% and 4.75% other variants in PC2 and PC3, respectively. The PCA analysis showed that all clusters underwent conformational changes. In terms of flexibility, the blue areas showed the most significant movement, and the white and red areas exhibited intermediate and least amount of flexibility movement respectively [72].
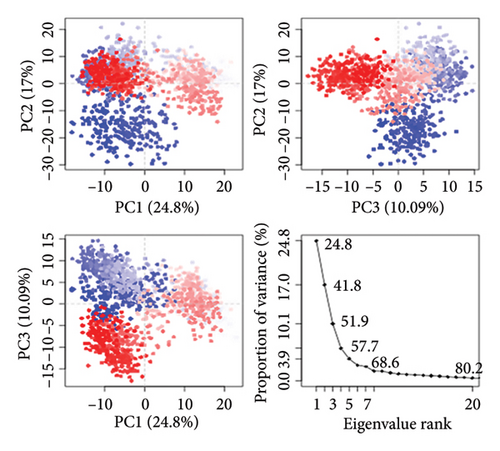
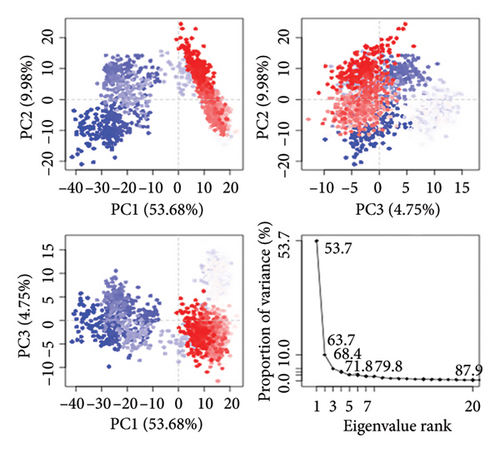
3.8.5. Cross-Correlation
A cross-correlation matrix (Figures 13(a) and 13(b)) was created to analyze the relationships between protein residues. Cyan color represents positively correlated residues, and magenta color represents anticorrelated residues. Most residues exhibited positive correlations, with anticorrelated residues being too minimal to be distinctly observed. The diagonal lines highlight positive associations among topologically adjacent residues. These findings demonstrate a robust correlation between protein residues during interactions with specific chemicals in the simulation.
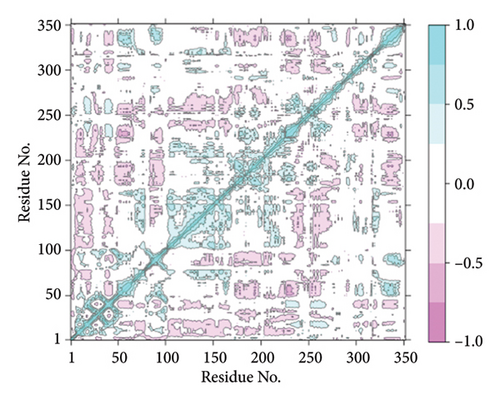

3.8.6. MM/GBSA
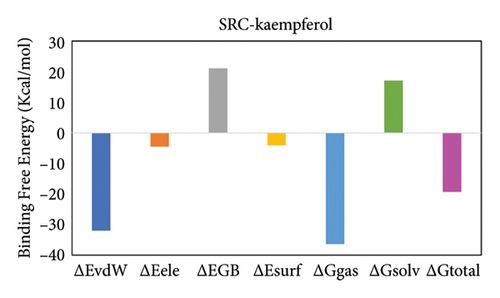
The total binding free energy (ΔG total) was calculated for both complexes using the MM/GBSA methodology. The overall values were frequently utilized to evaluate the protein–ligand complexes’ stability [73]. A higher ΔGtotal number indicates the less stable complex, whereas a lower value indicates the exact opposite. After deducing the energy of the ligand from the protein’s energy and adding it to the energy of the protein–ligand complex, we get the total energy. The MM/GBSA model utilizes various protein–ligand interactions to determine the total binding free energy, which includes the van der Waals energy (ΔEvdW), electrostatic energy (ΔEele), and the electrostatic contribution to solvation free energy by generalized Born (ΔGGB) formula. Table 4 displays the complexes’ complete binding free energy. Figures 14(a) and 14(b) show the total binding free energy in both complexes and how each energy component contributes to it.
| Energy components | MAPK-alpha-spinasterol | SRC-kaempferol |
|---|---|---|
| ΔEvdW | −48.94 ± 0.32 | −31.96 ± 0.38 |
| ΔEele | −8.41 ± 0.47 | −4.42 ± 0.47 |
| ΔEGB | 24.36 ± 0.51 | 21.11 ± 0.38 |
| ΔEsurf | −5.89 ± 0.03 | −4.01 ± 0.05 |
| ΔGgas | −57.35 ± 0.55 | −36.39 ± 0.71 |
| ΔGsolv | 18.46 ± 0.49 | 17.09 ± 0.34 |
| ΔGtotal | −38.89 ± 0.35 | −19.29 ± 0.52 |
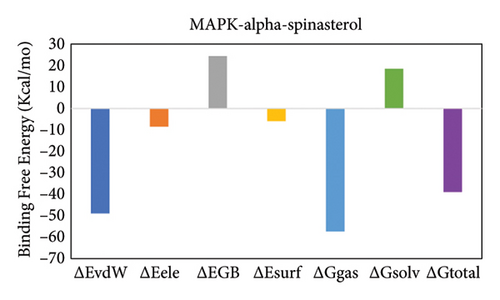
4. Discussion
NPs are biologically active compounds produced by marine organisms, fungi, and plants, and their research gained significant attention in recent years [74, 75]. Traditionally, these NPs have been the foundation for medical practices, offering powerful tools to combat biotic and abiotic stressors. Medicinal chemists utilize NPs to develop more effective medications with minimized side effects [76]. Due to their unparalleled chemical diversity, NPs, whether as pure compounds or standardized plant infusions, serve as promising supplementary sources for pharmaceutical development [77]. Among these, medical herbs have been pivotal in treating various ailments, with herbal medicine forming a cornerstone of modern therapeutics [78, 79]. A. hippocastanum (horse chestnut), a member of the Sapindaceae family, has been widely recognized for its therapeutic properties, particularly in CVI treatment [25]. The therapeutic effect of A. hippocastanum seed extract is thought to stem from its ability to suppress leukocyte activation, a significant pathological mechanism in CVI [80].
Recent advancements in network pharmacology provide a comprehensive method to understand the efficiency of the underlying molecular mechanism of A. hippocastanum. By identifying the interactions between active components, targets, and pathways, network pharmacology enhances our understanding of the therapeutic mechanisms. The main objective is to identify and analyze the connections between disease phenotypic targets and drug targets in the biomolecular network, uncovering the processes that govern these interactions [81].
Furthermore, bioinformatics, AI, and molecular biology are revolutionizing NP-based drug development by enabling the efficient discovery of therapeutic targets for various diseases [82]. These tools enable bioactive compound identification, potential target prediction, and exploration of synergistic effects, thereby enhancing therapeutic efficacy while minimizing adverse effects [83, 84]. As a result, the pursuit of novel therapies has increased in response to these advancements. In recent years, NPs and their derivatives have gained substantial attention as a possible source for targeted medications. This is due to their structural variety, ability to operate on several targets, and low toxicity. Approximately 50% of drugs used in clinical settings are derived from natural sources, emphasizing their significance in modern pharmacology. High-throughput techniques have further streamlined the evaluation of phytochemical pharmacological effects, particularly in drug discovery. Therefore, the identification of potentially active compounds that can halt the pathophysiology of diseases and ailments will turn out to be a defining characteristic of this current era.
In this study, the seed extract of A. hippocastanum was analyzed to identify its active components, with 105 chemical constituents screened, ultimately identifying 12 active phytochemicals. This crucial step establishes the protocol for evaluating the biologically active chemicals of A. hippocastanum and understanding their pharmacological mechanisms in treating CVI. Using datasets from GeneCards and OMIM, 3752 genes associated with CVI were identified. To identify the common genes between the disease and genes related to the compounds found in A. hippocastanum, we cross-referenced these genes using SwissTargetPrediction tool which revealed 147 common targets.
Using Cytoscape with the cytoHubba plugin, the top 10 hub genes were identified based on their degree of interaction, while isolated nodes without interactions were excluded from the analysis In terms of A. hippocastanum seed extract compounds, 12 compounds met the criteria for the DL index ≥ 0.18 and OB ≥ 30%. DL evaluates the probability of a chemical compound being developed into an oral medication based on its bioavailability [59, 60].
After the selection of 147 potential target genes involved in CVI, signaling pathways were made, aiming to control disease prevalence by targeting these genes. In the PPI network, a high degree of connectivity regions are particularly important as they are regarded to be the primary targets in the disease state. Therefore, by focusing on these specific nodes, we may accomplish the objective of treating the CVI. Hence, PPI analysis highlighted two potential target genes, SRC and MAPK3. Upon optimization in the Cytoscape, the PPI network revealed 10 isolated points (PFKFB3, CA9, ACP1, CXCR1, XDH, PPARD, TYMP, GPR35, AKR1A1, and GRK1). These isolated points, lacking interactions with other potential target genes, were eliminated from the network. Subsequently, among the hub genes, MAPK3 and SRC emerged as significant targets based on GO and pathway enrichment analyses. The MAPK3-alpha-spinasterol and SRC-kaempferol complexes emerged as potential therapeutic drugs. These chemicals from the seed extracts of A. hippocastanum hold promise as potential drugs, but clinical trials are necessary to uncover the underlying mechanisms through target hub genes. SRC plays a crucial role in cellular proliferation, differentiation, and angiogenesis, making it a critical target for addressing CVI pathogenesis. Moreover, SRC is a nonreceptor tyrosine kinase critical to various cellular functions, including proliferation, differentiation, migration, and angiogenesis. In the context of CVI, systemic risk factors contribute to vascular remodeling, endothelial dysfunction, and inflammatory mechanisms, all of which are central to CVI pathogenesis. This study demonstrates that kaempferol exhibits significant binding affinity for SRC, suggesting its potential to modulate SRC activity and mitigate specific clinical features of CVI. Kaempferol is mostly recognized for its significant anti-inflammatory properties. Kaempferol blocks vascular endothelial inflammation, upholds cardiac function, safeguards cranial nerve integrity, and addresses fibroproliferative disease, such as hypertrophic scar (HPS) [85]. Studies have highlighted the potential of kaempferol in modulating SRC activity, reducing vascular endothelial inflammation, and alleviating chronic inflammatory conditions [85]. Kratimenos et al. [86] reported that SRC kinase can suppress apoptotic cell death. Compared to low temperature alone in the pig brain, the suppression of SRC kinase further diminished the stimulation of CaM kinase IV over a brief period of low temperature following hypoxia. Another study [87] showed that SRC kinases can block several signaling pathways responsible for initiating focal adhesions and nuclei beneath the membrane. Additionally, they regulate calcium signaling and prevent cell death [88]. MAPK3, a member of the MAPK family, can control various cellular functions, including proliferation, differentiation, and growth [89]. The authors of [90] have been demonstrated that the stimulation of the EGFR/MAPK signaling pathway can promote the growth of stem cells and improve the healing process following chronic disease. Similarly, Tian et al. [91] found that SRC can reduce oxidative stress, prevent cell death, reduce inflammation, and eventually enhance neural recovery in mice. Similarly, our in silico approaches showed the same results and have demonstrated that SRC and MAPk3 exhibit a robust network and strong connections, as well as high binding affinities, as determined by molecular docking analysis and may prove potent phytochemicals against CVI treatment. While various studies on CVI have identified leukocyte activation as a key pathophysiological factor, the mode of action of A. hippocastanum seed extracts appears to be linked to inhibiting this process as well [92]. Studies have demonstrated its efficacy in reducing lower leg volume, calf, and foot girth, as well as alleviating signs of tiredness, soreness, and itching in patients [93]. It has been demonstrated that HCSE, or horse chestnut seed extract, can have a vasoactive impact by inhibiting an enzyme involved in proteoglycan degradation and inducing prostaglandin synthesis. The effectiveness of HCSE appears comparable to compression stockings treatment in reducing leg volume for people who have early-phase mild venous insufficiency Grade 1 [1, 94, 95].
We performed a more in-depth analysis of the pathways associated with these targets which showed the therapeutic effects of A. hippocastanum are mediated through its interaction with various biological processes, CCs, and MFs, as indicated by GO and KEGG analyses. The probable target genes were associated with protein/serine/tyrosine kinase activity, nuclear receptor activity, ligand-activated transcription activity, nuclear steroid activity, heme-binding, and steroidal binding. According to the GO analysis, 67 CCs were identified including membrane raft, neural cell body, vesicle lumen, presynaptic membrane, and integral and intrinsic components of the presynaptic membrane, in addition to 383 biological process such as response to peptide xenobiotic stimuli oxidative stress and external stimuli, and KEGG analysis exposed 120 pathways related to anti-CVI targets such as the signaling pathways enriched included chemical carcinogenesis, receptor activation, estrogen signaling pathway, HIF pathway, PD-L1 expression along with checkpoints, VEGF signaling pathway, and ErbB signaling pathway. The research contributes to comprehensive knowledge about the range of biological methods and molecular mechanisms which are likely responsible for the therapeutic potential of investigated target genes. Network pharmacology-derived critical targets and active constituents use receptor-ligand molecular docking to predict their docking positions.
Molecular docking provided additional evidence that active chemicals interacted with target genes, highlighting how network pharmacology bridges the gap between conventional herbal medicine and cutting-edge therapeutic research. This approach can provide information about the structure of the protein–ligand complex as well as the binding affinity between the ligand and protein. This information is valuable for lead optimization. Protein–ligand docking is a significant form of molecular docking that is valuable for its therapeutic applications in contemporary drug creation based on the structure of molecules. Multiple studies have confirmed the significance of molecular docking as a validation approach in network pharmacology [96, 97].
Liu et al. [98] conducted a study to elucidate the molecular targets and mechanism of Huangqi Guizhi Wuwu decoction by employing network pharmacology and molecular docking for the treatment of rheumatoid arthritis. Similarly, based on the “chemical-targets network,” we also selected compounds and 2 genes for docking analysis. Furthermore, docking analysis showed that alpha-spinasterol and kaempferol establish stable connections with the active sites of target genes, confirming our findings. This suggests that these compounds have the potential for treating CVI by inhibiting MAPK3 and SRC genes, as indicated by their binding scores of −9.3 and −9.0 kcal/mol respectively.
From a clinical perspective, several therapies can be used to eliminate varicose and inadequate veins. However, there is a lack of significant research on pharmacological treatments for this condition. Therefore, an extract derived from the seeds of horse chestnut is acknowledged as a natural treatment for CVI because of its antiedematous, anti-inflammatory, and venotonic properties [99]. In addition, recent discoveries in the field of flavonoid pharmacology have provided us with a fresh understanding of their function in the treatments. They gather in the innermost layer of human veins, maintain the function of the protective layer of cells lining the veins, and hinder substances that trigger inflammation at various stages of the inflammatory process. Therefore, they decrease the flow of plasma, which is the prelude to clinically visible swelling. When started early, seed extracts such as kaempferol and alpha-spinasterol can prevent the mechanisms that cause the progressive course of CVI, preserving the normal function of the venous vascular system [100].
This study investigates the active compounds, their possible targets, and related pathways for CVI treatment through network pharmacology. This provides a theoretical basis for further experimental investigations. While the findings of this study have been supported by the relevant literature, validation by experiment has not yet been conducted. In the future, it is possible to combine relevant experimental research on the mechanism of action of A. hippocastanum seed extract in CVI treatment with the results of this study to gain a further understanding of the pharmacological effects of A. hippocastanum seed extract. Furthermore, the data for this study were gathered from multiple prominent databases or acquired through software. We only deliberated on the potential mechanism of action linking the active components of the A. hippocastanum seed extract with CVI. The precise measurement of the level of movement between the two entities was not possible, and the potential impact of factors such as the source and potency of the drug could not be ruled out. Network pharmacology has proven to be effective in drug development, helping to revitalize natural treatments. Data analysis is the only option to get the essential pharmacological routes for treating CVI, considering the limitations of network pharmacology. However, network pharmacology uses diverse datasets for bioactive screening. However, there are certain limitations to using network pharmacology research in herbal medicine. Network pharmacology relies on multiple public databases to identify active components and disease-related targets. Despite their curation efforts, databases may contain contradictions arising from diverse information sources, ideas, and experimental findings. In addition, herbs that undergo pretrial procedures during their creation experience numerous chemical modifications that may occur. To resolve these issues, utilizing contemporary and effective methods for chemical identification, such as ultra-performance liquid chromatography-electrospray mass spectrometry, can be beneficial. Additionally, a prevalent issue arises in most GO analyses. Ontology concepts in GO are organized in a hierarchical structure that increases in detail, resulting in unnecessary redundancy of terminology. In addition, terms from several ontology sources can exhibit strong correlations. Due to the ability of functional enrichment analysis to detect overlapping or similar phrases, it is challenging to extract nonredundant and representative processes for inclusion in the analysis output. Although our findings present compelling evidence for the potential of A. hippocastanum in treating CVI, it is important to acknowledge that further investigation is necessary. Well-designed, large-scale clinical trials and animal experiments are essential to rigorously evaluate its therapeutic value and fully comprehend its pharmacological actions.
5. Conclusion
CVI is a common condition with considerable health and economic consequences. Prevention and early intervention remain the cornerstone of CVI management strategies. This study provides a robust framework for evaluating multicomponent, multitarget pharmacological therapies, identifying MAPK3 and SRC as pivotal targets in CVI treatment. Network pharmacology and molecular docking analyses revealed that A. hippocastanum contains active components, such as kaempferol and alpha-spinasterol, that interact with MAPK3 and SRC genes to modulate key pathways involved in CVI. The molecular mechanisms highlighted the involvement of MAPK and SRC pathways in inflammatory regulation, oxidative stress reduction, and vascular remodeling, which are critical to CVI progression and treatment. Molecular docking, coupled with MDS, highlighted MAPK3 and SRC as key targets in A. hippocastanum for CVI. Moreover, it was uncovered that the ligands of SRC-kaempferol and MAPK3-alpha-spinasterol complexes bind to the catalytic site, as evidenced by MM/GBSA and correlation analysis. To validate our findings, further investigation is necessary in the form of phytochemical and pharmacological research, due to the inherent limits of the current study. More investigation should prioritize conducting experimental validation studies to verify the therapeutic efficacy of the active constituents found in A. hippocastanum. Furthermore, investigating the practical uses of these discoveries could lead to the development of novel and efficient therapies for CVI. These study proposals will facilitate the ongoing advancement of targeted medicines, ultimately enhancing patient outcomes in the management of CVI.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Z.S., M.K., U.W., and U.A.A. conceptualized the study. A.A., N.A.A., and T.H.A. assisted with methodology and validated the study. Z.S., M.S., and U.W. conducted the investigation. A.A., N.A.A., and T.H.A. analyzed the data. Z.S., M.S., and U.W. drafted the original manuscript. M.K. and U.A.A. reviewed and edited the manuscript. U.A.A. and U.W. supervised the study. All authors have read and approved the final version of the manuscript for publication.
Funding
The authors received no funding for this work. The authors are thankful to the Researchers Supporting Project number (RSP2025R1035), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors are thankful to the Researchers Supporting Project number (RSP2025R1035), King Saud University, Riyadh, Saudi Arabia.
Open Research
Data Availability Statement
The research data supporting our findings can be obtained from the corresponding authors upon making a reasonable inquiry.




