Dynamic Monitoring and Network Analysis of Alcohol Withdrawal Symptoms: Implications for Psychiatric Nursing Practice
Abstract
Background: Alcohol withdrawal syndrome (AWS) is a complex condition characterized by a range of symptoms that can vary in severity. Effective management of AWS during the acute phase is crucial for patient safety and treatment outcomes. This study aimed to investigate the temporal changes in alcohol withdrawal symptoms and identify key symptoms relevant to nursing care using a dynamic monitoring approach and network analysis techniques.
Methods: The study included 82 inpatients with alcohol use disorder (AUD) admitted to Wenzhou Seventh People’s Hospital. Withdrawal symptoms were assessed using the Clinical Institute Withdrawal Assessment for Alcohol, Revised (CIWA-Ar) scale at baseline and every 8 h up to 40 h. Repeated measures ANOVA and network analysis were employed to examine symptom changes over time and the interrelationships between symptoms.
Results: Withdrawal scores significantly decreased over time, with the critical interval for symptom reduction identified between 8 and 32 h. Network analysis revealed visual disturbances as the most central symptom across both baseline and 40 h time points. Anxiety emerged as a crucial bridging symptom at 40 h, influencing the overall symptom profile.
Conclusions: This study highlights the dynamic nature of alcohol withdrawal symptoms and identifies visual disturbances and anxiety as key symptoms relevant to nursing care. The findings emphasize the importance of close monitoring and targeted interventions during the acute withdrawal phase, particularly within the first 32 h. Psychiatric nurses should prioritize the assessment and management of visual disturbances and anxiety to optimize patient outcomes.
1. Introduction
Alcohol withdrawal syndrome (AWS) represents a common and potentially fatal condition manifesting in individuals with alcohol use disorder (AUD) who abruptly discontinue or markedly decrease their alcohol consumption [1]. AWS represents a common and potentially fatal condition manifesting in individuals with AUD who abruptly discontinue or markedly decrease their alcohol consumption [2]. The prevalence of AWS among those diagnosed with AUD is substantial, with incidences reported between 50% and 80%. Research by Caputo [3] indicates that roughly half of those with AUD experience withdrawal symptoms following cessation of alcohol intake [3]. The clinical presentation of AWS includes a variety of symptoms, with diaphoresis (excessive sweating) being one of the most common manifestations, occurring in up to 90% of patients during withdrawal. Other typical symptoms include tremors, anxiety, agitation, hallucinations, insomnia, and additional features of autonomic hyperactivity such as tachycardia and hypertension [4, 5]. A comprehensive review by Mirijello et al. [1] described the timeline of AWS symptom onset, with minor symptoms such as anxiety and tremors appearing within 6–12 h after the last drink, followed by more severe symptoms such as seizures and hallucinations developing between 12 and 48 h [1]. Moreover, severe forms of AWS can progress to delirium tremens (DT), a potentially fatal complication characterized by hallucinations, disorientation, and severe autonomic instability, which can be life-threatening if not promptly recognized and treated [6]. A study by Monte et al. [7] reported that DT occurred in approximately 7.5% of patients hospitalized for AWS, with a mortality rate of up to 5.4% [7]. Healthcare professionals must have a comprehensive understanding of the syndrome and its management due to the high prevalence of AUD and the potential severity of AWS [8]. Effective care during the acute phase of AWS is crucial for minimizing the risk of adverse outcomes and promoting patient recovery [9]. Research focused on assessing, monitoring, and treating AWS during the acute phase, which is crucial to inform evidence-based practice and optimize patient care.
The acute phase of AWS is a critical period that requires close monitoring and appropriate care to prevent complications and ensure patient safety. Meticulous monitoring and management of withdrawal symptoms are essential components of clinical care during the acute phase of AWS. Existing research has emphasized the significance of withdrawal period care in the overall clinical treatment of AUD [10, 11]. Effective management of AWS symptoms during the acute withdrawal period is associated with increased patient engagement and retention in treatment programs [12]. A retrospective study by Mendoza [13] demonstrated that patients who received adequate symptom control during AWS were more likely to complete inpatient rehabilitation and maintain abstinence at follow-up compared to those who experienced poorly managed withdrawal [13]. Adequate control of withdrawal symptoms is associated with increased patient adherence to treatment plans and enhanced participation in subsequent rehabilitation and relapse prevention interventions [10]. Conversely, poorly managed AWS leads to premature treatment dropout and decreased motivation for ongoing care [14]. Research has indicated that successful management of AWS during the acute phase can increase the overall success rate of AUD treatment. Effective medical management of AWS is critical, often requiring specific pharmacological treatments to address withdrawal symptoms and prevent complications. Benzodiazepines are commonly prescribed for symptom control due to their efficacy in reducing withdrawal severity and preventing seizures [15]. In addition, mood stabilizers and other medications, such as anticonvulsants, have shown potential benefits in AWS management [16, 17]. Some of these pharmacological treatments also play a dual role as anticraving strategies, supporting long-term recovery from AUD [18, 19]. This multimodal approach to AWS treatment underscores the need for comprehensive medical intervention to enhance patient outcomes. Patients who complete medically supervised withdrawal are more likely to transition to postacute rehabilitation programs and maintain long-term abstinence compared to those who do not receive adequate withdrawal care [20]. A longitudinal study by Blondell et al. [21] followed patients with AUD for 2 years after inpatient detoxification, finding that those who received comprehensive withdrawal management and seamlessly transitioned to ongoing care had significantly higher rates of abstinence and improved psychosocial functioning compared to those who did not receive such care [21]. The acute phase of AWS represents a critical window of opportunity for engaging patients with AUD in ongoing treatment. By providing effective symptom management and support during this period, healthcare professionals can increase the likelihood of patients successfully completing withdrawal and transitioning to postacute rehabilitation, ultimately improving long-term outcomes in AUD treatment.
Despite advancements in the understanding and management of AWS, several gaps persist in the literature, particularly concerning the dynamic nature of withdrawal symptomatology and the identification of key symptoms relevant to nursing care. Existing studies have primarily focused on static assessments of withdrawal symptoms at discrete time points, overlooking the temporal evolution of symptoms during the acute withdrawal period [22]. Furthermore, there is limited research exploring the intricate interplay between different withdrawal symptoms and their implications for nursing practice. The present study seeks to address these knowledge gaps by employing a dynamic monitoring approach using the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) scale to assess withdrawal symptoms in patients with AUD during the acute withdrawal period. Through repeated measures analysis and network analysis techniques, this study aims to elucidate the temporal changes in withdrawal symptomatology and identify key nursing symptoms with centrality analysis.
1.1. Participant
The data for this study were collected from inpatients with AUD admitted to Wenzhou Hospital between June 2023 and January 2024. The inclusion criteria for the study were as follows: (1) diagnosed with AUD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) by two psychiatrists, (2) absence of other neurological diseases, and (3) 18 years or older. The exclusion criteria included the following: (1) comorbidity with other substance use disorders and (2) history of severe psychiatric disorders, such as acute psychosis, severe bipolar disorder with active manic episodes, or suicidal ideation. The sample size for this study was determined based on a power analysis conducted using G∗Power. To detect a within-subjects effect in the repeated measures analysis of variance (ANOVA), the following parameters were set: effect size (f) = 0.25, alpha (α) = 0.05, alpha (α) = 0.05, power (1 − β) = 0.80, and number of repeated measurements = 6. The analysis indicated that a minimum of 28 participants was required to achieve sufficient statistical power.
Before enrollment in the study, written informed consent was obtained from all participants after a detailed explanation of the study objectives, procedures, potential risks, and benefits. Participants were assured that their participation was voluntary, that they could withdraw at any time without affecting their standard medical care, and that their data would remain confidential and anonymized. The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Wenzhou Hospital (Approval no: EC-KY-2023019), ensuring compliance with ethical guidelines outlined in the Declaration of Helsinki.
1.2. Procedure
Upon admission to the hospital for inpatient treatment, all participants were diagnosed with AUD by two psychiatrists. The CIWA-Ar scale assessments were conducted every 8 h to balance the need for frequent monitoring with minimizing patient burden and ensuring feasibility within the clinical setting. This interval allowed for effective tracking of symptom progression while reducing potential stress for participants. The assessments were conducted by a team of four registered psychiatric nurses, each with a minimum of 5 years of clinical experience in managing alcohol withdrawal. Prior to the start of the study, both raters underwent a 2-day standardized training on the administration of the CIWA-Ar, facilitated by a registered psychiatrist. The training included theoretical instruction, video-based assessments, and supervised practice sessions to ensure consistency in scoring. All participants received standard medical management for AWS as per hospital protocols, including benzodiazepines for symptom control (with diazepam being the most commonly prescribed [76.8% of patients, n = 63], followed by lorazepam [23.2%, n = 19]). Additional supportive medications included thiamine supplementation (100% of patients) and electrolyte replacement as needed. To ensure consistency and stability in the assessment process, all participants involved in the study were pretrained on the proper use and scoring of the CIWA-Ar scale.
1.3. Measurements
1.3.1. Demographic Information
Basic demographic information of each patient, including gender and age, where gender is coded as a binary variable (0 = male; 1 = female) and age is a continuous value in years.
1.3.2. Alcohol Withdrawal Symptoms
The CIWA-Ar scale consists of 10 items that assess the severity of AWS, including nausea, tremor, diaphoresis, anxiety, agitation, tactile disturbances, auditory disturbances, visual disturbances, headache, and orientation [23]. Each item is scored on a scale ranging from 0 to 7 (the orientation item is scored on a scale from 0 to 4), with higher scores indicating more severe symptoms. The total score is calculated by summing the scores of all 10 items (ranging from 0 to 67). The CIWA-Ar has demonstrated strong validity and reliability in clinical settings. Previous research has reported good internal consistency (Cronbach’s α = 0.74–0.85) [24], with Cronbach’s α = 0.82 in the current study. The assessment typically requires 2–5 min to complete, depending on patient cooperation and symptom severity.
1.4. Statistical Analysis
Descriptive statistics were calculated for the demographic variables and alcohol withdrawal scores at each time point. Continuous variables were presented as means and standard deviations, and repeated measures ANOVA was conducted to examine the changes in alcohol withdrawal scores over time. The assumption of sphericity was assessed using Mauchly’s test, and if violated, the Greenhouse–Geisser correction was applied. Post hoc pairwise comparisons were performed using the Bonferroni correction to control for multiple comparisons [25]. Effect sizes were reported using partial eta squared (η2p) and Cohen’s d.
Network analysis was employed to investigate the associations between different withdrawal symptoms at baseline and after 40 h. The regularized partial correlation networks were estimated using the Gaussian graphical model (GGM) with the graphical lasso (glasso) algorithm [26]. The tuning parameter was selected using the extended Bayesian information criterion (EBIC). The network structures were visualized using the “qgraph” package in R. Centrality indices, including strength, betweenness, closeness, and expected influence, were calculated to identify the most influential symptoms within the networks. Strength centrality represents the sum of the absolute weights of the edges connected to a node, while betweenness centrality quantifies the number of shortest paths that pass through a node. Closeness centrality measures the inverse of the average shortest path length between a node and all other nodes in the network. Expected influence is a centrality measure that accounts for both positive and negative edge weights.
All statistical analyses were performed using SPSS Version 25 and R Version 4.2.1. The significance level was set at α = 0.05, and all tests were two-tailed. The network analysis was conducted using the “bootnet” and “qgraph” packages.
2. Result
2.1. Descriptive Analysis
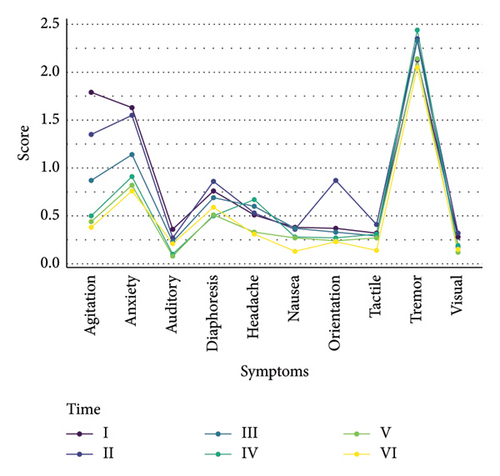
A total of 82 subjects were ultimately included in the study, with 75 (91.46%) being male. The average age of the participants was 47.68 years (SD = 9.66). Descriptive statistics for the alcohol withdrawal scores measured at baseline and every 8 h up to 40 h are presented in Table 1 and Figure 1. The mean withdrawal scores showed a decreasing trend over time, with the highest mean score observed at baseline (M = 8.51, SD = 6.11) and the lowest at 40 h (M = 4.90, SD = 4.66). A repeated measures ANOVA was conducted to examine the changes in alcohol withdrawal scores across the six time points. The results revealed a significant main effect of time (F(5, 405) = 18.577, p < 0.001, η2p = 0.19). This indicates that there were significant differences in the withdrawal scores across the measured time points, with time accounting for approximately 18.7% of the variance in the scores. Post hoc tests were conducted with the Bonferroni method. The results of the post hoc tests with the Bonferroni correction (p < 0.003) indicated several significant differences between the time points (as shown in Table 2). Compared to the baseline (Time I), the withdrawal scores were significantly lower at 24 h (Time IV) (p = 0.002), 32 h (Time V) (p < 0.001), and 40 h (Time VI) (p < 0.001). The scores continued to decrease at 32 h (Time V) and 40 h (t6), with significant differences observed when compared to earlier time points (p < 0.003). There was no significant difference found between the scores at 40 h (Time VI) and 32 h (Time V), suggesting that the withdrawal scores plateaued between these two time points (p = 0.05).
| M | SD | Min | Max | |
|---|---|---|---|---|
| Baseline | 8.51 | 6.11 | 1.00 | 27.00 |
| 8 h | 8.10 | 6.31 | 0.00 | 30.00 |
| 16 h | 7.13 | 5.91 | 0.00 | 27.00 |
| 24 h | 6.13 | 4.60 | 0.00 | 22.00 |
| 32 h | 5.15 | 4.24 | 0.00 | 20.00 |
| 40 h | 4.90 | 4.66 | 0.00 | 20.00 |
| Contrast | Diff | Se | t | p | Cohen’s d |
|---|---|---|---|---|---|
| 8-h baseline | −0.42 | 0.41 | 1.02 | 0.99 | 0.09 |
| 16-h baseline | −1.38 | 0.46 | 3.02 | 0.05 | 0.32 |
| 16–8 h | −0.96 | 0.42 | 2.29 | 0.37 | 0.22 |
| 24-h baseline | −2.38 | 0.59 | 4.04 | 0.002 | 0.53 |
| 24–8 h | −1.96 | 0.58 | 3.36 | 0.02 | 0.44 |
| 24–16 h | −1.00 | 0.44 | 2.26 | 0.40 | 0.22 |
| 32-h baseline | −3.37 | 0.55 | 6.11 | < 0.001 | 0.75 |
| 32–8 h | −2.95 | 0.56 | 5.25 | < 0.001 | 0.66 |
| 32–16 h | −1.99 | 0.43 | 4.65 | < 0.001 | 0.44 |
| 32–24 h | −0.99 | 0.23 | 4.40 | < 0.001 | 0.22 |
| 40-h baseline | −3.61 | 0.66 | 5.50 | < 0.001 | 0.81 |
| 40–8 h | −3.20 | 0.65 | 4.93 | < 0.001 | 0.71 |
| 40–16 h | −2.23 | 0.54 | 4.10 | 0.001 | 0.50 |
| 40–24 h | −1.23 | 0.32 | 3.89 | 0.003 | 0.28 |
| 40–32 h | −0.24 | 0.38 | 0.64 | 0.99 | 0.05 |
- Note: Bonferroni correction was applied to adjust for multiple comparisons (p < 0.003).
2.2. Network Analysis
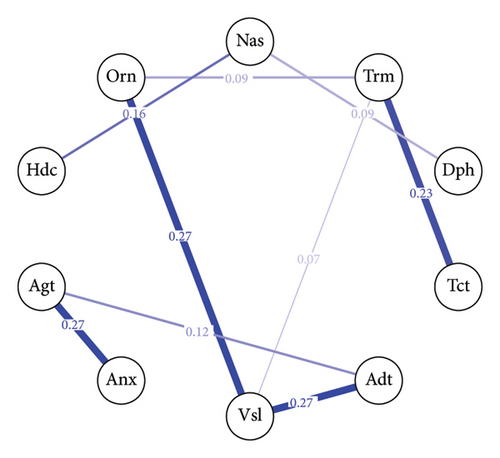
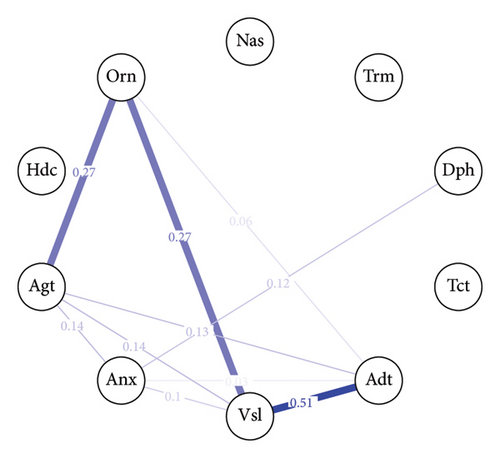
The baseline withdrawal symptom network, shown in Figure 2(a), consists of 10 nodes representing different withdrawal symptoms. Out of the 45 possible edges, 9 are nonzero, indicating the presence of associations between certain symptoms. The mean weight of the edges in the baseline network is approximately 0.03, the edge between “Vsl” (visual disturbances) and “Adt” (auditory disturbances) and the edge between “Vsl” (visual disturbances) and “Orn” (orientation) have the strongest connections, with an edge weight of 0.27 for both pairs. The 40-h withdrawal symptom network, depicted in Figure 2(b), also comprises 10 nodes. In this network, 10 out of the 45 possible edges are nonzero with a mean weight = 0.04. The strongest connection in the 40-h network is between visual disturbances and auditory disturbances, with an edge weight of 0.51.
2.3. Centrality Index
The centrality index (Figure 3) analysis reveals the relative importance of each withdrawal symptom within the network at baseline and after 40 h. “Vsl”(visual disturbances) emerge as the most central symptom across the strength and expected influence centrality measures at both time points (baseline: strength = 2.00, expected influence = 2.00; 40 h: strength = 1.74, expected influence = 1.74). Notably, there is a substantial difference in the betweenness centrality of “Anx” (anxiety) between the baseline and 40 h networks. In the 40-h network, anxiety exhibits the highest betweenness centrality in the entire network (betweenness = 2.03), indicating that it plays a crucial role in bridging connections between other symptoms at this stage of withdrawal.

3. Discussion
The present study aimed to investigate the temporal changes in alcohol withdrawal symptoms and identify key nursing symptoms using a dynamic monitoring approach and network analysis techniques. Our findings revealed significant reductions in withdrawal severity over time, with scores decreasing from baseline to 40 h. The network analysis highlighted the central role of visual disturbances and anxiety in the withdrawal symptom networks, suggesting their importance in the withdrawal process. These findings contribute to a better understanding of the dynamic nature of alcohol withdrawal symptomatology and provide valuable insights for nursing practice.
The repeated measures ANOVA revealed significant changes in alcohol withdrawal scores over time, with scores decreasing from baseline to 40 h. This finding aligns with previous studies that have demonstrated a gradual reduction in withdrawal severity during the acute phase of AWS [1, 8]. The post hoc tests provided further insights into the critical time interval for symptom reduction. Specifically, the results showed significant differences in withdrawal scores between 8 h and 32 h, indicating that this period represents a crucial window for monitoring and intervention. It is worth noting that agitation and anxiety were the symptoms that changed most significantly over time during the monitoring period of this study. This observation is consistent with previous research that has highlighted the prominence of these symptoms in the acute phase of AWS [27, 28]. The rapid decline in symptom severity during this interval, particularly for agitation and anxiety, suggests that the early stages of acute withdrawal, especially the first 32 h, are characterized by the most pronounced changes in symptomatology. This finding is consistent with the known clinical course of AWS, where the initial 24–48 h are often associated with the highest risk of severe symptoms and complications [29, 30]. This pattern of symptom progression is consistent with the known clinical course of AWS, characterized by an initial surge in symptom intensity followed by a gradual improvement as the body adapts to the absence of alcohol [29, 31].
The network analysis at baseline and 40 h provided valuable insights into the interrelationships between different withdrawal symptoms. Visual disturbances emerged as the most central symptom across both time points, suggesting their pivotal role in the withdrawal symptom network. This finding is consistent with previous research that has highlighted the prevalence and significance of visual disturbances in AWS [32]. The strong connection between visual and auditory disturbances in the networks further emphasizes the interrelated nature of perceptual disturbances during alcohol withdrawal. This co-occurrence of visual and auditory disturbances may be explained by the shared neurobiological mechanisms underlying these symptoms, such as the dysregulation of neurotransmitter systems and hyperexcitability of the central nervous system [33]. Interestingly, the betweenness centrality analysis revealed that anxiety played a crucial role in bridging connections between other symptoms at the 40-h time point. This finding suggests that anxiety may serve as a hub symptom, influencing the overall symptom profile and the relationships between other withdrawal symptoms. The central role of anxiety in the later stages of acute withdrawal is consistent with previous studies that have identified anxiety as a prominent and persistent symptom in AWS [34, 35]. The interconnectedness of anxiety with other withdrawal symptoms highlights the importance of targeting anxiety in the management of AWS. Addressing anxiety during the acute withdrawal phase may have a positive impact on the overall symptom burden and patient outcomes. The network analysis approach employed in our study provides a novel perspective on the complex interplay between withdrawal symptoms. By visualizing the symptom networks and identifying central symptoms, we can gain a deeper understanding of the underlying structure and dynamics of AWS. The centrality of visual disturbances and the bridging role of anxiety in the symptom networks offer valuable insights for clinical practice. Healthcare professionals should prioritize the assessment and management of these central symptoms, as they may have a substantial impact on the overall withdrawal experience and treatment response.
In conclusion, this study employed a dynamic monitoring approach and network analysis techniques to investigate the temporal changes in alcohol withdrawal symptoms and identify key symptoms relevant to nursing care. The repeated measures ANOVA revealed significant reductions in withdrawal severity over time, with the critical interval for symptom reduction identified between 8 and 32 h. The network analysis highlighted the central role of visual disturbances and the bridging role of anxiety in the withdrawal symptom networks. These findings contribute to a better understanding of the dynamic nature of alcohol withdrawal symptomatology and provide valuable insights for nursing practice.
4. Limitation
However, this study has several limitations that should be acknowledged. First, the sample size was relatively small, which may limit the generalizability of our findings. Future studies with larger and more diverse samples are needed to validate and expand upon our results. Second, while the CIWA-Ar scale is a well-established tool for assessing withdrawal severity, it relies on subjective patient reporting and clinical observation. AWS assessments are particularly challenging because patients often show diminished empathy and have difficulty establishing a therapeutic relationship [36]. This diminished empathic capacity can complicate clinical interactions, making it harder for healthcare providers to assess symptoms accurately and implement effective interventions. The incorporation of objective measures, such as biomarkers or physiological parameters, could provide additional insights into the withdrawal process. Despite these limitations, our study contributes to the growing body of literature on AWS and offers novel insights into the dynamic nature of withdrawal symptomatology.
Ethics Statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the IRB in Wenzhou Seventh People’s Hospital (EC-KY-2023019). Informed approbation was attained from all participants and/or their lawful guardian(s). Any participant under 16 years of age obtained the informed consent of their parents or legal guardian.
Consent
Please see the Ethics Statement.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Suilin Jia and Xuanru Wu conceived and designed the experiments. Guangdong Chen and JingJing Ding performed the experiments. Binwei Zhang conducted the data analysis. Suilin Jia and Qiqi Chen wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Wenzhou Municipal Science and Technology Bureau (Grant no. Y20220837).
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




