Glucose Variability and Postprandial Hyperglycaemia After Breakfast in Children and Young People With Type 1 Diabetes
Abstract
Aims: This study aimed to describe glucose variability (GV) and explore postprandial glycaemia and the association with food composition following breakfast in children and young people (CYP) with type 1 diabetes (T1D).
Methods: This was an observational study of CYP aged 1–17 years using continuous glucose monitoring (CGM). Retrospective CGM data were collected to assess GV along with questionnaires about the breakfast meal and 4-h postprandial period for 7 days. Statistical analysis included Student’s t-tests and linear mixed models.
Results: Ninety-six CYP were recruited, 89 shared their CGM data (mean age 10.1 ± 3.8 years) (44.9% females), of which 74 submitted questionnaires. Diurnal percentage coefficient of variation (%CV) (mean: 38.1% ± 4.3%) was significantly higher than nocturnal %CV (36.4 ± 5.2) (95% confidence interval [CI] [0.9, 2.5], Cohen d = 0.5, p < 0.001). Continuous subcutaneous insulin infusion (CSII) users had a significantly higher time in range (TIR) (mean: 61.7% ± 11.0%) than those using multiple daily injections (MDIs) (mean: 55.5% ± 15.0%) (95% CI [0.2, 12], Cohen’s d = 0.5, p = 0.04). Data on 387 breakfast meals were analysed. The preprandial glucose was significantly lower for CSII users (mean: 7.4 ± 2.3 mmol/L) compared with MDI users (mean: 9.5 ± 2.9 mmol/L) (95% CI [1.3,3.3], d = 0.9, p < 0.001). Preprandial glucose was significantly associated with mean postprandial glucose (R2 = 0.27, p < 0.001). Compared with breakfast meals containing a protein food (n = 71), ingested meals containing breakfast cereals only (n = 76) resulted in a significantly higher mean postprandial glucose (p = 0.01), peak excursion (p = 0.03), area under the curve (AUC) (p = 0.03) and time above range (TAR) (p < 0.001) and significantly shorter time to peak (p = 0.01) and lower TIR (p = 0.01). Ingested meals containing only breakfast cereals also resulted in significantly higher glucose excursion at 30, (p < 0.001), 60 (p < 0.001) and 90 min (p = 0.02) compared with breakfast meals containing a protein food.
Conclusions: GV is significantly higher in the diurnal period. Managing T1D with CSII and including a protein food in the breakfast meal may reduce postprandial hyperglycaemia after breakfast in CYP with T1D. The trial was registered retrospectively with Clinicaltrials.gov (10/08/2024): NCT06546657.
1. Introduction
Postprandial hyperglycaemia is a major cause of glucose variability (GV) in type 1 diabetes (T1D) [1], which has been shown to be more harmful than prolonged glucose elevations [2] and is associated with both macro and microvascular complications [3, 4]. Following its discovery, the challenge of matching insulin action with carbohydrate absorption to avoid both hypo- and hyperglycaemia was acknowledged by Banting et al. [5]. The advent of continuous glucose monitoring (CGM) highlighted the problem of GV and postprandial hyperglycaemia in children and young people (CYP) with T1D [6, 7]. There is some evidence that postprandial hyperglycaemia is more likely to occur after breakfast than other meals [8–10]. This is of concern as CYP are often actively learning during the postbreakfast period, and as hyperglycaemia can negatively affect cognitive ability [11], this may put CYP with T1D at a disadvantage compared with their nonT1D peers.
The dietetic management of postprandial glucose is complex. Whilst the preprandial insulin bolus and the quantity of carbohydrate in a meal are the principal influencers of glycaemic control [12], there are many other factors to consider, including the quality of carbohydrate [9], the glycaemic index (GI) of foods and the glycaemic load (GL) of the meal [13]. Diet quality is considered important as it has been suggested that postprandial hyperglycaemia after breakfast is mainly due to the consumption of highly processed carbohydrates [9]. Despite this, there is a paucity of evidence for the type of food that CYP with T1D consume for breakfast, however, national nutritional surveys report that, within the general population, CYP regularly consume ready-to-eat breakfast cereals that are highly processed [14, 15].
In recent years, most of the dietetic research in paediatric diabetes has focused on the impact of fat and protein on postprandial glucose, which has strengthened the empirical evidence that all three macronutrients need to be considered when calculating the prandial insulin dose [16]. One possible solution to managing postprandial hyperglycaemia at breakfast, which is alluded to in the International Society of Paediatric and Adolescent Diabetes (ISPAD) 2022 Nutritional Management Guidelines, is to add protein to the breakfast meal to lower the glycaemic response [13]. However, there is a lack of empirical evidence of the effectiveness of this strategy at breakfast, and most of what is known arises from clinical practice.
The aim of this study was to report on the following in CYP with T1D: (1) Glycaemic variability in the diurnal and nocturnal periods, (2) the composition of the breakfast meal, (3) the extent of postprandial hyperglycaemia at breakfast and (4) the relationship between postprandial glucose at breakfast and the breakfast meal composition.
2. Methods
This observational multicenter study of CYP with T1D who were recruited from 12 diabetes centres in the UK was conducted over a 1-year period in 2021. The inclusion criteria were age of 1–17 years, a diagnosis of T1D for more than 1 year and using insulin regimens of either multiple daily injection (MDI) or continuous subcutaneous insulin infusion (CSII) via an insulin pump along with Dexcom CGM. Those who had other medical conditions or were taking antihyperglycaemic medication, for example, metformin and/or antidepressants, were not eligible to participate in the study owing to the potential impact on glycaemic control. Potential participants brought their own Dexcom CGM G6 devices to the study, and this was approved by Dexcom Inc. Dexcom CGM G6 was the only CGM used in the study as per the inclusion criteria. No data were collected on the number of CYP using Dexcom CGM in the UK, nor how this was funded for those joining the study. However, the majority of Dexcom CGM was self-funded at the time of the study, as CGM was not part of standard treatment provided by the National Health Service (NHS).
The study was advertised to the CYP with T1D who were attending the diabetes centres located in the NHS Trusts that had joined the study. This was achieved by various methods, including waiting room posters, via NHS social media and during clinic appointments. Those CYP who were interested in joining the study had their eligibility assessed and were then recruited by their local dietitian and invited to complete, for 7-days, a twice daily questionnaire about their breakfast meal and the 4-h postprandial period along with the submission of a photograph of their breakfast meal and any food packaging where applicable. Participant information sheets were provided, and consent forms were obtained from the parents or carers and assent forms from the participants.
Baseline information was collected at recruitment from clinical records. This included: sex, date of birth, HbA1c and insulin regimen. The breakfast questionnaire sought information about the breakfast meal and details of diabetes management before and after the meal. This included the timing and duration of the meal as well as the type and amount of food and fluids consumed, and the specific brands of foods where applicable. Diabetes management information included the amount of insulin administered, and any adjustments required for the treatment of hypo- or hyperglycaemia in the preprandial period. The second daily questionnaire was about the 4-h postprandial period and included questions about insulin and food adjustments that were required for hypo- or hyperglycaemia. This questionnaire also sought information about any food and fluid intake and physical activities undertaken during the 4-h postprandial period.
The questionnaire submissions and meal photographs were reviewed and analysed by a dietitian using Nutritics (Nutrition Analysis Software, 2019) and from the food labels provided via photographs. The analysis included: energy (kcal), fats (g), saturated fats (g), carbohydrate (g), sugars (g), protein (g), fibre and salt (g) and GI and GL. Macronutrient intakes were compared with ISPAD recommendations [13].
The GI values of the carbohydrate foods contained within each breakfast meal were obtained using the International tables of GI [17, 18]. Where the GI was not known, the GI of a similar food was used. The total GI value of each breakfast meal was estimated by summing the means of the carbohydrate foods as described in Dodd et al. [19]. No adjustments were made for the impact on the GI of the fat and protein contained in the meals. The GI values of carbohydrate foods have been shown to correlate with the glucose response to mixed meals [20–22], and the GI is the main determinant of the glycaemic response to mixed meals [22]. The GL was calculated by multiplying the GI of the individual food by its available carbohydrate and dividing by 100 [23].
The breakfast meals were also categorised into two subgroups of breakfast cereal-only meals (cereal only) and those which included a protein food (added protein). The cereal-only subgroup included meals that contained a ready-to-eat cereal with milk and with no other foods included. The added protein subgroup included the meals which contained a food from the protein food group as per the UK Eat Well Guide [24], in addition to cheese from the dairy food group. Insulin dose timing was categorised into three types of lag time: ≥15 min and <15 min preprandial and postprandial dosing.
CGM data were collected retrospectively, via Dexcom Clarity, for up to 90 days to assess diurnal (06.00–22.00) and nocturnal (22.00–06.00) periods for analysis of GV and for the 7-day breakfast questionnaire recording period. For the assessment of GV, the CGM metrics included: mean glucose and percentage coefficient of variation (%CV) along with the time in range (TIR) metrics of time below range (TBR), TIR and time above range (TAR) [25].
For the breakfast meal analysis, the primary outcome measurement was the mean postprandial glucose, which was taken from the CGM sensor readings at 5-min intervals over 240 min. The following outcomes, which were taken from the CGM sensor readings over the 240 min period and the questionnaire responses, included (1) preprandial glucose (this was the closest reading taken prior to the breakfast meal commencing); (2) the timing of the insulin dose; (3) mean glucose excursion (defined as the change in glucose levels from baseline); (4) peak glucose excursion; (5) time to peak; (6) the mean area under the curve (AUC) (defined as the sum of the AUC of glucose excursions above baseline glucose over the 240 min period); (7) %CV (defined as the ratio of the standard deviation (SD) to the mean glucose) and (8) TBR, TIR and TAR. Only those breakfast meals which had ≥70% of CGM data were included in the analysis as recommended in Schnell et al. [26]. For the analysis of glucose excursions, only those breakfast meals which had a reading at baseline were included in the analysis. This applied to the analysis of the mean glucose excursion, peak excursion, time to peak and AUC. No adjustments were made for other missing CGM data points.
Statistical analysis was performed using Jamovi (The Jamovi project, Sydney, Australia, 2023). (Version 2.2.5 retrieved from https://www.jamovi.org). Figures were produced using GraphPad 9.5.0 (GraphPad Software, San Diego, California, USA). The incremental AUC was calculated using the trapezoidal method via GraphPad 9.5.0 (GraphPad Software, San Diego, California, USA). Parametric outcomes are presented as mean and SD, and for nonparametric outcomes, the median and interquartile range (IQR) are presented. GV outcomes were analysed using Student’s t-tests. For the breakfast postprandial period, paired Student’s t-tests and simple linear regression were performed to compare pre and postprandial glucose measurements. In order to account for the nonindependent data and correlations from repeated measurements from the same participants and the resultant risk of pseudo replication, the remaining outcomes from the postprandial breakfast period were analysed using linear mixed models. Furthermore, linear mixed models were used as these are superior at handling missing data compared with repeated measures analysis of variance (ANOVA). The linear mixed models were performed with restricted maximum likelihood (REML), the Wald method for confidence intervals and the Satterthwaite method for the degrees of freedom. The models included a fixed intercept and slope and a random intercept for the participants. Hypoglycaemic events and insulin correction events for hyperglycaemia were analysed using a generalised linear mixed model with logistic regression. The effect size for the linear mixed models was calculated using the suggested formula as described in Westfall et al. and Brysbaert and Stevens [27, 28].
Where appropriate, post hoc tests were performed using the Bonferroni correction to manage the risk of a type 1 error occurring with multiple statistical tests. p < 0.05 was considered statistically significant.
3. Results
3.1. Participants
Ninety-six participants consented to join the study and provided the baseline information. One was unable to share their CGM data, and six did not respond, resulting in 89 participants who shared their CGM data (Table 1), of which 74 submitted a total of 428 breakfast questionnaires with 368 corresponding postprandial questionnaires. Some meals had missing CGM data during the postprandial period, and this resulted in 387 breakfast meals remaining. Three of the 74 participants submitted a total of four breakfast questionnaires; however, of these, one did not consume any food at breakfast, and the other three meals had no corresponding CGM data, so these were excluded from the analysis, resulting in 71 meal participants.
| Characteristic | N = 89 |
|---|---|
| Age (years) | 10.1 ± 3.8 |
| Females (n) (%) | 40 (44.9%) |
| Males (n) (%) | 49 (55.1%) |
| Duration of T1D (years) | 4.0 (4.5) |
| Regimen – CSII (n) (%) | 66 (75%) |
| Of which - HCL, n (%) | 14 (16%) |
| Regimen – MDI, n (%) | 22 (25%) |
| HbA1c (mmol/mol) | 57.7 ± 9.3 |
- Note: Mean ± SD, median (IQR). Regimen of one participant was unknown.
3.2. GV
Data exported from Dexcom Clarity provided a mean of 79.3 days of CGM sensor readings. Mean percentage of %CV was 37.8% ± 4.2%; this was significantly higher in the diurnal (mean: 38.1% ± 4.3%) compared with the nocturnal period (mean: 36.4% ± 5.2%) with a mean difference of 1.7% (95% CI [0.9, 2.5], Cohen’s d = 0.5, p < 0.001) (Figure 1) and 36% of the participants met the %CV target of ≤36%. Diurnal %CV was significantly lower for those using MDI (mean: 36.4% ± 3.1%) compared with those using CSII via an insulin pump (mean: 38.6% ± 4.5%) with a mean difference of 2.3% (95% CI [0.5, 4.0], Cohen’s d = 0.6, p = 0.01). The mean percentage TIR was 60.1% ± 12.5% with no significant difference between the diurnal and nocturnal periods for this range of times (p > 0.05). CSII users had a significantly higher %TIR (mean: 61.7% ± 11.0%) compared with those using MDI (mean: 55.6% ± 15.0%), with a mean difference of 6.1% (95% CI [0.2, 12], Cohen’s d = 0.5, p = 0.04). The median %TBR was significantly lower for those using MDI (1.0% [1.8]) compared with CSII (all types) (3.0% [3.0]) (Mann Whitney U = 461, p = 0.01); this was significant for diurnal %TBR (Mann Whitney U = 486, p = 0.02) but not the nocturnal period (p = 0.05). For the %TIR target of ≥70%, 24.7% met this, whilst 77.5% met the target of <5% for TBR. There was no significant difference in those meeting and not meeting the %TIR target for ages, disease duration, sex or regimen (p > 0.05). Comparisons of GV between the CSII users who used either open loop or hybrid closed loop (HCL) systems were not analysed due to the small number of participants using HCL systems.
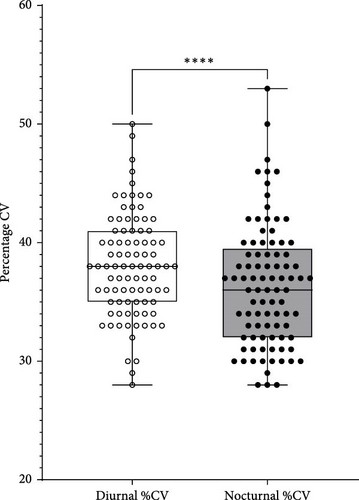
3.3. Preprandial Glucose
Of the breakfast meals which had CGM data and were included in the analysis (n = 387), 54.5% (n = 213) had a preprandial reading within the fasting glucose target of 4–8 mmol/L; 41.7% were above target, and the remaining 3.8% were below target. The preprandial glucose was significantly lower for the CSII users (mean: 7.4 ± 2.3 mmol/L) compared with MDI users (mean: 9.5 ± 2.9 mmol/L) with a mean difference of 2.2 mmol/L (95% CI [1.3, 3.3], d = 0.9, p < 0.001).
3.4. Correction Doses and Dose Timing
The odds of needing a correction dose of insulin before breakfast were 3.5 times greater for those using MDI compared with CSII (95% CI Exp [β] [1.1, 11.2], p = 0.03). The dose time was reported for 380 of the meals which had corresponding CGM data. Of these meals, 45.8% (n = 174) were managed with an insulin dose lag time of ≥15 min, 44.2% (n = 168) had a dose lag time of ≤15 min and 10% (n = 38) of the meals were managed with a postprandial dose.
3.5. Postprandial Glucose
Mean postprandial glucose was 9.2 ± 2.7 mmol/L and the mean glucose excursion was 1.4 ± 2.6 mmol/L. Two-thirds of the breakfast meals (n = 262, 67.7%) resulted in a mean postprandial glucose which was within the TIR target of 3.9–10 mmol/L and 32% of meals (n = 124) resulted in a sensor reading >10 mmol/L, of which 5.9% of meals (n = 23) were above >13.9 mmol/L. Mean postprandial glucose (mean: 9.2 ± 2.7 mmol/L) was significantly higher than the mean preprandial glucose (mean: 8.0 ± 2.6 mmol/L) with a mean difference 1.2 mmol/L (95% CI [0.9, 1.4], Cohen’s d = 0.5, p < 0.001) and this was observed for meals managed with both CSII and MDI.
There was a significant, positive association between pre and postprandial glucose (Figure 2). Every 1 mmol/L increase in preprandial glucose corresponded with a 0.5 mmol/L increase in mean postprandial glucose (95% CI [0.4, 0.6], d = 0.2, p < 0.001). This remained significant when adjusting to include covariates and factors of age, disease duration and sex.
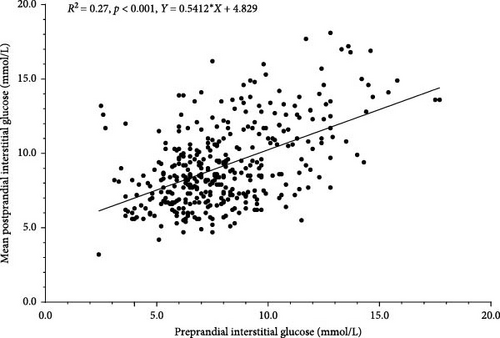
Mean postprandial glucose was significantly lower for the meals ingested by the CSII users (mean: 8.8 ± 2.5 mmol/L) compared with the MDI users (mean: 10.2 ± 3.2 mmol/L) with a mean difference of 1.5 mmol/L (95% CI [0.4, 2.6], d = 0.6, p < 0.01). This remained significant after adjusting the model with covariates and factors of age, disease duration and sex, albeit with a higher p value (p = 0.03); however, it was not significant when preprandial glucose was also added to the model as a covariate (p = 0.58). There was no significant difference in the postprandial glucose measurements across the three types of insulin dose timing.
3.6. The Breakfast Meal
The mean GI of the breakfast meals was 59% ± 10% and the mean GL was 26 ± 13. The low GI meals accounted for 36% (n = 139) whilst 49.5% (n = 191) were in the medium GI category and the remaining 14.5% of meals (n = 56) were in the high GI category. When categorised by GL, the majority of the breakfast meals, 65.6% (n = 252), were in the high GL category (≥20), followed by 28.1% of meals in the medium (n = 110) and 6.3% (n = 24) had a low GL category (<10). The proportion of energy from the three macronutrients for all meals was 52.9% ± 14.3% from carbohydrate, 31.2% ± 14.9% from fat and 14.2% ± 5.9% from protein. The mean fibre content was 3.0 ± 1.9 g. The low GI meals were the only breakfast meals to meet the ISPAD recommendations for macronutrient percentage from energy [13], although these recommendations are for all meals, not just breakfast. The protein content of the low GI meals was significantly higher than both the medium GI meals (95% CI [0.8, 4.0], adjusted p = 0.01) and high GI meals (95% CI [2.2, 6.9], p < 0.001).
The most popular main source of carbohydrate was breakfast cereals; this was the main carbohydrate source of 44.6% of meals (n = 191), followed by breads (33.4%, n = 143) and pastries (9.1%, n = 39). Only the breakfast cereals had a mean GI that was in the high GI category. Almost a fifth of meals (18.3%) contained cereal only as the main source of carbohydrate (plus milk). These cereal-only meals contained a significantly higher GI (mean: 67% ± 7%) than all the other meals (mean: 57% ± 9%) with a mean difference of 9% (95% CI [6.5, 11.1], d = 1.0, p < 0.001). They also contained a significantly higher GL (mean: 28 ± 15) than all the other breakfast meals (mean: 25 ± 13) with a mean difference of 3 (95% CI [0.1, 5], d = 0.2, p = 0.04). These cereal-only meals also contained significantly more carbohydrate per percentage of energy (mean: 63.3% ± 9.0%) than all the other breakfast meals (50.6% ± 14.3%), with a mean difference of 14.3% (95% CI [11, 18], d = 1.1, p < 0.001).
A fifth of breakfast meals (19.6%) included a protein food. The protein content was significantly higher (mean: 20.2 ± 9.3 g) than all the other meals (mean: 10.3 ± 6.0 g) with a mean difference of 10.6 g (95% CI [9.1, 12.1], d = 1.6, p < 0.01). The percentage of energy from carbohydrate was significantly lower for the added protein meals (mean: 38.8% ± 13.4%) compared with all the other breakfast meals (mean: 56.4% ± 12.3%) with a mean difference of 19.8% (95% CI [16.8, 22.8], d = 1.6, p < 0.001).
3.7. The Breakfast Postprandial Period
Ingestion of the high GI breakfast meals resulted in a significantly lower %CV (mean: 20.4% ± 7.8%) than the medium GI meals (mean: 24.7% ± 9.9% with a mean difference of 4.4% (95% CI [1.2, 7.5], d = 0.4, p = 0.01, adjusted p = 0.02). There were also fewer hypoglycaemic events following ingestion of the high GI meals (n = 9, 16%) than after ingestion of the medium GI meals (n = 62, 32.5%) with borderline significance (p = 0.06). Ingestion of the high GL meals resulted in a faster time to peak (mean: 118 ± 76 min) than both medium (mean: 132 ± 79 min) and low GL meals (mean: 148 ± 87 min) combined with a mean difference of 22 min (95% CI [0.6, 44], d = 0.3, p = 0.04). There were no other significant differences in the glucose outcome measurements, nor any difference in the excursion at 30-min intervals between the categories of GI and GL (p > 0.05).
The ingestion of the cereal-only meals resulted in a significantly higher mean postprandial glucose over the 240 min (mean: 9.6 ± 2.7 mmol/L) compared with the added protein meals (mean: 8.5 ± 2.0 mmol/L) with a mean difference of 1.3 mmol/L (95% CI [0.4, 2.1], d = 0.5, p = 0.01).
The peak excursion was also significantly higher following the ingestion of the cereal-only meals (mean: 5.2 ± 3.2 mmol/L) compared with the added protein meals (mean: 4.5 ± 2.6 mmol/L) with a mean difference of 1.2 mmol/L (95% CI [0.6, 2.3] d = 0.4, p = 0.03). The time to peak was significantly shorter following the ingestion of the cereal-only meals (mean: 102 ± 71 min) compared with the added protein meals (mean: 135 ± 77 min) with a mean difference of 35 min (95% CI [9, 62], d = 0.5, p = 0.01). The mean AUC was also significantly higher following the ingestion of the cereal-only meals (mean: 515 ± 463 [mmol/L] ∗min) compared with the added protein meals (mean: 442 ± 346 [mmol/L] ∗min) with a mean difference of 167 (mmol/L) ∗min (95% CI [20, 313], d = 0.4, p = 0.03). The ingestion of the cereal-only meals resulted in a less TIR (mean: 136 ± 76 min) than the added protein meals (mean: 167 ± 70 min) with a mean difference of 38 min (95% CI [11, 64], d = 0.5, p = 0.01) and more TAR with a mean difference of 31 min, (95% CI [17, 46], d = 0.7, p < 0.001) (Table 2).
| Glucose measurements | Cereal only | Added protein | p |
|---|---|---|---|
| Preprandial glucose (mmol/L) | 8.5 ± 2.7 | 7.6 ± 2.5 | ns |
| Mean postprandial glucose (mmol/L) | 9.6 ± 2.7 | 8.5 ± 2.0 | 0.01 |
| Mean excursion (mmol/L) | 1.3 ± 2.6 | 1.3 ± 2.0 | ns |
| Peak excursion (mmol/L) | 5.2 ± 3.2 | 4.5 ± 2.6 | 0.03 |
| Time to peak (min) | 102 ± 71 | 135 ± 77 | 0.01 |
| AUC ([mmol/L] ∗min) | 515 ± 463 | 442 ± 346 | 0.03 |
| %CV | 25.3 ± 10.4 | 22.3 ± 10.8 | ns |
| Hypo events (n) (%) | 21 (29.6) | 18 (23.7) | ns |
| Correction events (n) (%) | 17 (27.4) | 16 (22.2) | ns |
| TIR (min) | 136 ± 76 | 167 ± 70 | 0.01 |
| TAR, high (min) | 59 ± 49 | 54 ± 58 | ns |
| TAR, very high (min) | 35 ± 51 | 10 ± 26 | <0.001 |
- Note: Mean ± SD. Linear mixed model fit by REML with fixed intercept and random intercept (subjects). Cereal-only meals n = 71, added protein meals n = 76 (number with primary outcome measure of mean postprandial glucose).
- Abbreviation: ns: nonsignificant.
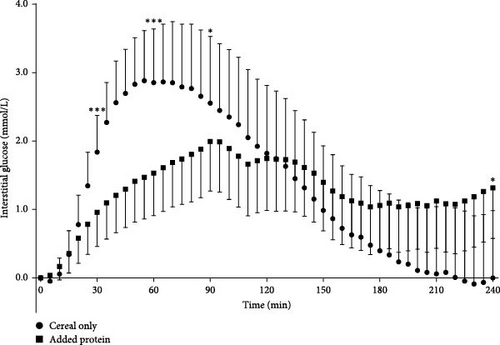
At 30 min, the glucose excursion was significantly higher after ingestion of the cereal-only meals (mean: 1.8 ± 2.3 mmol/L) compared with the added protein meals (mean: 1.0 ± 2.2 mmol/L) with a mean difference of 1.5 mmol/L (95% CI [0.7, 2.3], d = 0.6, p < 0.001). This remained significantly higher at 60 min with a mean difference of 2.3 mmol/L (95% CI [1.2, 3.3], d = 0.7, p < 0.001) and at 90 min with a mean difference of 1.5 mmol/L (95% CI [0.2, 2.7], d = 0.4, p = 0.02). These differences at these times remained significant when the model was adjusted to include the type of regimen. There was no other significant difference in the glucose excursions until 240 min when the glucose excursion was significantly lower after ingestion of the cereal-only meals compared with the added protein meals with a mean difference of 1.4 mmol/L (95% CI [0.1, 2.8], p = 0.04); however this was not significant when the model was adjusted to include type of regimen (p = 0.44) (Figure 3).
3.8. Snacks and Physical Activities
The mean postprandial glucose was significantly lower where the participant had a snack during the postprandial period (mean: 8.5 ± 2.4 mmol/L) compared with those who did not snack (mean: 9.5 ± 2.8 mmol/L) with mean difference of 1 mmol/L (95% CI [0.5,1.6], d = 0.4, p < 0.001). The TIR (min) was also significantly higher for those who snacked during the postprandial period (mean: 163 ± 72 min) compared with those who did not snack (mean: 138 ± 79 min) with a mean difference of 26 min (95% CI [9, 43], d = 0.3, p = 0.01). The activity duration was significantly longer for those who snacked (mean: 45 ± 58 min) compared to the nonsnack group (29 ± 53 min) with a mean difference of 14 min (95% CI [0.5, 28], d = 0.3, p = 0.04). Activity duration of those who took a snack without insulin was significantly longer (mean: 62 ± 69 min) than those who took insulin with their snack (31 ± 42 min), with a mean difference of 36 min (95% CI [16, 57], d = 0.6, p < 0.001).
4. Discussion
4.1. GV
Although only 36% of the participants met the %CV target of ≤36% [25], which is the level of stable glycaemia [29], the mean %CV is lower than others have reported [30–34], suggesting that GV for CYP with T1D is improving. This improvement reflects the use of real time CGM (rtCGM) by all the participants in the present study, as this is known to be superior at improving glycaemic control compared with intermittentscan CGM (isCGM) [33], which was used in some of the other studies reporting on GV [30, 33, 34]. Furthermore, more recent studies have also reported similar levels of %CV to the current finding [35–38].
The higher diurnal %CV compared with the nocturnal period was also observed by both Tansey et al. [7] and Edge et al. [30]. Although in the present study the mean diurnal glucose was lower than in both these previous studies [7, 30], the higher diurnal %CV demonstrates that managing GV in the diurnal period, when food consumption and physical activity occur, remains a challenge. The significantly lower diurnal %CV observed for the MDI users was due to them spending less TBR than the CSII users. This conflicts with findings showing that hypoglycaemia tends to occur less often in those using CSII, however, this is observed for severe hypoglycaemic events [39] and for mild hypoglycaemia, there appears little difference between MDI and CSII users [40]. Other studies have also reported a lower %CV in MDI users when using rtCGM [35, 41]. It is also known that the use of rtCGM, regardless of regimen, reduces the risk of hypoglycaemia [42], which may explain the current findings.
Achieving a TIR of ≥70% is more challenging. In this present study, the mean TIR was 60.1%, and only 24.7% of the participants met the ≥70% target. Several studies have observed a similar level of %TIR for CYP with T1D [31, 32, 35, 43], and the higher %TIR for the CSII users compared with MDI users was also observed by Cherubini et al. [41].
The low number of participants meeting the %TIR target demonstrates how difficult this target is to achieve; in a study based at a diabetes camp an improvement in %TIR during camp was observed from 58% to 67% however the authors noted that this involved “great professional effort” and during camp only 30.8% met the %TIR target [44]. However, in this previous study, some of these CYP were using isCGM and none were using HCL systems, which have been shown to significantly improve glycaemic control, including TIR, from both randomised controlled and single arm trials [45–49]. Similarly, in the present study, few participants were using HCL systems, which likely explains the low number of those who met the TIR target. As increasing numbers of CYP in the UK have access to HCL systems, it is expected that this TIR target will be more achievable for CYP with T1D.
4.2. The Preprandial Period
Similar levels of preprandial glucose before breakfast have been reported elsewhere [6, 50, 51]. This present study suggests that CSII users experience more optimal preprandial glucose levels, however, Heptulla et al. [50] did not observe any difference in the preprandial glucose when the participants in their study moved from using MDI to CSII. Despite this, the “Dawn Phenomenon” [52] is the likely cause of the higher preprandial glucose observed in the MDI users as basal insulin, administered via injections, cannot be adjusted to meet the diurnal and nocturnal variability of insulin requirements unlike CSII via an insulin pump and particularly HCL systems [45, 53].
Preprandial glucose is an important factor for postprandial glycaemia. It is known that in adults with noninsulin-dependent type 2 diabetes, preprandial glucose is strongly positively correlated with postprandial glucose [54], although once glucose levels exceed the renal threshold for reabsorption, there is an inverse association with postprandial glucose [55]. There is also some evidence that preprandial glucose is associated with postprandial hyperglycaemia in adults with T1D [56], as was observed in this study.
The mean insulin dose timing met the ISPAD 2022 guidance of 10–15 min preprandial [57] although national registry data report that up to a third of CYP do not adhere to these recommendations [58, 59] and none of the dose timings resulted in a difference in glucose outcomes which contrasts with the review by Mozillo et al. [60] who concluded that preprandial dose timing was associated with lower peak glucose compared with doses given postprandial.
4.3. The Postprandial Period
Reporting on data collected using the first Food and Drug Administration (FDA) approved CGM, Boland et al. [6] highlighted the issue of GV caused by postprandial hyperglycaemia in CYP with T1D, however, they did not observe this to be significantly worse after breakfast than after other meals. In contrast, other studies, also using CGM, observed that postprandial glucose was worse after breakfast compared to other meals [8–10].
The findings of this present study highlight that postprandial hyperglycaemia can occur after breakfast, however, two-thirds of the breakfast meals analysed resulted in a postprandial glucose within the TIR target, therefore, good glycaemic control after breakfast is possible. The glucose levels were also lower than others have reported [6, 9, 10, 50]. In Boland et al. [6], in their study of 56 CYP with T1D, the majority of whom were using CSII, the peak postprandial glucose after breakfast was 16.3 ± 4.7 mmol/L. This was a similar finding to Heptulla et al. [50] who observed a significant reduction in postprandial glucose after breakfast in their subjects when they were moved from injection therapy to CSII therapy, although this remained above target at 14.8 ± 0.9 mmol/L (p < 0.003). In Gandrud et al. [9], a study involving children with T1D aged under 7 years, the glucose excursion after breakfast was also above target at a mean of 7.5 ± 3.9 mmol/L although some of the subjects had higher levels of HbA1c than in the present study, which these same authors observed to be associated with postprandial hyperglycaemia after breakfast.
In the present study, the mean glucose excursion did not exceed recommendations [61]. and as this was also lower than reported elsewhere [6, 9], this suggests that postprandial hyperglycaemia after breakfast may be improving for CYP with T1D. However, this contrasts with the findings of Monzon et al. [10], who reported the mean postprandial glucose after breakfast to be 14 mmol/L, and this was higher after breakfast compared with both lunch and dinner. These findings likely differ because the study involved CYP from the USA, who have been shown to experience higher levels of poor glycaemic control [62]. Furthermore, postprandial hyperglycaemia seems to affect some CYP more than others; in the present study, a third of meals resulted in a mean glucose reading above 10 mmol/L, which is not acceptable, and as those using MDI had a higher preprandial reading, they also experienced higher postprandial glucose.
4.4. The Breakfast Meal
Some of the foods in the present study were like those reported by Mackey et al. [63] for children aged 2–5 years with T1D, although breakfast cereals did not appear to be commonly consumed in Mackey et al., unlike in the present study. The consumption of high GI breakfast cereals is observed in clinical practice. Lower GI cereals are available and account for over a third of those reported in the international tables of GI [18]; however, not all these products are available in the UK.
The percentage of energy from carbohydrate of 52.9% ± 14.3% is like the findings of Fisher et al. [64] (2023), who reported this to be 52.3% for breakfast in their sample of 48 Australian CYP with T1D. In the present study, the percentage of energy from protein was 14.2% ± 5.9%, which was lower than in Fisher et al. [64], who reported these to be a mean of 16.8%, although none of the breakfast meals in Fisher et al. were high in protein. Studies including CYP with T1D from the USA have reported both higher and lower amounts of carbohydrate with Monzon et al. [10] observing an intake of 62% of energy from carbohydrates at breakfast for children aged 2–6 years with T1D, which was higher than the 48.5% reported in Mackey et al. [63] in a similar age group of CYP with T1D. These studies demonstrate that carbohydrate intake at breakfast in CYP with T1D is variable.
The mean GI of 59% ± 10% is higher than reported by Mackey et al. [63], who observed a GI of 51.6% in breakfast meals consumed by children aged 2–5 years with T1D. As only the low GI breakfast meals met the ISPAD recommendations for macronutrient distribution for all meals [13], this appears to substantiate the recommendation for the GI to be incorporated into carbohydrate counting education. In contrast, the low GL meals only contained 36% of carbohydrate from energy, which would be classed as a low carbohydrate diet if this was followed for all meals [65, 66]. Low intakes of carbohydrate without increases in protein and fat will result in inadequate energy intake and weight loss in CYP [67]. It appears more clinically beneficial to focus on the GI rather than GL for ensuring the ISPAD macronutrient recommendations are met.
4.5. Postprandial Glucose and Food Composition
In the present study, the ingestion of the high GI breakfast meals resulted in a significantly lower %CV compared with medium GI meals and fewer hypoglycaemic events were observed with borderline significance. This infers that the consumption of lower GI meals may increase the risk of hypoglycaemia. There is conflicting evidence in the literature for this. Two studies of adult subjects with T1D did not observe more frequent hypoglycaemic events following consumption of lower GI meals [68, 69] although the latter did report that hypoglycaemic events occurred earlier in the postprandial period following the ingestion of a low GI meal. In their study of CYP with T1D, Nansel et al. [70] observed mild hypoglycaemia and the need for lower doses of insulin when low GI meals were consumed compared to high GI meals. As TBR correlates with %CV [71], hypoglycaemia caused by the consumption of lower GI meals may result in higher levels of GV, and these meals may require insulin adjustment [13].
The largest difference in glycaemic response was observed between the meals which contained only breakfast cereal and those which included a protein food. The significantly higher glucose excursions after the ingestion of the breakfast cereal-only meals are also consistent with other findings [72–74]. Parents also report that breakfast cereals are a problem food, causing high glucose levels [75]. The breakfast cereal-only meals had a significantly higher GI. Foods with a high GI can cause a large rise in the early postprandial period [72, 76, 77] followed by a drop in the later postprandial period, which can cause hypoglycaemia [73]. Although there was no difference in hypoglycaemic events, the breakfast cereal meals did result in a significantly lower glucose excursion in the later postprandial period.
The breakfast cereal meals also had a significantly larger carbohydrate load as per percentage of energy, which may negatively impact longer-term glycaemic control. Mackey et al. [63] observed a trend towards an HbA1c above target for those children who consumed higher amounts of carbohydrate for breakfast as per percentage of energy, however, this was not significant.
In contrast to high GI foods, meals which contain fat or protein can result in a lower glucose excursion in the early postprandial period [78, 79], and this was observed after ingestion of the “added protein” breakfast meals. CYP with T1D who spend more than 70% of their TIR have been observed to consume more protein than those with lower TIR [80], and in the present study, adding protein to the breakfast meal also resulted in the longest TIR with significantly less time spent above 13.9 mmol/L. In nonT1D, the addition of protein to a high GI carbohydrate meal has been shown to reduce the AUC by almost half [81]. This can be beneficial in lowering the GI of a meal, however, in CYP with T1D, high intakes of protein and fat can cause delayed hyperglycaemia between three and 5-h postprandial [78, 82, 83] with an additive effect in meals which are high in both these macronutrients [82]. This effect can increase the need for more correction doses of insulin during the postprandial periods after ingestion of high-protein meals [84]. However, this was not observed in the present study and following the ingestion of the “added protein” meals, the glucose excursion started to fall by 150 min with no delayed hyperglycaemia and no significant difference in the number of correction events for any type of meal, suggesting that this amount of protein which was a mean of 20.2 ± 9.3 g was not enough to cause delayed hyperglycaemia.
The glucose levels were lower for those who snacked during the postprandial period, and the physical activity of these participants was also of a longer duration. This suggests that much of the snacking that took place was to manage glucose levels during activity, especially for those who did not cover the snack with insulin. Fisher et al. [64], in a diet survey study, found no association between snacks and HbA1c, and furthermore, GV has been shown to be lower for those who snack between meals [10], which is likely due to fewer hypoglycaemia events.
There are several limitations to this study. As an observational study, it lacks randomisation, which can lead to bias and the overestimation of treatment effects when compared with randomised controlled trials [85]. There is also potential for confounding factors as these are not controlled for between groups in observational studies [86]. There were potential confounding factors in the present study arising from the participants’ socioeconomic status and food choice, which could have affected glycaemic control. The sample size was also small, and although bias was controlled for by not limiting recruitment to those with good glycaemic control, this may have arisen from only recruiting those using Dexcom CGM. At the time of recruitment, fewer CYP had access to CGM, and those who did have access to CGM were more likely to be from a higher socioeconomic status, as there was a socioeconomic disparity in access to technologies [87]. Furthermore, although only those CYP who had T1D for at least 1 year were recruited to the study, it is not known if any were in partial remission, which would have affected their glycaemic control. The intake of meals was also self-reported, and although the accuracy of this was likely increased via the collection of photographs, the accurate analysis of the breakfast meals did rely on correct submissions. Although this study highlights that high GI ready-to-eat cereals are a commonly consumed breakfast meal, the study did not explore other commonly consumed breakfast foods among different ethnic groups and the impact these have on glycaemic control after breakfast.
5. Conclusions
This study provides some evidence of the impact that breakfast meal composition has on postprandial glucose. There are several facets to this, including the GI, the GL and carbohydrate load and the macronutrient composition of the meal. Many CYP with T1D choose to consume high GI ready-to-eat cereals for breakfast. This study provides evidence of the extent of this and the association between these cereals and high levels of postprandial hyperglycaemia, and it supports the use of protein to manage postprandial glycaemia after breakfast in CYP with T1D.
The impact of increasing protein intake at breakfast in T1D requires further investigation in order to establish the amount of protein required to dampen the glucose rise after breakfast and to establish the optimal breakfast meal composition. One such intervention study has now been undertaken with results pending. Given their ability to improve overall glycaemic control, HCL systems may be better able to manage postprandial hyperglycaemia regardless of meal composition and further research is required to establish their effectiveness in managing postprandial glycaemia after breakfast in CYP with T1D.
Ethics Statement
The study was approved by the University of Stirling (NHS, Invasive & Clinical Research Committee) and the West of Scotland (5) Research Ethics Committee (20/WS/0123).
Disclosure
This paper formed part of a Clinical Doctorate thesis for Julie Johnson titled ‘The Breakfast Rise Education and Knowledge Study (The BREAK study).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Julie Johnson: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, visualization, writing—original draft, writing—review and editing. Victoria Franklin: Conceptualization, methodology, supervision, writing—review and editing. Ashley Shepherd: Conceptualization, methodology, project administration, supervision, writing—review and editing. Grace Chau: Investigation, project administration, writing—review and editing. Kate Keen: Investigation, project administration, writing—review and editing. Sophie Lennon: Investigation, project administration, writing—review and editing. Maria Leveridge: Investigation, project administration, writing—review and editing. Kirsty Maclean: Investigation, project administration, writing—review and editing. Julie Nicol: Investigation, project administration, writing—review and editing. Vanessa Phillipson: Investigation, project administration, writing—review and editing. Sue Roach: Investigation, project administration, writing—review and editing. Adele Swart: Investigation, project administration, writing—review and editing. Stuart Galloway: Conceptualization, methodology, project administration, supervision, validation, writing—review and editing.
Funding
No funding was received for this research.
Acknowledgments
We wish to thank all the children and young people and their families who participated in this study. The abstract of this study was presented at the 50th Annual Conference of the ISPAD (2024) [88].
Open Research
Data Availability Statement
The data to support these findings are available from the corresponding author on request.




