Effects of Pyridine and Ionic Liquid ([Bmim]Cl) Extraction on the Structure of Mongolian Bituminous Coals
Abstract
Organic solvents such as pyridine and ionic liquid (IL) ([Bmim]Cl anion) have been used to extract soluble parts from Mongolian sub-bituminous and bituminous coals for the first time. The extracts and insoluble coal residues were analyzed in detail by FTIR spectroscopy. The paper highlights the types of coal that influence traditional and innovative environmentally friendly solvents-based extraction. Bituminous type of coal IL-based extraction provides more promising results, such as many types of hydrogen bonds to be revealed, a shortage of aliphatic chain, a breakage of oxygen-containing structures, and removal of minerals or oxygen-containing groups, compared to sub-bituminous coal. The traditional solvent also has the advantage of being processed easily, a room temperature procedure producing virtually similar results to IL treatment in the low-frequency region. Quantitatively one can confirm some results such as the aromatization of extracts and the aliphatic chain length shortage.
1. Introduction
The climate condition of long winters with extremely low temperatures (up to −40°C) together with its current economic circumstance and lifestyle of traditional dwelling inhabitants demand an environmentally friendly and clean way of using coal as an energy source.
Sufficient resources of up to 162 billion tons in as many as 200 or more coal occurrences [1] are endowed through the country of Mongolia to provide an appropriate domestic utilization to keep up effective and broad features. Solvent extraction is widely used in the study of coal compositional or structural characteristics with traditional solvents such as benzene, toluene, methanol, chloroform, dichloromethane, pyridine, and N-methyl pyrrolidone [2–8]. In the solubilizing of coal, given the disassociation and rearrangement of functional groups, particularly, an increased number of polycyclic aromatic hydrocarbons or formation of hydrogen bonding in the coal structure. For example, pyridine as a solvent of extraction forms strong hydrogen bonds in coal from which rearrangement of the coal macromolecular structure makes it denser [9, 10]. Visible morphology changes followed by macromolecular structural mobility were observed in the contact of coal with organic solvent pyridine [5, 9–12].
In 2010 Painter et al. [13] first introduced an ionic liquid (IL) with various cations and anions in raw coal treatment, where the efficiency of solvation yield has reached as high as 40%. Extraction yields the highest possible percentage and the finest dispersion of coal particles when chloride-based ILs are used. Later Lei et al. [14] reported Chinese lignite with a carbon content of 64.75% that was given 74% extract yield with chloride-based IL. Behind such an efficient yield of extraction stands ILs that own unique properties such as flexible structural building through different combinations of the constituent cations and anions, low volatility, low extraction temperature (< 100°C), and negligible vapor pressure [15–17].
Among various chloride-based ILs, imidazolium-based 1-butyl-3-methyl-imidazolium chloride ([Bmim]Cl) can dissolve coal fractions with a high percentage [13, 14, 18]. The extraction yield percentage presents an interaction efficiency between coal and chloride-based ILs. Additionally, it has been established that the extraction yield depends on the coal type [9, 14].
Bituminous and sub-bituminous types of coals are found to be high availability and low-cost in Mongolia for energy commodities [1]. However, the utility of coal in the country is limited by a lack of innovative technology and a clear view of optimal consumption or coal production. More like lignite, sub-bituminous, and bituminous humic type Mongolian coals with non-marine influences, high volatile matters, and high ash tendency are still systematically not studied. To obtain accurate features into the structures, namely the functional groups′ rearrangement by solvent-based coal extraction we chose two types of Mongolian coals with different metamorphisms and two types of solvents (such as pyridine and IL ([Bmim]Cl anion)). The experiment of solvent-based extraction on Mongolian coals was conducted for the first time with the current project.
The method of the current study is Fourier Transform infrared spectroscopy (FTIR) which is widely used for revealing renovations and rearrangements in the functional groups of coal structures. The method is reliable among analytical chemical techniques for determining the structural characteristics of coal [14, 16, 17, 19–24]. Qualitative and quantitative data on the distribution and contents of the functional groups can be obtained using the Beer–Lambert theory-based FTIR method.
2. Samples and Methods
Tavantolgoi coal deposit in Western south of the Ulaan-Nuur valley in Umnu-Gobi aimag consists of I (0 + I) to XVI coal seams in 191 m-deep length. Tavantolgoi IV seam coal is defined as a bituminous coal [1, 25] with the resource of a continuation up to hundreds of years.
Alagtogoo coal [1] deposit is in Dornogobi aimag, Central Mongolia, 330 km from southeast Ulaanbaatar with an estimated reserve of 872 Mt of sub-bituminous coal. Deposit is open-pit mining that produces 5 Mt per annum.
Sample preparation for the experiment was performed following standard procedures of coal chemistry: A piece of coal from each deposit was crushed initially and then milled to a size of 0.05 mm or less by using agate mortar in an argon flow glove box. At last, milled samples were kept in a Schlenk flask under argon.
Pyridine, IL, and methanol were purchased from Sigma-Aldrich and applied without further purification. Solid at room temperature 1-butyl-3-methylimidazolium chloride anion ([Bmim]Cl) (> 98.0% HPLC, ≤ 1.0% H20 impurity), pyridine (≥ 99.9% HPLC), methanol (≥ 99.8% ACS) were used.
2.1. Solubility of Coal in Pyridine
Nearly 1 g of coal sample was refluxed in 25 mL of pyridine at 408 K under the pressure of 1.33 Pa for 24 h, afterward, it was centrifuged at a speed of 6000 rpm for 1.5 h. An extract was separated from the insoluble solid part using a 0.4 m diameter pore size of Teflon filtration. Subsequently, it was dried by evaporating an excess liquid pyridine under a vacuum at 323 K. The sample was labeled pyridine extract (PE). The solid part of the insoluble coal residue (ICR) was washed with methanol several times to remove solvent leftover and then the isolation was made from liquid methanol by evaporation. The final solid part was dried in a vacuum at 323 K under nitrogen flow for weeks. The sample is labeled the pyridine-treated ICR (PICR).
2.2. Solubility of Coal in an IL of [Bmim]Cl Anion
Approximately 1 g of coal is placed in a tube with 10 g of IL ([Bmim]Cl anion). The mixture was placed, capped in two necked flasks, heated to 373 K, and stirred at 1250 rpm under a pressure of 1.33 Pa for 48 h. The procedure was comparatively well enough for a sufficient amount of coal to be dissolved. The suspension was kept at 373 K in the centrifugation for 6 min at 4000 rpm. After the first centrifugation round, we separated the supernatant from the insoluble part of the coal. A supernatant solution was separated from the insoluble part of the coal and labeled as [Bmim]Cl− E (IL extract). The extract was stored under nitrogen gas in an oil bath heated up to 373 K. Methanol (approximately 70 mL) was poured into the insoluble part of coal in a flask inside an oil bath at 333 K and kept for 1 hour. There it was stirred at 1250 rpm without interruption until the IL was fully dissolved. Afterward, being in a steady state for 12 h, the supernatant was removed, and a residue was dried by opening the flask neck edge in the presence of nitrogen blowing in an oil bath at 333 K. The dry insoluble coal part was labeled as [Bmim]Cl− IL-treated ICR.
2.3. Scanning Electron Microscope (SEM/EDS) for Elemental Analysis
Coal samples were analyzed by SEM (JOEL) equipped with backscattered and secondary electron detectors with energy dispersive X-ray spectrometry (EDS). The SEM-EDS provides detailed imaging information about the morphology and surface texture of individual particles, as well as the elemental composition of samples.
In this study, backscattered electron imaging (BSE) which provides visual information based on gray-scale intensity between chemical phases and EDS was used to characterize heavy elements in coal samples. At the same time, secondary electron imaging (SEI) images of the coal samples also were taken with magnification up to 105 at a resolution of 10−6 m.
The elemental composition of coal samples is determined using a characteristic X-ray spectrum up to 20 kV on the surface of the coals. The elemental analysis was performed in a “spot mode” in which the beam is localized on a single area manually chosen within the field of view. The EDS detector was capable of detecting elements with an atomic number equal to or greater than six.
2.4. FTIR Spectroscopy
Functional groups of coals were detected by an in-situ FTIR spectrometer of Nicolet Avatar 330 in transmittance mode. Raw coal samples were first dried in an oven at 383 K for 72 h. Then it was ground with KBr to a fine powder in a mortar and prepared into a 10 mm size tablet under the pressure of 4.14 × 107 Pa. In the spectrometer, the tablet was scanned 64 times in the range from 400 to 4000 cm−1 with a resolution of 4 cm−1. Spectra were transformed to absorption to present an intensity proportional to the number of functional groups. The calculations of peak areas and heights were performed using OriginLab.
3. Results and Discussion
The main elemental contents in coal are carbon, oxygen, hydrogen, nitrogen, and sulfur. Carbon, oxygen, and sulfur contents in the raw coals were determined as shown in Table 1. The carbon content of Alagtogoo coal indicates its metamorphism tendency as a sub-bituminous type. Tavantolgoi coal is a bituminous type containing a high amount of carbon. Oxygen containment in sub-bituminous Alagtogoo coal is sufficiently greater than in bituminous Tavantolgoi coal.
| Coals | Element content, wt.% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Al | Si | Ca | S | Fe | Se | Mn | Mg | Ag | Ba | |
| Alagtogoo | 73.62 | 15.99 | 0.83 | 2.38 | 1.66 | 1.08 | 0.66 | 2.48 | 0.37 | 0.03 | 0.09 | 0.77 |
| Tavantolgoi | 82.05 | 7.19 | 1.72 | 3.42 | 1.53 | 0.63 | 0.11 | 1.68 | 0.23 | 0.08 | 0.35 | 0.78 |
As determined by EDS, the predominant heavy elements in the coals are silicon, selenium, aluminum, calcium, manganese, and iron (Table 1). The existence of magnesium, manganese, and sulfur was observed. Aluminum was primarily associated with silicon. Calcium and silicon exist on brighter spots of coals (Figure 1.).
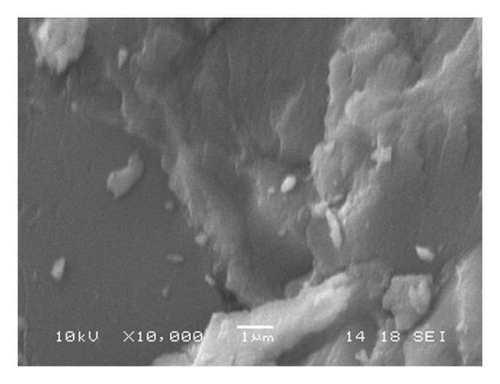
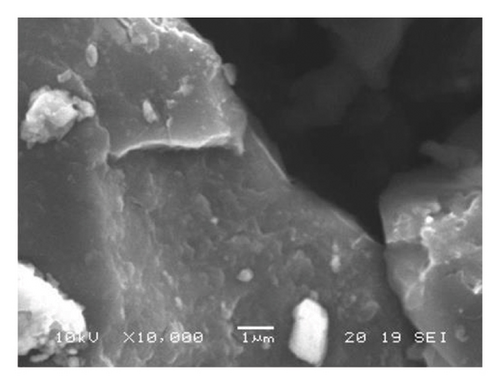
Properties of Tavantolgoi Seam IV maceral composition can be referred from [1], as vitrinite (74.5 vol% m.m.f.) dominated coal. Alagtogoo coal deposit is in the East Gobi basin and is known basically as the period of the major coal seam, Lower Cretaceous rocks.
Analytical data are provided by FTIR spectroscopy results that offer considerable potential for quantitatively determining major functional groups present in coals and their productions.
For the precise difference between the infrared spectra of as-received coal and its solvent-treated productions (extract (E) and ICR), the infrared wave zone was divided into the three regions of 4000-2500 cm−1, 1800–900 cm−1 and 900–400 cm−1 (Figures 2 and 3) allowing a more complete and accurate analysis.
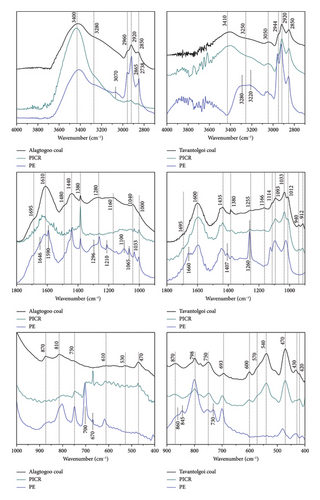
Numerous studies [14, 16, 17, 19–24] and our previous publications have proposed raw coal functional group assignations, including Mongolian coals [26–28] as follows:
In the high frequency region (4000–2500 cm−1) self-associated n-mers (polymeric or cyclic) OH groups (3400 cm·cm−1), OH⋯ether hydrogen bonds (3300 cm−1), cyclic OH hydrogen bonds (3200 cm−1), and OH⋯N hydrogen bonds (3100–2800 cm·cm−1), stretching vibrations of the CH groups (2800–3100 cm−1) are available.
In the low frequency region (1800–900 cm−1) carbonyl stretching (C=O group) occurs. This is one of the easiest absorptions to recognize in an infrared spectrum because of its intensity. Usually, it is around 1600 cm−1 for coal. Carboxyl group vibration (aldehydes, ketones, and unsaturated aryl aldehydes) occurs in 1700–1690 cm−1 intervals. Minerals existence, various oxygen containing defects in the coal structure appear as oxygen-containing groups such as C–O, Si–O groups, Al-OH groups, S–O groups, and OH translations.
Two of the bending methyl C-H absorptions at 1440 cm−1 (CH2) and 1380 cm−1 (CH3) from methylene groups in the structure are registered.
The absorption bands in the region of 900-400 cm−1 are associated with out-of-plane vibrations of the C–H group. There are usually three peaks associated with out-of-plane bending vibrations of isolated (850–890 cm−1 range), two adjacent (815–830 cm−1), and three adjacent (775–800 cm−1) aromatic CH bonds [26, 27, 29].
Figure 2 presents the spectra of PE and PICR compared to the spectrum of the as-received coals for Alagtogoo and Tavantolgoi. As-received coal infrared spectrum and its peak positions labeled by literal wavenumber are shown in the uppermost spectrum.
Shifted or newly yielded peak positions, except for the original sample, are also given in the figures (Figures 2 and 3). As shown in Figures 2 and 3, spectra of as-received coals and both solvent-treated insoluble residues (PICR and [Bmim]Cl− ICR) are similar except for the intensity.
In the short-wavelength region of the infrared spectrum, an extra band at 3070 cm−1 from hydrogen associated with aromatic carbon [30] and C–H stretching bands from aliphatic compounds at 2865 cm−1 and 2738 cm−1 occurred.
The tendency of pyridine treatment is to a certain extent mineral-related than aromaticity. In the region of 1200–400 cm−1, we observe several absorptions (1296, 1210, 1100, and 1065 cm−1), however, these were solvent-influenced bands (comparison done with pure pyridine, raw coal, and Tavantolgoi PE spectra) [11]. This phenomenon is true for IL-treated sub-bituminous coal too (1126 and 1090 cm−1). Ts. Amartaivan et al. [31] have established that several coals including Tavantolgoi and several coals from Eastern Mongolia, contain various trace elements. This finding may relate to solvent and coal surface admicelles parts that can be retained after extraction in the supernatant because of bond dissociations [17].
The intensity of the spectrum in the frequency area of 4000–3000 cm−1 is quite enhanced and basic absorptions (3410 cm−1 and 3250 cm−1) are shifted and overlapped in 3280–3220 cm−1 (see Figure 2). This type of change is caused often by π electrons, namely OH-π hydrogen bond formation [15].
Similarly, sub-bituminous coal Alagtogoo, but significantly fewer absorptions were registered in the region of 1200–400 cm−1 for the sample PE and [Bmim]Cl−E of Tavantolgoi.
Bituminous coal Tavantolgoi is well aromatized even in the extraction with pyridine showing the prominent occurrence at 1660 cm−1 in the spectrum. Barely seen shoulder absorption at 1407 cm−1 declares CH bending vibration in the interaction between coal and pyridine.
For the quantification view, OH and aromatic ring stretch ratio (3400/1600 cm−1) gives an increase (see. Table 2) that indicates a good interaction of pyridine with OH group of bituminous Tavantolgoi coal. In contrast, the value is decreased for the sub-bituminous Alagtogoo coal. A similar contradictory result was received for the ratios of 1450/1600 cm−1 (aliphatic and oxygen-containing group) and 1650/1600 cm−1 (carbonyl and aromatic ring stretch). Niu et al. [32] demonstrated that the highest heterogeneity in the fundamental structure of coal makes contradictory results to be achieved.
| Sample | CH2/CH3 | (CH2 + CH3)/Car | Aliphatic C-H/aromatic C-H | OH/Car | C = O/Car | |
|---|---|---|---|---|---|---|
| Alagtogoo | Raw coal | 1.28 | 0.68 | — | 0.84 | 1.04 |
| PICR | 1.10 | 0.40 | — | 2.13 | 0.47 | |
| PE | 0.74 | 0.60 | — | 0.43 | 0.32 | |
| Tavantolgoi | Raw coal | 1.50 | 0.88 | 1.96 | 0.27 | 0.55 |
| PICR | 1.77 | 0.87 | 2.02 | 0.32 | 0.61 | |
| PE | 1.38 | 0.91 | 1.46 | 0.44 | 0.76 | |
Some ratios remain similar despite the coal type. For instance, a ratio of 2800–3000/3050 cm−1(aliphatic and aromatic C-H) [33] and a semi-quantitative parameter value of CH2/CH3, the aliphatic chain length shortage (see Table 2) [34].
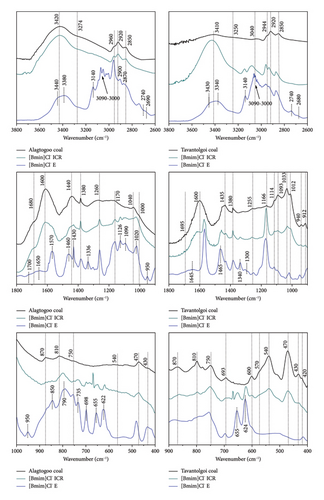
Figure 3 demonstrates major infrared absorptions for the [Bmim]Cl−-treated coal productions and the as-received coals for Alagtogoo and Tavantolgoi.
As a consequence of the IL treatment, an extra band at 3380 cm−1 from hydrogen associated with aromatic carbon was formed. This band has strongly overlapped with self-associated n-mers OH groups 3420 cm−1. Additionally, in this range of 4000–3000 cm−1, there are 3140 cm−1 and 3090–3000 that are associated with OH⋯N hydrogen bonds (see Figure 3).
C–H stretching bands from aliphatic compounds at 2740 cm−1 and 2690 cm−1 were reproduced. Since these peaks are observed in the spectra of both [Bmim]Cl−E of Alagtogoo and Tavantolgoi, referring from a spectrum of pure [Bmim]Cl− substance, we conclude it is evidence of residual solvent.
In the low frequency region basic absorption bands of the original coal are rearranged to a certain extent, for example, carbonyl absorption at 1600 cm−1 is decreased crucially in intensity with shifting to 1650 cm−1, CH bending shifted to 1460 cm−1 with extra absorption at 1430 cm−1, CH bending around 1300 cm−1 covers original absorption at 1380 cm−1, and an extra two of peaks at 1335 cm−1 and 1300 cm−1 and finally, 1040 cm−1 of the basic band has blue shifting. Shifting of basic bands indicates structural bond disruptions, essentially carboxyl group renovation [34]. Bending CH area activation is remarkable only after IL-based treatment. This can indicate the presence of hydrogen bonding in this area at a certain level. But again, the bituminous coal structure contains more hydrogen bonding than the sub-bituminous one. Because after pyridine treatment we could observe only one peak at 1407 cm−1 for Tavantolgoi coal.
In the region of 1500–400 cm−1, Alagtogoo coal in contrast to Tavantolgoi demonstrates many mixed intense absorptions indicating that a rigid coal network limits bond disruption. This apparent difference in the extraction spectra reveals minerals still present but, in that sense, a fundamental site of the coal structure.
IL makes the coal more aromatic evidenced by a new intense absorption band with a maximum near 1570 cm−1. This band location corresponds to bending vibrations of C=C groups in the aromatic ring [22] typical for sp2 bonds between carbon atoms.
After the extraction, absorptions in the wave number interval of 4000–2500 cm−1 are crucially rearranged. Intensity has been increased significantly, and bands are quite broad (for broad and shifted absorption bands we created a fitting curve using OriginLab program for the determination of certain peaks).
Several types of hydrogen bonds released absorption bands, including a band at 3340 cm−1 that may arise from OH⋯ether hydrogen bonds or cyclic OH hydrogen bonds, 3145 cm−1, 3040 cm−1, and 3090–3000 cm−1 are assigned to OH⋯N hydrogen bonds.
The nature of absorptions observed in the frequency area of 1600–400 cm−1 is similar to [Bmim]Cl−E of sub-bituminous Alagtogoo. The main difference is the number of infrared absorption bands of [Bmim]Cl−E of Tavantolgoi that are roughly fewer than in sub-bituminous Alagtogoo. The point is on the elemental containment of trace or oxygen enrichment of sub-bituminous Alagtogoo coal. Oxygen containment of Tavantolgoi coal was measured as half of that of Alagtogoo coal. Aromatic absorption around 1570 cm−1 is always prominent for bituminous coal extract. Due to Lei et al. [35] a crucial affection of IL treatment to the complexities of minerals caused by the mostly organic constituents of raw coal extracted during the process.
A small magnitude of CH2/CH3 ratio [24, 36] that indicates a shortage of aliphatic chains, has been observed for the [Bmim]Cl− E samples. In Tables 2 and 3, these are 1.10 and 1.77 (PICR), 0.74 and 1.38 (PE), 1.11 and 1.15 ([Bmim]Cl− ICR), and 0.53 and 0.94 ([Bmim]Cl− E).
| Sample | CH2/CH3 | (CH2 + CH3)/Car | Aliphatic C-H/aromatic C-H | OH/Car | C=O/Car | |
|---|---|---|---|---|---|---|
| Alagtogoo | Raw coal | 1.26 | 0.86 | — | 0.55 | 0.41 |
| [Bmim]Cl− ICR | 1.11 | 0.93 | — | 0.72 | 0.50 | |
| [Bmim]Cl− E | 0.53 | 2.21 | — | 1.71 | 0.64 | |
| Tavantolgoi | Raw coal | 1.43 | 0.88 | 1.88 | 0.28 | 0.56 |
| [Bmim]Cl− ICR | 1.15 | 0.64 | 0.85 | 1.37 | 0.69 | |
| [Bmim]Cl− E | 0.94 | 0.60 | 1.20 | 0.44 | 0.23 | |
The C=O/Car ratio for original coal and [Bmim]Cl− ICR is increased (Table 3). This is one of the indications that ILs are more effective than pyridine. As discussed by Niu et al. [32] an aromaticity increase is mainly related to the rough disruption of C=O bonding by converting to C-O.
The value of (CH2+CH3)/Car ratio (aliphatic to aromatic C-H), is a trend of aliphatic group change during the extraction. Table 3 presents these values were decreasing in both [Bmim]Cl− ICR and [Bmim]Cl− E for the Tavantolgoi, suggesting that aliphatic groups tend to be eliminated by the IL.
4. Conclusion
The solubility of two types of Mongolian coals, bituminous Tavantolgoi and sub-bituminous Alagtogoo coals was investigated by FTIR spectroscopy.
Two kinds of solvents pyridine and IL ([Bmim]Cl−) were applied for the structural characterization and a solubility degree in Mongolian coals. The main characteristic point of Mongolian coals is their great content of trace elements or various minerals inclusion. Some heavy elements including silicon, aluminum, sulfur, and selenium were prominent in the coal mass containment. Oxygen content in coal plays a key role in the solvent-based extraction production character.
Both bituminous and sub-bituminous types of coals [Bmim]Cl−-based extraction provides more promising results, such as many types of extra hydrogen bonds occurring to confirm good solubility and, an excellent removal of minerals or oxygen-containing groups in the low-frequency region. The low content of oxygen in the fundamental structure of Tavantolgoi coal plays an important role in this extraction process. Quantitatively confirmed a shortage of aliphatic chains and a high degree of aromatization for IL-treated bituminous coal. The ionic liquid is unique for creating hydrogen bonding even in sub-bituminous coal.
The traditional solvent also has advantages in coal extraction, such as room temperature processing, the capability to form OH bonding in bituminous coal and a shortage of aliphatic chains.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was within the framework of the project (P2024-4682) supported by the Asia Research Center at the National University of Mongolia and Chey Institute for Advanced Studies Korea.
Acknowledgments
This work was within the framework of the project (P2024-4682) supported by the Asia Research Center at the National University of Mongolia and Chey Institute for Advanced Studies Korea.
The authors would like to thank W. Jeannine for the English language improvement.
Open Research
Data Availability Statement
The authors declare that all experimental data in the frame of the current work are available at any time.




