Angiotensin II Use in Postcardiac Surgery Vasoplegic Syndrome Patients: A Single-Center Descriptive Experience
Abstract
Objectives: We evaluated real world use of angiotensin II (AT II) in patients with vasoplegic syndrome (VS) following cardiac surgery.
Design: A retrospective chart review was performed to describe and evaluate VS following cardiac surgery under cardiopulmonary bypass (CPB) for AT II use and associated outcomes. Among these outcomes examined were death, stroke, myocardial infarction, acute kidney injury (AKI), tracheostomy need, ventilator hours, and hospital and cardiovascular intensive care unit (CVICU) lengths of stay (LOS). These outcomes were compared across patients with VS who received AT II vs. patients who did not receive AT II using Wilcoxon rank sum and Chi-square testing, as appropriate.
Setting: Academic medical center.
Participants: Adult postcardiac surgery VS patients.
Interventions: AT II vs. non-AT II receiving VS patients.
Measurements and Main Results: Of 2013 included patients undergoing cardiac surgery under CPB during the study period, 52 met criteria for VS, 11 (21.2%) received AT II, and 41 (71.8%) did not. The incidence of AKI, tracheostomy, CVICU LOS, and hospital LOS was higher in the AT II group (Tables 1 and 2). The median maximum postoperative NEE dose within 24 h following surgery was higher in the AT II group: 0.44 mcg/kg/min (IQR 0.39, 0.57) versus 0.23 mcg/kg/min (IQR 0.21, 0.26, p < 0.001).
Conclusions: AT II use was rare among cardiac surgical patients. AT II use was associated with increased resource use. AT II patients were on higher pressure dosing and may have had worse outcomes without AT II. Larger, prospective studies are needed to understand the impact of AT II on outcomes in this population.
1. Introduction
Vasoplegic syndrome (VS) occurs in up to 50% postcardiac surgery patients [1] and is associated with worse outcomes [2]. Although the definition varies [1, 3, 4], postcardiac surgery VS is characterized by severe shock accompanied with normal or high cardiac output state (cardiac index (CI) > 2.0 L/kg/m2) and low peripheral vascular tone (typically systemic vascular resistance (SVR) < 800 dyne/s/cm2), despite vasopressor dependence or unresponsiveness to conventional vasopressors, typically defined as a norepinephrine equivalent (NEE) > 0.2 mcg/kg/min [5, 6]. It usually presents within 24–72 h of cardiopulmonary bypass (CPB) [1, 7]. VS in postcardiac surgery patients is associated with increased mortality (up to 25%) and morbidity [8].
Norepinephrine has long been considered first line therapy for shock [1]. In postcardiac surgery VS, however, there are several mechanisms by which patients may respond poorly to norepinephrine [1]. Angiotensin II (AT II) has recently been introduced as a novel therapy for refractory distributive shock. Its benefit in VS following CPB is attributed to several well-described mechanisms [1, 9]. Accordingly, a recently conducted post hoc analysis of the AT II for the treatment of high-output shock (ATHOS-3) demonstrated significant vasopressor sparing and improved mean arterial pressure (MAP) in postcardiac surgery vasoplegic patients who received AT II [10]. This analysis, however, included a small number of patients and could only provide limited insight into the safety outcomes, including mortality and adverse events. Moreover, there is limited published literature and information on the use of AT II for postcardiac surgery VS, especially in a real-world clinical environment. Furthermore, to the best of our knowledge, this is the largest data set on AT II use in postcardiac surgery patients.
The aim of this retrospective observational study is to describe our single center experience with the use of AT II in a real-world clinical setting. We additionally sought to compare short term outcomes in patients with and without AT II use, with the hypothesis that the patient group receiving AT II would experience lower hospital mortality and shorter intensive care unit (ICU) and hospital length of stay (LOS).
2. Materials and Methods
Institutional Review Board (IRB) approval was obtained (University of Miami Human Subject Research IRB #20221193). The requirement for written informed consent was waived by the IRB. Applicable strengthening the reporting of observational studies in epidemiology (STROBE) guidelines were followed in the preparation of this manuscript [11].
2.1. Patient Selection
Our clinical database was retrospectively queried for all patients who underwent cardiac surgery with CPB at our institution between 2/2/2019 and 10/30/2022. All EMR data were extracted, transformed, and loaded into a relational database (EPIC Clarity) that we queried using MySQL. Our final datasets (based on the consort diagram of inclusion/exclusion criteria) were analyzed. Exclusion criteria included patients under 18 years of age, off-pump-cardiac surgical procedures, and any cardiac surgical procedure other than the first open heart surgery (either via sternotomy or minimally invasive approach) of a given hospitalization. We then proceeded to identify patients experiencing VS using the following institutional criteria in line with our clinical practice: CI greater than or equal to 2.0 L/minute/m2, along with SVR of less than or equal to 800 dyn/seconds/cm−5 (at any point of time in the first 24 postoperative hours) despite maximum vasopressor infusion of greater than or equal to 0.2 mcg/kg/minute, expressed in NEE [12]. A published vasoactive infusion score (VIS) system was utilized in determining the NEE [12]. The maximum vasopressor infusion rate in the first 24 postoperative hours was based on summation of infusion rates of individual vasopressors (norepinephrine, vasopressin, AT II, and epinephrine) within 60 min of the highest documented norepinephrine infusion rate. Patients in whom data were missing and who did not meet criteria for VS were excluded from the analysis.
2.2. Exposures
The AT II group comprised of patients with primary exposure to AT II within the first 24 h following cardiac surgery. Our control (non-AT II group) consisted of patients treated with only conventional vasopressor agents during the first 24 h. In our institution, AT II is typically administered in patients on two conventional vasopressors (norepinephrine, vasopressin, epinephrine, or phenylephrine) with escalating requirements. We attempt to wean AT II off first due to cost and concern for adverse outcomes.
2.3. Outcomes
In comparing the two groups, we investigated a primary outcome of mortality; we also considered secondary outcomes during hospital admission including stroke, myocardial infarction, thrombosis, acute kidney injury, need for tracheostomy, postoperative hours spent on ventilatory support, as well as hospital and CVICU LOS. ICD 10 codes (Table 3) with appropriate ‘present on admission’ indicators were used to capture hospital outcomes and differentiate these from pre-existing illnesses. Baseline data including age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) score, race/ethnicity, preoperative left ventricular ejection fraction (LVEF), preoperative use of angiotensin-converting enzyme inhibitors (ACEI) or AT II receptor blockers (ARB), preoperative creatinine, and Elixhauser comorbidities were also collected using ICD 10 diagnosis codes (Tables 1 and 2). Also recorded was the type of surgery (coronary artery bypass grafting (CABG), valvular surgery, and both or none), perioperative anticoagulation use, perioperative deep vein thrombosis (DVT) prophylaxis use, hospital and CVICU LOSs, CPB, and aortic cross clamp durations.
| AT II | Non-AT II | p value | |
|---|---|---|---|
| Total patients, n (%) | 11 (21.2%) | 41 (71.8%) | |
| Age, years, median (IQR) | 67 (58, 73) | 64 (58, 74) | |
| Age, ≥ 65 years, n (%) | 7 (64%) | 20 (49%) | |
| Female gender, n (%) | 2 (18%) | 10 (24%) | |
| Race/ethnicity, n (%) | |||
| Hispanic Black | 0 (0%) | 1 (2.4%) | |
| Hispanic White | 4 (36%) | 27 (66%) | |
| Non-Hispanic Black | 2 (18%) | 5 (12%) | |
| Non-Hispanic White | 5 (45%) | 6 (15%) | |
| Other/unknown | 0 (0%) | 2 (4.9%) | |
| LVEF ≤ 40% ∗∗∗, n (%) | 5 (45%) | 16 (44%) | |
| ACEI or ARB, n (%) | 5 (45%) | 16 (39%) | |
| Anticoagulant use, n (%) | 1 (9.1%) | 0 (0%) | |
| Prophylaxis use, n (%) | 0 (0%) | 0 (0%) | |
| Creatinine (mg/dL) ∗, median (IQR) | 0.87 (0.81, 1.21) | 1.60 (1.11, 3.13) | |
| BMI, median (IQR) | 30.5 (24.1, 36.6) | 28.1 (26.3, 31.2) | |
| ASA score, median (IQR) | 4.0 (4.0, 4.0) | 4.0 (4.0, 4.0) | |
| Surgery type, n (%) | |||
| Aortic | 0 (0%) | 0 (0%) | |
| CABG only | 1 (9.1%) | 12 (29%) | |
| Valve only | 6 (55%) | 18 (44%) | |
| 2+ | 4 (36%) | 10 (24%) | |
| Neither | 0 (0%) | 1 (2.4%) | |
| CPB Time, min, median (IQR) | 167 (132, 214) | 125 (97, 160) | 0.077 |
| CPB time > 180 min, n (%) | 5 (45%) | 9 (22%) | 0.14 |
| Aortic cross clamp time, min, median (IQR) | 143 (111, 180) | 86 (61, 108) | 0.004 |
| Aortic cross clamp time > 120 min, n (%) | 6 (55%) | 8 (20%) | 0.05 |
| Max SOFA score∗∗, median (IQR) | 10.00 (8.00, 11.50) | 10.00 (8.50, 11.50) | 0.8 |
| NEE, median (IQR) | 0.44 (0.39, 0.57) | 0.23 (0.21, 0.26) | < 0.001 |
| Hospital LOS, days, median (IQR) | 23 (13, 31) | 9 (6, 11) | 0.008 |
| ICU LOS, days, median (IQR) | 17 (9, 27) | 3 (3, 7) | 0.001 |
| Expired | 2 (18%) | 2 (4.9%) | 0.2 |
| Atrial fibrillation/flutter | 5 (45%) | 19 (46%) | > 0.9 |
| AKI | 8 (73%) | 13 (32%) | 0.019 |
| AKI with hemodialysis | 5 (100%) | 3 (50%) | 0.2 |
| Hyperbilirubinemia | 2 (18%) | 5 (12%) | 0.6 |
| Arrhythmia | 4 (36%) | 22 (54%) | 0.3 |
| Cardiac arrest | 0 (0%) | 4 (9.8%) | 0.6 |
| Cardiogenic shock | 9 (82%) | 26 (63%) | 0.3 |
| Chronic kidney insufficiency | 3 (27%) | 17 (41%) | 0.5 |
| Ischemic digits | 0 (0%) | 0 (0%) | |
| Myocardial infarction | 1 (9.1%) | 4 (9.8%) | > 0.9 |
| Strokes | 0 (0%) | 2 (4.9%) | > 0.9 |
| Thrombosis | 1 (9.1%) | 0 (0%) | 0.2 |
| Trach | 5 (45%) | 3 (7.3%) | 0.007 |
| Vent hours, median (IQR) | 65 (41, 350) | 16 (5, 31) | 0.012 |
- Note: Bold values represent p values which are statistically significant.
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, American Society of Anesthesiologists; AT II, angiotensin II; BMI, body mass index; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass time; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; LVEF, left ventricular ejection fraction; Min, minutes; NEE, norepinephrine equivalents; SOFA, sequential organ failure assessment.
- ∗Missing data for 24 cases.
- ∗∗Missing data for 22 cases.
- ∗∗∗Missing data for 5 cases.
| AT II n (%) | Non-AT II n (%) | |
|---|---|---|
| Total patients | 11 (21.2%) | 41 (71.8%) |
| Elixhauser comorbidities | ||
| AIDS | 0 (0%) | 0 (0%) |
| Alcohol abuse | 0 (0%) | 1 (2.4%) |
| Deficiency anemia | 4 (36%) | 20 (49%) |
| Rheumatoid arthritis/CVD | 1 (9.1%) | 0 (0%) |
| Blood loss anemia | 0 (0%) | 1 (2.4%) |
| Congestive heart failure | 9 (82%) | 22 (54%) |
| Chronic pulmonary dis. | 2 (18%) | 7 (17%) |
| Coagulopathy | 11 (100%) | 34 (83%) |
| Depression | 2 (18%) | 6 (15%) |
| Diabetes mellitus | ||
| Uncomplicated | 0 (0%) | 11 (27%) |
| Complicated | 2 (18%) | 15 (37%) |
| Drug abuse | 0 (0%) | 2 (4.9%) |
| Hypertension | 9 (82%) | 36 (88%) |
| Hypothyroidism | 2 (18%) | 9 (22%) |
| Liver disease | 0 (0%) | 5 (12%) |
| Lymphoma | 0 (0%) | 0 (0%) |
| Fluid and electrolyte dis. | 0 (0%) | 0 (0%) |
| Metastatic cancer | 1 (9.1%) | 2 (4.9%) |
| Other neurologic dis. | 0 (0%) | 2 (4.9%) |
| Obesity | 8 (73%) | 15 (37%) |
| Paralysis | 0 (0%) | 0 (0%) |
| Peripheral vascular dis. | 4 (36%) | 14 (34%) |
| Psychoses | 0 (0%) | 2 (4.9%) |
| Pulmonary circulation disorder | 9 (82%) | 21 (51%) |
| Renal failure | 7 (64%) | 20 (49%) |
| Solid tumor without metastasis | 1 (9.1%) | 3 (7.3%) |
| Peptic ulcer disease | 1 (9.1%) | 4 (9.8%) |
| Valvular disease | 11 (100%) | 31 (76%) |
| Weight loss | 3 (27%) | 4 (9.8%) |
- Abbreviations: AT II; angiotensin II; CVD, collagen vascular disease; NEE, norepinephrine equivalents.
| ICD version | ICD code | |
|---|---|---|
| Cardiac arrest (27) | ICD 10 | 197.x |
| Ischemic digits (14) | ICD 9 | 433.x and 434.x |
| Cardiogenic shock (15, 16) | ICD 10 | R570.x and 150.x |
| Myocardial infarction (16, 17) | ICD 10 | 121.x and 122.x |
| Atrial fibrillation/flutter (18, 19) | ICD 9 and ICD 10, respectively | 427.3x and I148.0-I148.9 |
| Arrhythmia (20) | ICD 9 and ICD 10, respectively | 427.x, I146.x, I147.x, I149.x, and R96.x |
| Acute kidney injury (13, 21, 22) | ICD 9 and ICD 10, respectively | 584.x and N17.x |
| Chronic kidney insufficiency (13, 23) | ICD 9 and ICD 10, respectively | 585.x and N18.x |
| Stroke (24, 25) | ICD 10 | 160.x, 161.x, 163.x, 164.x, and 165.x |
| Thrombosis (26) | ICD 10 | 174.x and 180.x |
- Abbreviation: ICD, international classification of diseases.
2.4. Statistical Analysis
Standard summary statistics were used to describe the cohort. Comparisons were made between the AT II and non-AT II patients using Chi-square and Wilcoxon rank-sum testing as appropriate. p < 0.05 was considered to be statistically significant.
3. Results
A total of 2929 cardiac surgical procedures were performed over the study period, of which 2103 were performed as the first procedure of a given hospitalization under CPB. Among these, 52 patients met our criteria for VS, of which 11 (21.2%) patients received AT II and 41 (78.8%) patients did not (Figure 1).
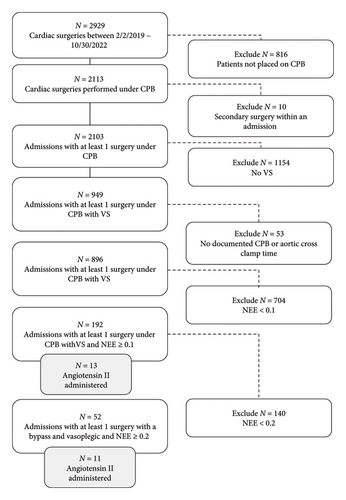
Epidemiology of AT II use (Tables 1 and 2).
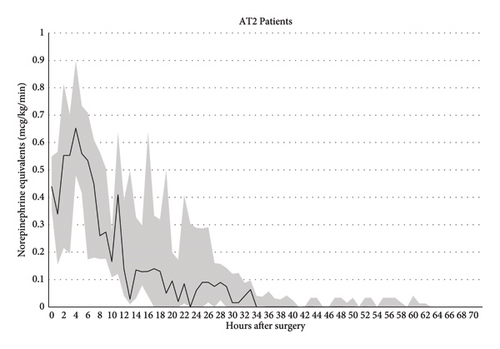
Among patients receiving AT II in the setting of VS, 7 (64%) were over 65 years in age, and 9 (82%) were male. The incidence of preoperative chronic renal insufficiency and diabetes mellitus in this group was 7 (64%) and 2 (18%) respectively. The AT II group was similar to the control group in terms age, gender, race/ethnicity, BMI, baseline creatinine, and most Elixhauser comorbidities. Five (45%) patients who received AT II were on preoperative ACEI or ARB. Although the use of ACEI or ARB was numerically higher in the AT II group, this was statistically comparable with the non-AT II control group (16 [39%], p = 0.7). 5 (45%) AT II patients had a low (< 40%) preoperative LVEF, which was comparable to the control group. The proportion of patients undergoing isolated CABG, isolated valve surgery, and combined CABG and valve surgery was also comparable between the groups. While the median CPB duration in patients receiving AT II was comparable to the non-AT II group, the aortic cross clamp duration exceeded 120 min in 6 (55%) patients (vs. 8 [20%]) in non-AT II patients, p = 0.05. The median aortic cross clamp duration was significantly higher in AT II patients (143 vs. 86 min, p = 0.004). The median maximum postoperative NEE dose within 24 h following surgery was higher in the AT II group: 0.44 mcg/kg/min (IQR 0.39, 0.57) versus 0.23 mcg/kg/min (IQR 0.21, 0.26, p < 0.001) (Figure 2).
The hospital mortality for patients receiving AT II (18%) was numerically higher; unfortunately, we were underpowered to detect statistical differences in mortality. The ClinCalc calculator returned a posthoc power value of 35%. This indicates that, given the observed proportions and sample sizes, our study had a 35% probability of detecting a statistically significant difference at the 0.05 alpha level. There was also no significant difference in the incidence of myocardial infarction, cardiac arrest, cardiogenic shock, arrhythmia episodes, stroke, thrombosis, and hyperbilirubinemia. Notably, the incidence of AKI was significantly higher in the AT II group (8 (73%) vs 13 (32%), p = 0.019) as was incidence of tracheostomy (5 (45%) vs 3 (7.3%), p = 0.007). The patients receiving AT II also spent a significantly longer time on invasive mechanical ventilation in the postoperative period and experienced significantly longer hospital and CVICU LOS.
Additional details on individual AT II cohort patients can be found in Table 4. All these patients received a maximum vasopressor dose (expressed in NEE) of greater than 0.3 mcg/kg/min. The total duration of vasopressor administration exceeded 23 h in all but one. Several of these patients had multiple procedures performed during surgery, associated with CPB time exceeding 120 min in all but two. Most patients also experienced complicated postoperative courses with events including respiratory failure, AKI needing continuous veno-venous hemodialysis, and atrial fibrillation, among others. Two patients developed heparin-induced thrombocytopenia, with one requiring above knee amputation for limb ischemia. Most of the AT II patients experienced prolonged periods of mechanical ventilation, as well as ICU and hospital LOS, pointing to the overall underlying illness. Among the two patients who died, one (patient 4) had acquired hospital acquired Coronavirus disease 2019 (COVID-19) pneumonia, and another (patient 5) suffered from urinary tract infection, pneumonia, and sepsis. The COVID-19 pneumonia patient developed COVID-19 in hospital. This patient subsequently had a prolonged hospitalization, developed multi organ system failure, and had a withdrawal of care performed and subsequently expired. The second patient became septic from a urinary tract infection and ventilator-associated pneumonia in the postoperative period. The patient had a prolonged hospitalization and was made DNR and a withdrawal of care and expired.
| Age | Sex | Surgery performed | CPB time (min) | Cross clamp time (min) | DHCA | Max NEE (mcg/kg/min) | Total pressure duration (h) | AT II max dose | Total AT II duration (h) | PRBC (units) | Vent (h) | ICU LOS (days) | Hospital LOS (days) | Postoperative course | Disposition/outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | M | MVr CABG | 137 | 115 | No | 0.577 | 25.5 | 20 | 20.5 | 5 | 528 | 27 | 28 | IABP placement and dialysis for AKI | LTAC |
| 2 | 73 | M | AVR, MVr, TVr, and LAAL | 251 | 173 | No | 0.369 | 23.15 | 40.089 | 19.5 | 3 | 65 | 5 | 8 | AKI and AF | Rehab |
| 3 | 54 | M | CABG | 152 | 79 | No | 0.444 | > 24 | 5 | 22 | 1 | 40 | 13 | 18 | Respiratory failure | Home |
| 4 | 73 | F | CABG | 126 | 107 | No | 0.509 | > 24 | 5 | 5.3 | 5 | 500 | 12 | 31 | COVID-19 pneumonia | Death |
| 5 | 76 | F | CABG, AVR, and LV aneurysm resection | 167 | 118 | No | 1.041 | > 72 | 80 | 47.5 | 10 | 464 | 19. | 23 | Respiratory failure and sepsis | Death |
| 6 | 69 | M | Ascending aorta replacement, AVR, MVR, TVr, LAAL, PFO closure, septal myectomy, and MAZE | 232 | 186 | Yes | 0.427 | 14.75 | 9.975 | 12.1 | 16 | 159 | 17 | 17 | Dialysis for AKI, respiratory failure, and major bleeding | Rehab |
| 7 | 60 | M | AVR, aortic root/ascending aorta replacement, reimplantation of coronaries, and MVr | 69 | 225 | No | 0.361 | > 24 | 5 | 27.5 | 5 | 42 | 5 | 8 | AF with RVR | Home |
| 8 | 45 | M | CABG, MVr, and PFO closure | 188 | 143 | No | 0.561 | > 24 | 70 | 20 | 8 | 236 | 36 | 40 | Respiratory failure, HITT, and fungal sepsis | Home |
| 9 | 67 | M | AVR, MVr, MAZE, LAAL, and ascending aorta replacement | 196 | 152 | No | 0.419 | 95.5 | 5 | 11.35 | 7 | 522 | 28 | 31 | Respiratory failure | Home |
| 10 | 67 | M | Redo AVR, aortic root, and ascending aorta replacement | 292 | 201 | No | 0.858 | 40 | 50 | 9.75 | 13 | 912 | 38 | 39 | Respiratory failure, dialysis for AKI, HITT, and limb ischemia | LTAC |
| 11 | 77 | M | MVR | 74 | 51 | No | 0.334 | 34.5 | 5 | 5.2 | 7 | 41 | 4 | 8 | Bleeding requiring chest washout | Home |
- Note: Italic cells indicate patients with death as hospital outcome.
- Abbreviations: AF, atrial fibrillation; AKI, acute kidney injury; ATII, angiotensin II; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; HITT, heparin-induced thrombocytopenia/thrombosis; IABP, intra-aortic balloon pump; ICU, intensive care unit; LAAL, left atrial appendage ligation; LOS, length of stay; LTAC: long-term acute care; LV, left ventricle; MVr: mitral valve repair; MVR, mitral valve replacement; NEE, norepinephrine equivalent; PRBC, packed red blood cells; TVr, tricuspid valve repair.
4. Discussion
This retrospective observational study describes our real world, academic institutional experience with AT II for management of postcardiac surgery-related VS. Given the overall paucity of published data, our study adds to the growing evidence base pertaining to AT II use in this specific context. We provide one of the largest studies of AT II use in postcardiac surgery patients and important outcomes associated with its use. We have also added further details on patients who received AT II (Table 4).
Overall, use of AT II in VS patients at our institution was uncommon, being administered in 11 out of a total of 52 VS patients who received vasopressor doses exceeding 0.2 mcg/kg/min (in NEE). Although the hospital mortality in the AT II population was numerically higher than the control group, we were underpowered to detect mortality differences. However, as noted, the postoperative course in the two AT II patients who died was complicated by COVID-19 pneumonia and sepsis, respectively, which may have disproportionately contributed to mortality. The patient with COVID-19 pneumonia acquired COVID-19 in the hospital. This patient subsequently had a prolonged hospitalization, developed multiorgan system failure, and had a withdrawal of care. The second AT II patient became septic from a urinary tract infection and ventilator-associated pneumonia in the postoperative period. The patient had a prolonged hospitalization and was made DNR and a withdrawal of care and expired.
The AT II group was, also, noted to have a higher incidence of adverse secondary outcomes (namely AKI, hospital and CVICU LOS, tracheostomy use, and ventilator hours). This association with worse secondary outcomes, in part, is potentially attributable to underlying differences in severity of chronic illness. Specifically, we could not adequately account for selection bias (who did versus did not receive AT II). Our AT II group had significantly higher median maximum NEE infusion rates, almost twice that of the non-AT II group (Figure 2), which may in part have been attributed to increased CPB and cross clamp durations. Longer CPB and aortic cross clamp durations, both of which occurred in patients subsequently administered AT II, are risk factors for VS [5]. Studies on animal models and humans have indeed demonstrated that production of inflammatory mediators and generation of NO are functions of time spent on CPB [3, 13, 14].
CPB contributes to vasoplegia through numerous mechanisms. These include systemic inflammatory response syndrome from exposure to the circuit, oxidative stress, ischemia, reperfusion syndrome, surgical trauma, gut endotoxin release, cell saver reinfusion, and hemolysis. Furthermore, CPB leads to low plasma vasopressin levels, adrenergic receptor desensitization, vascular smooth muscle cell hyperpolarization, sustained baroreceptor simulation with cytokine release, and alteration of endothelial glycocalyx with subsequent vasoplegia [15].
Additionally, angiotensin converting enzyme (ACE) is responsible for converting angiotensin I (AT I) to AT II. This enzyme is primarily found in the pulmonary capillary endothelium. During CPB, the pulmonary vasculature is bypassed, and it is possible that some of the postcardiopulmonary vasoplegia is due to ACE deficiency from bypass of the pulmonary vasculature [16]. Lack of AT II and increases in AT I ultimately lead to increased vasodilation which, in turn, lead to increased nitrogen oxygen synthase, vasodilation, and worsening shock [16]. Theoretically, the shock should improve with increased AT II and a shift away from a high renin state. As such, it would be expected (as shown in our results) that longer coronary bypass times are associated with worse outcomes and higher pressure requirements possibly due to more ACE deficiency in addition to the numerous other causes of vasoplegia from CPB [16].
The differences in outcomes between groups are likely confounding by indication. The patients in the AT II group had higher vasopressor requirements, increased comorbidities, and longer bypass and cross clamp times. These differences likely suggest a sicker baseline population. It is therefore likely that any worse outcomes we have seen in the AT II group are driven by worse underlying illness rather than the direct effect of AT II. And perhaps, without AT II administration, we would have seen even worse outcomes in these patients.
These significantly elevated vasopressor requirements likely prompted the critical care team to initiate AT II in the first place. The amount of vasoactive and inotropic pharmacological support has previously been shown to be an independent predictor of mortality, morbidity, and LOS in the cardiac surgical patient [17, 18]. While high vasoactive support following cardiac surgery may itself be a cause of poor outcomes, it also serves as a marker of severe underlying illness [18]. It is therefore conceivable that the patients receiving AT II had worse compromise of cardiovascular physiology at baseline or following surgery than patients who did not. AKI, a higher incidence of which was found in patients receiving AT II, is by itself an independent predictor of postcardiac surgery mortality [19, 20]. As such, patients in the AT II group would be expected to fare worse.
While our results are inconclusive with regards to causality, it is possible that patients in the AT II group would have performed much worse in the absence of the initiation of AT II. Indeed, a posthoc subgroup analysis of the postcardiac surgical patients in the ATHOS-3 trial, the initial randomized controlled trial allowing for Food and Drug Administration approval of AT II, demonstrated a rapid improvement in MAP following AT II initiation [10]. While we were unable to reliably obtain and compare data on MAP, we anecdotally experienced a relative vasopressor sparing effect with avoidance of further deterioration in MAP and hemodynamic parameters in our patient population receiving AT II. Whether AT II conferred any intrinsic survival benefit in this presumably sicker group of patients needs to be evaluated in studies with larger cohort size and better optimized matching.
Our study has several limitations. We initially used a CI > 2.0 L/kg/m2, SVR < 800 dyne/s/cm2, and a NEE of > 0.1 mcg/kg/min to define VS to capture all the AT II patients over the specified time period. We later changed this to a CI > 2.0 L/kg/m2, SVR < 800 dyne/s/cm2, and NEE cutoff to greater than 0.2 mcg/kg/min in order to better align with previously published criteria [21]. We lost two patients using this more stringent cutoff. We list the data from a NEE > 0.1 in Table 5. This change in selection criteria, however, did not significantly affect our findings.
| AT II | Non-AT II | p value | |
|---|---|---|---|
| Patients, n (%) | 13 | 179 | |
| Age, years, median (IQR) | 69 (60, 74) | 67 (59, 74) | |
| Age, ≥ 65 years, n (%) | 9 (69%) | 103 (58%) | |
| Female gender, n (%) | 3 (23%) | 54 (30%) | |
| Race/ethnicity, n (%) | |||
| Hispanic Black | 0 (0%) | 2 (1.1%) | |
| Hispanic White | 4 (31%) | 95 (53%) | |
| Non-Hispanic Black | 2 (15%) | 12 (6.7%) | |
| Non-Hispanic White | 7 (54%) | 50 (28%) | |
| Other/unknown | 0 (0%) | 20 (11%) | |
| Hospital LOS, days, median (IQR) | 18 (8, 31) | 7 (5, 10) | < 0.001 |
| ICULOS days, median (IQR) | 13 (5, 27) | 3 (1, 5) | < 0.001 |
| AT II duration, hours, median (IQR) | 19 (5.21) | NA | |
| Anticoagulant use, n (%) | 1 (7.7%) | 2 (1.1%) | |
| Prophylaxis use, n (%) | 0 (0%) | 0 (0%) | |
| BMI, median (IQR) | 30.3 (24.7, 35.0) | 28.1 (25.6, 30.9) | |
| Surgery type, n (%) | |||
| Aortic | 0 (0%) | 0 (0%) | |
| CABG only | 1 (7.7%) | 55 (31%) | |
| Valve only | 8 (62%) | 84 (47%) | |
| 2+ | 4 (31%) | 36 (20%) | |
| Neither | 0 (0%) | 4 (1.7%) | |
| CPB time, min, median (IQR) | 167 (126, 205) | 114 (85, 147) | 0.006 |
| CPB time > 180 min, n (%) | 6 (46%) | 26 (15%) | 0.01 |
| Aortic cross clamp time, min, median (IQR) | 143 (107, 175) | 81 (59, 107) | < 0.001 |
| Clamp time > 120 minutes, n (%) | 7 (54%) | 32 (18%) | 0.006 |
| Creatinine, mg/dL, median (IQR)∗ | 0.89 (0.84, 2.03) | 1.22 (0.96, 2.01) | |
| Max SOFA score, median (IQR)∗∗ | 10.0 (9.0, 13.0) | 7.0 (5.0, 10.0) | 0.02 |
| LVEF ≤ 40%∗∗∗ | 5 (38%) | 40 (27%) | |
| ACEI or ARB | 6 (46%) | 59 (33%) | |
| NEE, mcg/kg/min, median (IQR) | 0.42 (0.25, 0.54) | 0.14 (0.11, 0.20) | < 0.001 |
| ASA score, median (IQR) | 4.0 (4.0, 4.0) | 4.0 (4.0, 4.0) | |
| Outcomes | |||
| Expired, n (%) | 2 (15%) | 4 (2.2%) | 0.055 |
| Atrial fibrillation/flutter, n (%) | 6 (46%) | 74 (41%) | 0.7 |
| AKI, n (%) | 10 (77%) | 51 (28%) | < 0.001 |
| AKI with hemodialysis, n (%) | 5 (38%) | 10 (6%) | 0.12 |
| Hyperbilirubinemia, n (%) | 2 (15%) | 13 (7.3%) | |
| Arrhythmia, n (%) | 5 (38%) | 83 (46%) | 0.6 |
| Cardiac arrest, n (%) | 0 (0%) | 26 (15%) | 0.2 |
| Cardiogenic shock, n (%) | 10 (77%) | 88 (49%) | 0.053 |
| Chronic kidney insufficiency, n (%) | 3 (23%) | 47 (26%) | > 0.9 |
| Ischemic digits, n (%) | 0 (0%) | 1 (0.6%) | > 0.9 |
| Myocardial infarction, n (%) | 1 (7.7%) | 13 (7.3%) | > 0.9 |
| Stroke, n (%) | 0 (0%) | 7 (3.9%) | > 0.9 |
| Thrombosis, n (%) | 1 (7.7%) | 0 (0%) | 0.068 |
| Tracheostomy, n (%) | 5 (38%) | 8 (4.5%) | < 0.001 |
| Ventilator hours, median (IQR) | 42 (29, 236) | 4 (2, 11) | < 0.001 |
| Elixhauser comorbidities | |||
| AIDS, n (%) | 0 (0%) | 0 (0%) | |
| Alcohol abuse, n (%) | 0 (0%) | 3 (1.7%) | |
| Deficiency anemia, n (%) | 5 (38%) | 43 (24%) | |
| Rheumatoid arthritis/CVD, n (%) | 1 (7.7%) | 7 (3.9%) | |
| Blood loss anemia, n (%) | 0 (0%) | 3 (1.7%) | |
| Congestive heart failure, n (%) | 10 (77%) | 81 (45%) | |
| Chronic pulmonary disorder, n (%) | 2 (15%) | 39 (22%) | |
| Coagulopathy, n (%) | 13 (100%) | 151 (84%) | |
| Depression, n (%) | 2 (15%) | 18 (10%) | |
| Diabetes mellitus, n (%) | |||
| Uncomplicated | 0 (0%) | 36 (20%) | |
| Complicated | 3 (23%) | 57 (32%) | |
| Drug abuse, n (%) | 0 (0%) | 2 (1.1%) | |
| Hypertension, n (%) | 11 (85%) | 149 (83%) | |
| Hypothyroidism, n (%) | 2 (15%) | 34 (19%) | |
| Liver disease, n (%) | 1 (7.7%) | 18 (10%) | |
| Lymphoma, n (%) | 0 (0%) | 5 (2.8%) | |
| Fluid and electrolyte disorder, n (%) | 0 (0%) | 0 (0%) | |
| Metastatic cancer, n (%) | 1 (7.7%) | 3 (1.7%) | |
| Other neurological disorders, n (%) | 0 (0%) | 12 (6.7%) | |
| Obesity, n (%) | 10 (77%) | 60 (34%) | |
| Paralysis, n (%) | 0 (0%) | 3 (1.7%) | |
| Peripheral vascular disorder, n (%) | 5 (38%) | 46 (26%) | |
| Psychoses, n (%) | 0 (0%) | 4 (2.2%) | |
| Pulmonary circulation disorder, n (%) | 11 (85%) | 68 (38%) | |
| Renal failure, n (%) | 8 (62%) | 62 (35%) | |
| Solid tumor without metastasis, n (%) | 1 (7.7%) | 12 (6.7%) | |
| Peptic ulcer disease, n (%) | 1 (7.7%) | 10 (5.6%) | |
| Valvular disease, n (%) | 13 (100%) | 141 (79%) | |
| Weight loss, n (%) | 3 (23%) | 11 (6.1%) |
- Note: Bold p values are ones that are considered statistically significant.
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, American Society of Anesthesiologists; AT II, angiotensin II; BMI, body mass index; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass time; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; LVEF, left ventricular ejection fraction; Min, minutes; NEE, norepinephrine equivalents; SOFA, sequential organ failure assessment.
- ∗Missing data for 115 cases.
- ∗∗Missing data for 76 cases.
- ∗∗∗Missing data for 30 cases.
Our database query revealed missing data in several patients. The missing data included documentation of CPB and start/stop times and hemodynamic data preventing us from establishing the presence of VS. As such, this missing information resulted in exclusion of several patients from the analysis, reducing cohort size and potentially introducing bias. Out of a total of 2929 surgical encounters over our study period only, only 2103 were included, in part due to absent CPB time. Additionally, due to the retrospective nature of our study, we did not have routine renin levels on our patients; this would be potentially helpful to differentiate patients who would respond more favorably to AT II. Finally, our study reports on the use of AT II in our single center in Miami, Florida, where most cardiac surgeries are performed by a single surgeon and are minimally invasive in nature. It is unknown how externally generalizable our findings are.
5. Conclusion
In our retrospective chart review of patients undergoing cardiac surgery with CPB, AT II was used in 21.2% VS patients. Suboptimal outcomes in patients exposed to AT II may have been attributed to worse underlying illness and selection bias. We continue to advocate for the use of AT II as salvage therapy in cardiac surgical patients with persistent VS despite escalating doses of two vasopressors. Future research utilizing larger cohorts or randomized controlled trials will help to better elucidate the benefits and risks of AT II in this patient population.
Nomenclature
-
- AT II
-
- Angiotensin II
-
- AT I
-
- Angiotensin I
-
- CPB
-
- Cardiopulmonary bypass
-
- ACE
-
- Angiotensin converting enzyme
-
- CVICU
-
- Cardiovascular intensive care unit
-
- ICU
-
- Intensive care unit
-
- SVR
-
- Systemic vascular resistance
-
- CI
-
- Cardiac index
-
- CS
-
- Cardiac surgery
-
- US
-
- United states
-
- CAD
-
- Coronary artery disease
-
- CABG
-
- Coronary bypass graft surgery
-
- LIMA
-
- Left internal mamillary artery
-
- LAD
-
- Left anterior descending artery
-
- SVG
-
- Saphenous vein graft
-
- RPL
-
- Right posterior lateral artery
-
- OM
-
- Obtuse marginal artery
-
- VS
-
- Vasoplegic syndrome
-
- LOS
-
- Length of stay(s)
-
- COVID-19
-
- Coronavirus disease 2019
Ethics Statement
IRB approval was obtained.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors read and approved the final manuscript. All listed authors have substantially contributed to this article and the interpretation of the data. All listed authors have contributed to drafting this work and have approved the final version of this article. All authors are agreeable to be accountable for all aspects of this work and will investigate and resolve questions in regard to this.
Funding
No funding was received for this research.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author after obtaining a data usage agreement with the University of Miami.