Exploring Photocatalytic and Photoelectrochemical Applications of g-C3N4/Metal Sulfide Quantum Dots Nanocomposites: A Review of Recent Trends and Innovations
Abstract
Global environmental contamination will worsen, requiring innovative solutions based on cutting-edge research. Photocatalysis has advanced in recent years by using metal sulfide quantum dots (QDs), graphitic carbon nitride (g-CN), and their nanocomposite heterojunctions. This systematic study examined the latest developments in this revolutionary area. The article begins by describing metal sulfide QDs and the optical, electrical, and structural characteristics of metal sulfide QDs and g-CN. Appreciating the tremendous potential of these materials to solve environmental issues begins with these realizations. The major attraction, nanocomposite heterojunctions, was next examined. We examine the fascinating world of g-CN–metal sulfide QD composites, including their synthesis, cooperative behavior, and heterojunction topologies such as Type I, Type II, Zscheme, and S-scheme. These heterojunctions unlock the photocatalytic efficiency hitherto unreachable. We learn how these heterostructures boost photocatalytic efficiency, revealing their revolutionary potential. This review shows how these advances are used in real life. These materials have accelerated the shift toward a cleaner, sustainable future by degrading organic contaminants and converting CO2 into useful compounds.
1. Introduction
In the modern era of relentless industrialization, the need for technological advancement and economic growth is evident [1]. Our society has witnessed remarkable progress, significantly improving the quality of life by billions [2, 3]. However, this progress has come at a significant environmental cost, with industrialization leading to pervasive water and air pollution, posing grave threats to our planet’s ecological balance [4, 5]. In the face of these formidable challenges, photocatalysis has emerged as a beacon of hope, offering a potent and sustainable solution to combat environmental pollution and usher in a cleaner and more sustainable future. The concept of photocatalysis, at its essence, harnesses the power of light-absorbing materials to drive chemical reactions capable of breaking down noxious pollutants into harmless compounds [6]. This process embodies the essence of sustainable and environmentally friendly science as it taps into the boundless energy of sunlight, which is a clean and renewable resource. Over the years, scientists and researchers have explored an array of photocatalysts, each with unique attributes and potential, in a relentless pursuit of enhanced efficacy and adaptability [7]. g-CN is one of the 2D materials that have become important factors in the field of photocatalysis among these photocatalysts [5, 8, 9].
It has been an incredible journey for g-CN in the field of photocatalysis. Because of its special qualities, this adaptable 2D material has drawn interest from academics worldwide, making it an extremely attractive contender to address urgent environmental concerns [8, 10]. In this review, we thoroughly examine several precursors and preparation methods utilized in the synthesis of g-CN. Additionally, we investigated the basic properties that support its remarkable photocatalytic activity, which has recently garnered a great deal of interest. Despite its immense potential, g-CN is not without restrictions. One of the main issues that can lower its photocatalytic efficiency is the rapid recombination of electron–hole pairs generated during photosynthesis.
Integrating quantum dots (QDs) is one of the key strategies for improving the efficiency of g-CN photocatalysts [11]. Metal sulfide QDs are a subset of nanoscale semiconductor materials that have garnered significant attention owing to their unique electrical and optical properties, which are caused by size-related quantum confinement effects [12]. These QDs, which are composed of sulfur anions and metal cations, frequently from transition metals, are highly sought-after materials in a range of applications owing to their amazing optical properties and tunable bandgaps. Beyond the well-known cadmium sulfide (CdS), other examples of metal sulfide QDs include molybdenum disulfide (MoS2), zinc sulfide (ZnS), tin sulfide (SnS2), and silver sulfide (Ag2S). In addition, photocatalytic carbon dioxide (CO2) reduction is aided by metal sulfide QDs [13–15]. These QDs help to convert CO2 into useful hydrocarbons and oxygenates by harnessing photogenerated charge carriers. This has great potential to lower greenhouse gas emissions and use CO2 as a sustainable feedstock for chemical synthesis. All things considered, metal sulfide QDs have been named for their photocatalytic applications, proving their mettle in a variety of fields such as CO2 reduction, pollutant degradation, and hydrogen generation. They are essential cocatalysts in photocatalytic systems because of their adjustable characteristics and effective solar energy absorption, which has advanced the search for clean, sustainable energy sources and environmental remediation [16]. We expect more advancements and uses of metal sulfide QDs in photocatalysis as this field of study develops, helping to create a more sustainable and environmentally friendly future.
Even though g-CN and metal sulfide QDs are efficient, techniques must be devised to maximize solar light use and upconversion [17]. Fast charge recombination is the biggest issue in g-CN/metal sulfide nanocomposites. An efficient heterojunction process is needed to improve the charge transfer efficiency by lowering charge recombination [18]. In photoelectrochemical applications, research on the stability of g-CN/metal sulfide heterostructures has not been thoroughly explored. Therefore, it is essential to pay attention to both potential degradation processes and long-term stability [19].
Salunkhe et al. reported advancements in photocatalytic water treatment using g-CN-based metal oxide QD nanocomposites, which is mainly focused on pollutant degradation [20]. On the other hand, the present work highlights the special benefits of using g-CN in combination with metal sulfide QDs. Since earlier research primarily focused on metal oxide QDs, we investigate the distinct optical, electrical, and structural properties of metal sulfide QDs. Together with their strong absorption characteristics and effective charge carrier behavior, sulfide QDs’ bandgap adjusting ability improves their photocatalytic effectiveness in visible light.
Ren et al. described interfacial effect between transition metal sulfides (TMS) and g-CN heterostructures, but they have not focused on particular synthesis method for QDs or how their size affects TMS characteristics [21]. By offering thorough details on the essential physical characteristics of metal sulfide QDs, followed by insights into their binding mechanisms with g-CN and the ways heterostructures of various types (Type I, Type II, Z-scheme, and S-scheme) increase photoreaction rates, our analysis surpasses previous research in this field. Despite showing a number of heterojunction topologies, the Ren et al. article does not describe how these systems use QDs to promote pollutant degradation and CO2 conversion.
In this comprehensive analysis, we delved deeply into the function of metal sulfide QDs in photocatalysis to fill in the previously noted research gaps. We elucidate the fundamental properties that render these QDs’ formidable companions for effective environmental restoration. A new method for attaining the best photocatalytic performance in nanocomposites is the synergistic impact that g-CN and metal sulfide QDs create together. Through a comprehensive analysis of the most current scientific literature, we demonstrate the major advantages offered by this synergistic combination and provide insights into the underlying processes driving their higher photocatalytic activity. These insights not only point the way toward cleaner air and water, but also bring us one step closer to a sustainable future with greater awareness of the environment. Throughout this comprehensive review, we will highlight groundbreaking findings and innovative achievements that have profoundly altered the field of g-CN and metal sulfide QD nanocomposites for photocatalytic applications. These remarkable advancements have not only expanded our understanding of photocatalysis, but also provided viable solutions to the pressing worldwide issue of environmental pollution. By examining the performance of g-CN/metal sulfide QD heterostructures, which actively aid in environmental cleanup efforts by destroying organic pollutants and converting CO2 to energy conversion systems, this article bridges the research gap. The impact of material structure on band energy profiles and charge transport mechanisms is thoroughly examined in this work, which also outlines directions for further investigation.
2. g-CN Photocatalyst
Graphitic carbon nitride (g-CN) is a 2D carbon-based material that has garnered considerable attention in the field of photocatalysis. Like graphene, it is composed of carbon and nitrogen atoms organized in a hexagonal lattice. Because of its exceptional thermal resistance, chemical stability, and distinct electronic structure, it is a desirable photocatalytic material owing to a number of important characteristics [22]. For example, the large surface area of g-CN offers many active sites for photocatalytic processes [17, 23]. With a bandgap of typically 2.7 eV, the optical characteristics of g-CNs are essential to their photocatalytic activity because they enable them to absorb visible light and start photochemical reactions [24]. Figure 1 shows the various applications of g-CN in the field of photocatalysis.
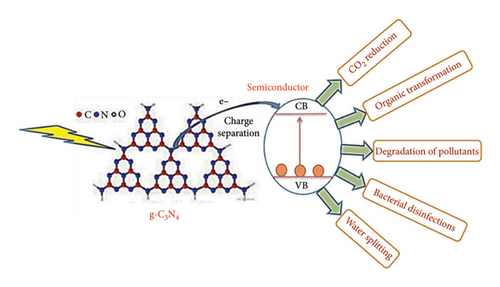
The visible light responsiveness, chemical stability, abundance of active sites, and high charge carrier mobility of g-CN are important features that make it an ideal photocatalyst for a variety of applications. Because of its capacity to absorb visible light, sunlight can be used for photocatalytic reactions in a practical manner. The durability of g-CN in photocatalytic applications is guaranteed by its resistance to chemical degradation, and its two-dimensional (2D) structure offers a multitude of active sites for reactant adsorption and effective photocatalysis. Furthermore, the high charge carrier mobility of g-CN makes it easier for photogenerated electron–hole pairs to separate and migrate, which is essential for effective photocatalysis [26]. Heat polycondensation of nitrogen-rich precursors is the process used to manufacture g-CN. For synthesis to have the required qualities, regulated conditions are usually used [25]. Temperature, temperature duration, and technique selection are important synthesis parameters that affect the g-CN morphology, bandgap, surface area, and ultimately its photocatalytic efficacy.
2.1. Melamine-based Synthesis of g-CN
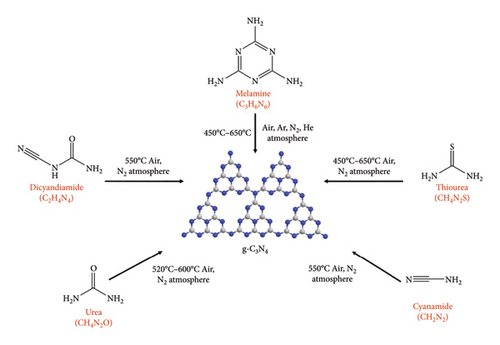
Melamine is the most widely used precursor because it is readily available and inexpensive. One popular technique is the thermal condensation of melamine in inert gas atmospheres, such as nitrogen, at moderate temperatures (approximately 550°C–600°C) for a few hours (usually 2–6 h) [27]. This process forms g-CN nanosheets (NSs) with a layered structure. This method is suitable for photocatalytic applications because of its relatively low synthesis temperature, which maintains the bandgap in the desired visible light range [28]. The large surface area of the resulting g-CNs facilitated efficient reactant adsorption and encouraged photocatalytic reactions.
2.2. Thiourea-based Synthesis of g-CN
Utilizing precursors such as dicyandiamide or thiourea, which can be heated to temperatures exceeding 600°C for up to 12 h, is another method for forming bulk g-CN [29]. Using this technique, g-CN with a more ordered structure and higher crystallinity is produced, which may have various bandgap characteristics. With this method, the bandgap may move into the UV range, but the material still has strong photocatalytic activity, which is beneficial for applications that require UV light [30].
2.3. Urea-based Synthesis of g-CN
Urea is a commonly used nitrogen-rich precursor that is both affordable and readily available for the synthesis of g-CN. The procedure entails the controlled thermal polymerization of urea at high temperatures. The precursor mixture is heated to temperatures in the range of 500°C–600°C (or higher) in a furnace or tubular reactor. Thermal polymerization of urea is started by this heating step [31]. The finished product is frequently allowed to anneal for a predetermined amount of time at high temperatures (usually a few hours to overnight). This process enabled the production of g-CN with enhanced crystallinity. The characteristics of g-CN can be greatly influenced by the catalyst or template selection, as well as the temperature and length of the synthesis process. After urea-based synthesis, the resultant g-CN usually has a layered structure and bandgap that is appropriate for absorbing visible light, making it a useful photocatalyst for a variety of applications [32]. The surface area and crystallinity of g-CN are also susceptible to changes in the synthesis conditions, which may influence the photocatalytic activity of the compound.
In summary, the production of g-CN involves the carefully regulated thermal polycondensation of nitrogen-rich precursors, primarily melamine. Figure 2 illustrates the optimized parameters such as temperature and annealing environment for the synthesis of g-CN using different precursors. Temperature, time, and precursor selection affect the morphology and bandgap of g-CN. These elements also affect the surface area and photocatalytic effectiveness of the compound. Whether visible light absorption or UV light sensitivity is required for a particular photocatalytic application, researchers can customize the synthesis parameters to create g-CN materials that are optimized.
Notwithstanding its encouraging characteristics, g-CN has many drawbacks that may affect its performance as a photocatalyst. A few significant challenges are the rapid recombination of electron–hole pairs generated during photosynthesis, limited absorption of UV light, and inefficient utilization of photogenerated charge carriers. These drawbacks may limit their effectiveness in specific photocatalytic processes. To overcome the limitations of g-CN, researchers have suggested a number of methods. These include metal/nonmetal doping, controlled morphology, bandgap engineering, heterostructure formation, and formation of dual heterojunctions. Doping with metal (gold/silver) or nonmetal (boron/sulfur) ions alters the electronic band structure of g-CN, resulting in superior light absorption characteristics and improved charge carrier separation. Controlled fabrication of 2D g-CN NSs with an enlarged surface area leads to minimization of the carrier transport distance, thus reducing the recombination rate and improving photocatalytic performance. During the synthesis of g-CN, the bandgap energy can be adjusted to increase light absorption in the visible light range. Combining g-CN with other semiconductors like metal oxides, metal sulfides, or reduced graphene oxide to form heterojunctions, which can greatly increase its photocatalytic performance by maintaining a strong redox ability while improving the charge separation efficiency. In addition, the formation of dual heterostructures by the codeposition of semiconductors will further improve the photocatalytic activity of g-CN NSs [33, 34]. It has been shown that the presence of other semiconductors has a major impact on the photocatalytic performance of g-CN in nanocomposites. The distinct qualities of each component, such as enhanced charge separation and higher light absorption, are utilized in this synergy. One example is the addition of metal sulfide QDs (MoS2 or CdS) to g-CN to boost its photocatalytic activity for water splitting and pollutant degradation [12]. When g-CN is combined with other semiconductors in heterojunctions, several components work together to produce synergy. Initially, the two materials’ aligned energy levels promote effective charge transfer at the interface, reducing electron–hole recombination. Second, the special qualities of the cocatalysts, such as their metal sulfide QDs, can improve the charge carrier mobility and increase the range of light absorption. Compared to either material alone, this combination produces a very effective system for photocatalytic processes, improving the performance. In summary, g-CN is a potential photocatalyst with special qualities that allow it to be used in a variety of ways. Researchers have suggested several changes and tactics to improve its performance despite its inherent constraints, such as the creation of nanocomposites with other semiconductors. By utilizing the complementary properties of g-CN and other materials, these nanocomposites produce heterojunctions with strong photocatalytic activity. As research in this area continues, we may expect more developments in the creation and use of g-CN-based photocatalysts for clean energy production and environmental remediation.
3. Metal Sulfide QDs
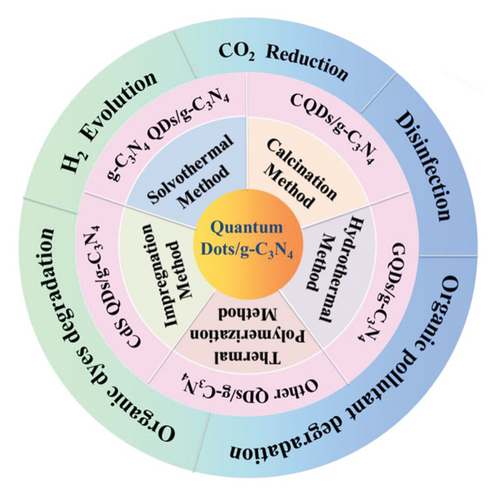
Metal sulfide semiconductors are a broad class of materials with intriguing electrical characteristics that render them useful for a number of applications, such as photocatalysis [35]. Metal sulfides are a class of photocatalysts that have drawn a lot of interest because of their huge specific surface areas, suitable band structure, and small bandgaps. These characteristics make them ideal for achieving rapid hydrogen evolution and superior photodegradation of organic pollutants in the presence of visible light. Typically, sulfur anions and metal cations constitute these complexes. Examples including silver sulfide (Ag2S), zinc sulfide (ZnS), cadmium sulfide (CdS), nickel disulfide (NiS2), cobalt sulfide (Co3S4), and molybdenum disulfide (MoS2). You et al. reported the fabrication of Ag2S QDs anchored g-CN heterostructures by self-assembly technique and observed a significant improvement in the H2 production rate [36]. According to Zahid et al., the RhB dye degradation efficiency of g-CN was significantly improved after the successful codeposition of ZnS and In2S3 QDs [37]. Khan et al. reported the solid-state synthesis of 0D/2D CdS/g-CN nanostructures and achieved superior photocatalytic performance over orange II dye and improved photocatalytic H2 generation under visible light illumination [38]. Li et al. obtained higher H2 evolution rate by the deposition of NiS2 QDs on g-CN NSs [23]. Yang et al. reported the highest H2 production rate of 20,536.4 µ mol g−1 h−1 by the deposition of Co3S4 QDs on g-CNNSs, which is 555 times higher than the bare g-CN NSs [39]. Azhar et al. reported that the bandgap of g-CN was significantly reduced from 2.8 to 2.2 eV by the deposition of MoS2 QDs, resulting in the increase in charge separation and charge transportation; thereby, photocatalytic RhB degradation efficiency was greatly improved [40]. The synthesis methods and applications of different metal sulfide QDs/g-CN nanocomposites are illustrated in Figure 3.
Their distinct electrical configurations make them attractive options for utilizing solar energy in photocatalytic processes [42]. Owing to quantum confinement phenomena, QDs are nanoscale semiconductor materials with size-dependent electrical and optical properties. High absorption and emission efficiency, photostability, tunable bandgaps, and the capacity to absorb and emit light at particular wavelengths are important properties. Owing to these characteristics, QDs are appealing for a variety of applications, including electronics and biological imaging [16]. A subclass of QDs made up of sulfur anions and metal cations is known as metal sulfide QDs. Their size and composition allow them to display distinctive characteristics. These characteristics include exceptional charge carrier mobility, strong photoluminescence quantum yields, and tunable bandgaps [13]. Metal sulfide QDs are multipurpose materials used in sensors, LEDs, photovoltaics, and photocatalysis [43].
The quantum confinement actions of metal sulfide QDs give rise to their optical characteristics. The size-dependent bandgaps of these nanocrystals allow for efficient light absorption and emission. All metal sulfide QDs have their own merits and challenges in the field of photocatalysis. For instance, MoS2 QDs show interesting optical features in the UV and visible spectra, whereas CdS QDs absorb and emit light in the visible range. Hence, MoS2, CdS, NiS2, and Co3S4 exhibit high photocatalytic efficiency under visible light. In particular, these QDs are very strong in H2 production via water splitting, but their low chemical stability and toxic nature are challenging factors. The nontoxic nature of ZnS and Ag2S QDs offers environment-friendly applications, but their lower photocatalytic performance under visible light limits their application potential. However, the visible and NIR light absorption capacities of Ag2S QDs make them suitable candidates for CO2 reduction and H2 production. Moreover, ZnS QDs exhibit excellent photocatalytic performance under UV light because of their wide bandgap. Hence, the selection of QDs for photocatalysis depends on the exact necessity of the purposes like light absorption capacity, long-term stability, environmentally friendly nature, cost-effectiveness, etc. New strategies need to be developed for the fabrication of novel materials to mitigate challenges such as improved stability, reduced toxicity, extended light absorption capacity, and enhanced charge separation to improve the overall performance of photocatalysts. Table 1 shows the advantages and disadvantages of various metal sulfide QDs for photocatalytic applications. Their ability to absorb and emit specific wavelengths of light is a crucial factor for their application, particularly in photocatalysis [12]. Metal sulfide QDs possess several characteristics that render them suitable for photocatalysis, such as high quantum efficiency for photogenerated charge carriers and tunable bandgap, allowing for efficient light absorption; surface engineering capabilities for enhanced catalytic activity; and compatibility with a wide range of photocatalytic reactions. Despite their advantages, metal sulfide QDs have limitations, such as limited stability under prolonged irradiation, challenges in controlling size and size distribution, potential toxicity concerns, and photocorrosion, especially with certain heavy metal-based QDs.
| MS QDs | Advantages | Disadvantages |
|---|---|---|
| Ag2S | Narrow bandgap, low toxic nature, and visible and NIR light absorption capacity | Low stability and limited photocatalytic efficiency |
| ZnS | High stability, wide bandgap, and efficient photocatalyst under UV light | Limited quantum efficiency, fast charge recombination, and lower visible light absorption capacity |
| CdS | High stability, tunable bandgap, great H2 evolution efficiency | Limited long-term stability, toxic nature, and photocorrosion |
| NiS2 | Cost-effectiveness, effective charge transfer, and high catalytic performance | Toxicity and limited light absorption capacity |
| Co3S4 | Excellent photocatalytic activity, presence of multiple active sites, better stability, and durability | Toxicity, expansive precursors, and challenging synthesis for controlled size and morphology |
| MoS2 | Narrow bandgap, effective charge separation, and outstanding photocatalytic performance under visible light | Photocorrosion, low stability, and complex synthesis |
The broad spectrum of light absorption and intrinsic photoelectric qualities of metal sulfides make them promising photocatalysts. Owing to strong carrier recombination and photocorrosion, metal sulfide photocatalysts have very poor chemical stability, which significantly reduces their photocatalytic activity [35]. When photogenerated holes oxidize surface sulfide ions (S2–) to create sulfate () or sulfur (S), metal sulfide compounds are prone to corrosion under light irradiation, which deactivates the photocatalysts. The following are the causes of photocorrosion in metal sulfide-based photocatalysts. First metal sulfide photocatalysts produce photoelectron–hole pairs when exposed to light, and the photogenerated holes spontaneously react with S2–. Soon after, the characteristics of metal sulfide photocatalysts deteriorated and their ability to catalyze reactions was impeded. Another reason is that metal sulfide photocatalysts exhibit a range of surface flaws. Under aerobic conditions, these flaws cause photoelectrons and holes to be trapped, which aids in the redox process. Inhibiting the photocorrosion of metal sulfide materials is necessary for photocatalytic processes, since the lifetime of photoelectron–hole pairs and chemical stability are criteria for highly effective photocatalysts [35]. Consequently, efforts have been undertaken to reduce carrier recombination and create a practical idea to lessen photocorrosion [44]. According to recent research, there are a number of ways to prevent photocorrosion, such as integrating metal sulfide photocatalysts into other layered oxides, mixing them with other ions or cocatalysts, and creating heterostructures. Heterojunction fabrication is thought to be the most successful way to improve the photocatalytic performance. P-N junction, Type I, Type II, Z-scheme, S-scheme, etc. are several types of heterostructures that have been designed and categorized so far. The distinctive staggered band structures between the two semiconductors in Type II heterostructures differ from those in the other heterostructures. This may greatly encourage the spatial separation of electrons and holes to slow their recombination [21].
Various strategies have been proposed to address these limitations, including surface passivation, ligand exchange, and doping with other elements. These modifications can improve the stability, control the size, and mitigate toxicity concerns, enhancing the overall photocatalytic performance of metal sulfide QDs. The combination of metal sulfide QDs with g-CN in nanocomposites has demonstrated synergistic effects on the photocatalytic performance [45]. These nanocomposites leverage the unique properties of each component to enhance the charge separation and utilization of solar energy, leading to improved photocatalytic activity. For example, MoS2 QDs and g-CN have demonstrated remarkable efficacy in the creation of hydrogen and the breakdown of pollutants. The complementary qualities of metal sulfide QDs and g-CN are the basis of their cooperative ability to create heterojunctions [46]. Metal sulfide QDs allow light to be absorbed in the ultraviolet spectrum, whereas g-CN is an effective absorber of visible light. When coupled, they constitute a powerful photocatalytic system that can achieve high photocatalytic performance while utilizing a wide range of light. To maximize the performance of these heterojunctions in a range of photocatalytic activities, their exact design is essential. In conclusion, there is a lot of promise in the field of photocatalysis with metal sulfide QDs owing to their special qualities and potential for synergy with materials such as g-CN. Their adaptability to different photocatalytic reactions and their tunable properties make them excellent options for producing clean energy and tackling environmental issues. The incorporation of metal sulfide QDs into sophisticated photocatalytic systems is anticipated to yield inventive and environmentally friendly solutions for a variety of applications as research proceeds.
4. Metal Sulfide QDs–g-CN Nanocomposites
To elucidate the mechanisms underlying the synergistic effects observed in g-CN/metal sulfide QD composites, details of the mechanisms of charge separation and light absorption enhancement and schematics that effectively illustrate these processes are proposed [5]. Band structure of various types of heterojunctions in a photocatalytic hybrid nanocomposite is depicted in Figure 4. Type II heterojunction formation: In g-C3N4/metal sulfide QD composites, a Type II heterojunction is often formed, where the conduction band of the metal sulfide QDs is higher than that of g-C3N4, and the valence band of the metal sulfide QDs is lower than that of g-C3N4. This band alignment facilitates the transfer of electrons from the conduction band of g-C3N4 to the metal sulfide QDs, while holes transfer in the opposite direction. This spatial separation of charge carriers across the interface of the two materials reduces recombination and increases the lifetime of these carriers, thereby enhancing photocatalytic activity. Mechanism of Extended Light Absorption: Broadening Absorption through Composite Action: The integration of metal sulfide QDs, which have size-dependent optical properties due to quantum confinement effects, with g-C3N4, extends the light absorption range of the composite. g-C3N4 absorbs visible light, while the metal sulfide QDs can absorb both UV and visible light, depending on their bandgap. This complementary absorption profile allows the composite to utilize a broader spectrum of solar radiation, thereby increasing the photocatalytic efficiency.
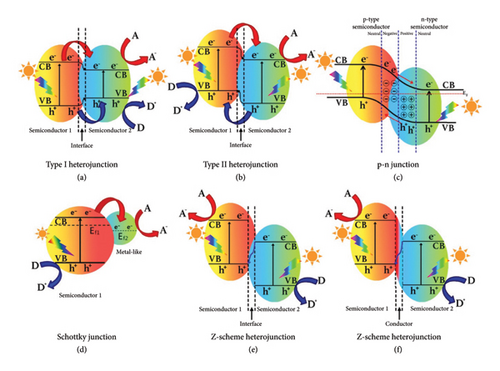
In particular, when exposed to visible light, the g-CN/CdS heterostructures demonstrated exceptional photocatalytic efficiency. Compared to g-CN (−1.1 and + 1.54 eV), the relative band edge potentials of the valence band (EVB) and conduction band (ECB) of CdS are more negative at +1.98 and −0.36 eV, respectively [47]. There is no question that this band orientation promotes Type II heterostructure production. When g-CN and CdS couple, Type II heterostructures with a lower charge recombination rate and a quicker charge transfer rate are formed. The holes (h+) from the CB of CdS migrate to the VB of g-CN at the same time that the excited electrons (e–) from the CB of g-CN move the CB of CdS. Superoxide radicals may readily form from the reduction of O2 molecules because the CB edge potential of CdS is more negative than that of O2/• (−0.3.3 eV). According to the aforementioned interpretations, a Type II heterostructure is formed, which increases the photocatalytic activity and decreases the recombination of the generated e––h+ pairs.
4.1. CdS QDs
One well-known semiconductor material with a direct bandgap is cadmium sulfide (CdS). Its superior optical and electrical qualities make it suitable for a number of applications, such as photocatalysis [48]. It is possible to create CdS in nanoscale forms, such as QDs, nanorods, and nanoparticles (NPs). Owing to their enormous surface area and quantum confinement effects, these nanomaterials offer special qualities that make them excellent choices for photocatalytic applications [49]. Nanoscale CdS particles with size-dependent quantum confinement phenomena are known as CdS QDs. These materials possess several advantages when used as photocatalysts. Tunable bandgap for efficient light absorption. The high surface area provides numerous active sites for catalytic reactions. High photostability ensures longevity during photocatalysis [50]. Efficient charge carrier generation and separation are crucial for photocatalytic processes. CdS QDs have achieved significant milestones in photocatalysis, including the degradation of organic pollutants, hydrogen production from water, and carbon dioxide reduction [51, 52]. They have been proven to exhibit strong photocatalytic activity in visible light, utilizing solar energy for the production of renewable energy and environmental remediation. However, CdS QDs also have limitations, such as rapid charge carrier recombination and potential toxicity concerns due to the presence of cadmium, which can hinder their photocatalytic performance and environmental acceptability. To overcome these limitations, researchers have explored strategies such as surface passivation, doping with other elements, and coupling with other semiconductor materials to enhance charge separation and mitigate toxicity concerns, thereby improving the photocatalytic performance of CdS QDs. Creating heterojunctions with other semiconductors is one such tactic [53]. By fusing the special qualities of CdS QDs with those of other materials, these heterojunctions produce beneficial effects that improve charge separation and photocatalytic activity [54, 55]. A promising combination for photocatalytic applications is the combination of the g-CN and CdS QDs. g-CN functions as a visible light absorber in conjunction with UV-responsive CdS QDs [56, 57]. This combination improves charge carrier separation and increases the range of light absorption, which improves the photocatalytic activity [58, 59]. Figure 5 depicts the morphological information of CdS/KPW/meso-g-C3N4 nanostructures.
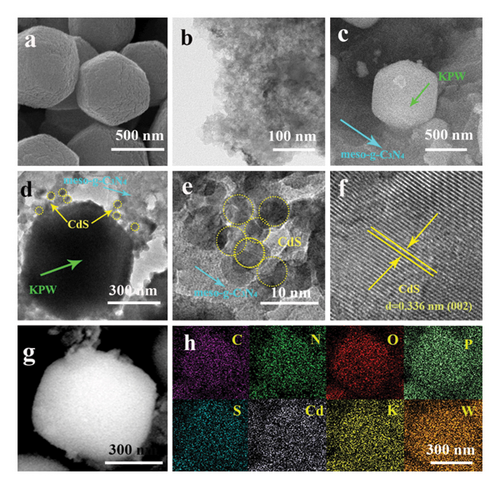
To enhance the photocatalytic activity of materials based on titanium dioxide, Zhao et al. created a hierarchical structure using CdS QDs, g-CN NS, and TiO2-x nanobelts. Ternary heterostructures were created in g-CN NS, TiO2-x nanobelts, and CdS QDs using a novel technique that combined thermal polymerization and chemical bath deposition with in situ reduction by NaBH4 [60]. The b-TiO2/C3N4/CdS samples demonstrated the most efficient photocatalytic H2 evolution rate of 254.8 mmol h−1. This rate was significantly higher than that of TiO2, g-CN, and CdS, being 45.5, 3.03, and 2.78 times higher, respectively. Additionally, the degradation rate of bisphenol A (BPA) for b-TiO2/C3N4/CdS was the highest, reaching up to 95%. The enhanced photocatalytic performance can be attributed to the efficient charge separation, suitable energy band position, and synergistic effect of the TiO2−x, g-CN, and CdS QDs. These factors collectively extend the photoresponse to the visible light region and promote the spatial separation of photogenerated charge carriers. This research successfully demonstrated the fabrication of TiO2-x nanobelts/g-CN NS/CdS QDs’ ternary heterojunctions, which showed remarkable photocatalytic performance. The hierarchical structure significantly enhanced the photocatalytic H2 evolution activity and degradation rate of BPA under visible light irradiation. Numerous recent studies have shown the potential of heterostructures for environmental cleanup and sustainable energy generation owing to their adjustable features, efficient charge separation, and synergistic effects.
4.2. Ag2S QDs
Ag2S, known as silver sulfide, is classified as a compound semiconductor within the realm of inorganic semiconductors. This material is categorized as a direct bandgap semiconductor owing to its ability to facilitate efficient electron–hole pair formation following photon absorption, as indicated by the energy gap between its valence and conduction bands. The application of photocatalysis is contingent on this characteristic. Ag2S exhibited an orthorhombic crystal structure. Sulfur (S) anions surround silver (Ag) cations in this structure [61]. The crystallinity of Ag2S, which varies according to the synthesis technique, is determined by its crystal structure. High crystallinity is preferred to enhance the charge carrier mobility and photocatalytic activity. The optical characteristics of Ag2S are essential for photocatalytic applications. The bandgap energy is adjustable, typically ranging from 0.9 to 1.2 eV. Ag2S can effectively absorb photons within the visible light spectrum owing to its bandgap [62]. Ag2S NPs are known as Ag2S QDs. Owing to their small size, these QDs have special qualities such as quantum confinement effects, which affect their optical and electrical properties [63].
Ag2S QDs serve as photocatalysts and offer several advantages: The light absorption characteristics of Ag2S QDs can be precisely controlled owing to their tunable bandgap. This enhances their absorption range and allows for flexibility in various photocatalytic activities. The large surface area, resulting from nanoscale size, increases the number of active sites available for catalysis. This characteristic enhances their photocatalytic effectiveness. Ag2S QDs demonstrate efficient charge separation, thereby inhibiting the premature recombination of photogenerated electrons and holes. This attribute is essential for the effectiveness of photocatalysis. Ag2S QDs have significantly enhanced the field of photocatalysis. These have been utilized for various purposes, including the reduction of carbon dioxide, the synthesis of hydrogen from water, and the degradation of organic contaminants. These accomplishments illustrate their capacity for clean energy generation and sustainable environmental restoration. Ag2S QDs offer multiple advantages; however, they also present certain limitations [64].
A notable limitation is the potential toxicity associated with the silver content. Surface passivation or encapsulation, which reduces the release of silver ions, can mitigate this issue. Moreover, enhancements in charge separation and stability can mitigate the limitations in their photocatalytic effectiveness. The formation of heterojunctions with alternative semiconductors represents a promising strategy for enhancing the photocatalytic activity of Ag2S QDs. The integration of the unique properties of Ag2S with other materials results in heterojunctions that improve charge separation and overall photocatalytic activity. The integration of Ag2S QDs with g-CN offers multiple advantages for photocatalytic applications. The light-absorbing capabilities of Ag2S QDs are improved through the efficient absorption of visible light by g-CN [65]. This combination enhances charge carrier separation, extends the range of light absorption, and boosts photocatalytic activity. Ag2S QDs and g-CN create a heterojunction that facilitates efficient charge transfer and solar energy utilization, positioning it as a promising candidate for diverse photocatalytic applications.
The researcher combined highly dispersed Ag2S QDs with the nanomaterial in a novel way to enhance the photocatalytic activity of g-CN NSs. Ag2S QDs were produced hydrothermally, and g-CN NSs were created by thermally polymerizing melamine [36]. The goal of this complex procedure was to produce a synergistic nanocomposite with enhanced photocatalytic properties. The weight percentage of Ag2S QDs put onto the g-CN NSs was adjusted, among other experimental conditions, in an effort to maximize the composite’s photocatalytic efficiency. The optimal weight % of Ag2S QDs on g-CN NSs was determined to be 3.0 weight percent after a thorough analysis of the data and a thorough debate. This composition’s exceptional performance in photocatalytic hydrogen evolution can be ascribed to its enhanced light absorption capacity and its ability to effectively separate photogenerated electron–hole pairs. The materials’ photocatalytic activity was significantly increased by purposely adjusting their bandgap to boost their absorption capacity of light. Furthermore, the incorporation of Ag2S QDs led to morphological changes in the g-CN NS, which in turn produced a 0D/2D heterojunction. This structural alteration significantly contributed to the augmented performance. Enhanced electrical properties of the g-CN NS, attributed to the incorporation of Ag2S QDs, facilitated the efficient separation of photogenerated carriers within the composite material. The mechanism underlying the remarkable enhancement in photocatalytic performance was elucidated, with a focus on the efficient separation of photogenerated electron–hole pairs at the 0D/2D heterojunction and the heightened light absorption capacity conferred by the composite material.
The researchers used Ag2S QDs’ catalytic potential as electron mediators in the Z-scheme heterojunction construction process in order to enhance the photocatalytic activity of SnS2/g-CN NS composites. The synthesis process unfolded in two distinctive steps. Initially, SnS2/g-CN NS composites were meticulously crafted employing a two-step hydrothermal method. Subsequently, the researchers embarked on the deposition of Ag2S QDs onto the composites, employing a successive ionic layer adsorption and reaction (SILAR) method [66]. Experimental variations came into play as the number of SILAR cycles during the deposition of Ag2S QDs was altered, providing insights into the influence of this parameter on photocatalytic efficiency. Figure 6 illustrates the synthesis of g-CN/Ag2S QDs’ heterojunction.
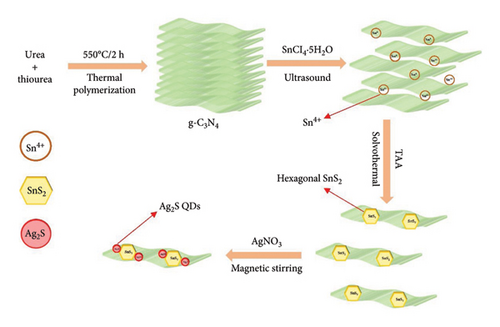
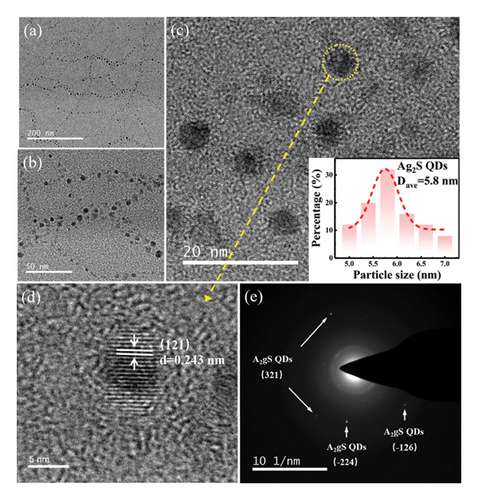
Figure 7 depicts the TEM and HTEM images of Ag2S QDs and the fast Fourier–transform (FFT) pattern of Ag2S QDs/g-CN. This ingenious structural arrangement facilitated an extraordinary efficiency in the separation of photogenerated charge carriers, marking a pivotal advancement in photocatalysis. The results of the study displayed the supremacy of the composite with 5 SILAR cycles, which achieved a staggering 98% degradation of RhB in just 40 min. This performance was 6.8 times better than that of pure g-CN. The phenomena were explained by the Z-scheme heterojunction’s perfect electron and hole separation caused by photogeneration. Notably, as reference points, the bandgap values for SnS2 (2.2 eV) and g-CN (2.7 eV) were given. The composites’ layered structure was revealed by morphological investigations, which showed that Ag2S QDs were evenly distributed throughout the surface of SnS2/g-CN NS. The underlying mechanism responsible for the superior performance of the composites could be traced back to the Z-scheme heterojunction. This configuration acted as a formidable force in ensuring the efficient separation of photogenerated electrons and holes, curtailing their recombination, and ultimately culminating in the remarkable enhancement of photocatalytic activity.
4.3. MoS2 QDs
Because molybdenum disulfide (MoS2) is categorized as a 2D semiconductor, its electrical characteristics are very different from those of conventional three-dimensional (3D) semiconductors. MoS2 is a member of the P63/mmc space group and has a unique hexagonal crystal shape known as the “2H” phase. This layered structure is made up of layers of molybdenum atoms stacked hexagonally between sulfur atoms, creating a special crystalline lattice [67]. A key element affecting MoS2 photocatalytic efficacy is its crystallinity. For effective charge carrier transport, high-quality MoS2 has a clearly defined 2H phase with few flaws and grain boundaries. It is possible to modify the lateral size and layer thickness of MoS2 to maximize its photocatalytic activity. The optical characteristics of MoS2 are essential to its photocatalytic uses. Because of its direct bandgap, photons are either absorbed or emitted during electron transitions between the valence and conduction bands. Depending on its thickness, MoS2 can have a direct bandgap in monolayer MoS2 or an indirect bandgap in bulk MoS2. Photocatalysis benefits from this direct bandgap property because it facilitates effective light absorption and charge production [68]. MoS2 QDs are nanoscale MoS2 particles with special characteristics. They are good candidates for photocatalysis and effective light absorbers because they inherit the direct bandgap of monolayer MoS2. Increased catalytic activity and quick charge transfer are made possible by their compact size and large surface area. MoS2 QDs have proven to be highly effective in photocatalytic applications, such as the reduction of CO2 and the breakdown of organic contaminants and hydrogen produced through water splitting [14]. Their efficacy in photocatalysis is attributed to their distinct optical and electrical characteristics. MoS2 QDs have drawbacks in terms of stability and charge recombination despite their encouraging characteristics. Surface functionalization, the creation of composites with different materials, and the creation of heterojunctions are methods to get around these obstacles. To improve photocatalytic activity, combining MoS2 QDs with other semiconductors through heterojunction creation is a potential strategy. MoS2 and another semiconductor can interact to provide certain photocatalytic processes and charge separation. For photocatalytic applications, g-CN and MoS2 QDs provide a number of benefits [69]. MoS2 can benefit from the cocatalyst role of g-CN, which increases charge transfer and overall photocatalytic efficiency [70]. Improved catalytic performance may result from the two materials working together harmoniously.
This study aimed to optimize the electrocatalytic hydrogen evolution reaction (HER) by incorporating MoS2 nanostructures into g-CN as nanoflakes (NS) and QDs. The synthesis process occurred systematically. Initially, bulk g-CN powder was synthesized from melamine powder at a temperature of 650°C. This powder was subsequently dispersed in ethanol, subjected to sonication, and centrifuged to produce the g-CN (NS/QD) suspension. MoS2 powder was combined with a water–ethanol solution, followed by stirring, sonication, and centrifugation, leading to the formation of the MoS2 suspension. This study primarily examined the deposition of the ersusposites, particularly g-CN (NS/QD) with MoS2 nanostructures, onto a flexible, conductive, 3D carbon cloth substrate [71]. This research uniquely employed a binder-free electrophoretic technique for the deposition of the ersusposites onto carbon cloth. This innovative method enabled accurate regulation of the deposition process. The analysis of results and subsequent discussions indicate that the electrode made from this ersusposites demonstrated a Tafel slope of 88 mV/dec and an overpotential of 0.28 V versus RHE at a current density of −2 mA/cm2. This performance exceeded that of g-CN in its unmodified state. The enhanced electrocatalytic performance can be ascribed to the synergistic effects of the MoS2 and g-CN nanostructures integrated into the 3D carbon cloth. The effects encompassed an expansion of surface area and an increase in the number of exposed active sites, which were crucial in catalyzing the HER. Morphological observations revealed that the carbon cloth substrate was successfully coated with the agglomerated form of g-CN (NS/QD). Elemental mapping analysis verified the presence of carbon, nitrogen, and oxygen, confirming the composition of the modified electrode. The integration of MoS2 nanostructures into the heterostructure enhanced charge transport, proton adsorption, and the kinetics of hydrogen evolution on the electrode. The enhancements resulted in improved electrocatalytic performance of the HER. The improved performance can be attributed to the synergistic effects of integrating MoS2 and g-CN nanostructures onto a 3D carbon fabric substrate. This combination led to an increased surface area and exposed active sites, facilitating charge transport, proton adsorption, and the HER.
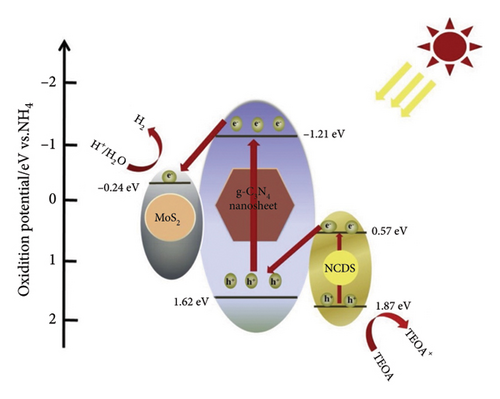
This study aimed to develop a novel photocatalyst by leveraging the properties of MoS2 QDs within a Z-scheme heterostructure composed of nitrogen-doped carbon dots and g-CN NSs [72]. This project aimed to enhance the efficiency of photocatalytic hydrogen evolution, an essential process in the conversion of renewable energy sources. The production method of this novel photocatalyst was not clearly outlined in the extracted information. Conversely, various techniques were not disclosed and involved the assembly of a composite material consisting of g-CN, NCDS, and MoS2 QDs. The proposed photocatalytic mechanism for hydrogen evolution over g-C3N4/NCDS/MoS2 under visible light irradiation is depicted in Figure 8. A systematic investigation was conducted to assess the hydrogen evolution activity through photocatalysis. A CEL-SPH2N photolysis water production system facilitated the execution of this experiment. The researchers systematically conducted their investigations using a specially designed glass vessel that functioned as the photocatalytic reactor. The experimental conditions were maintained consistently throughout the investigation to ensure precision and uniformity. The photocatalytic reaction occurred at a stable temperature, accompanied by a continuous flow of condensed water. Mechanical stirring was utilized to maintain uniform suspension of the photocatalyst in the reaction vessel. This research is innovative due to the integration of MoS2 QDs into a Z-scheme heterostructure, which includes graphitic carbon nitride NSs and nitrogen-doped carbon dots. This integration aimed to improve the photocatalytic hydrogen evolution process by utilizing the distinctive properties of MoS2 QDs. Photoelectrochemical measurements were performed using a standard three-electrode system.
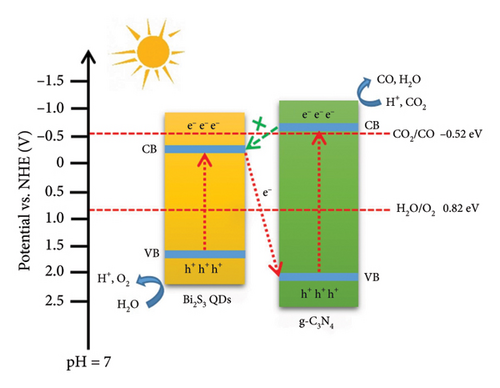
4.4. Bi2S3 QDs
Bi2S3 is a direct bandgap semiconductor with a rhombohedral crystal structure and belongs to space group D3d. It exhibits good crystallinity and structural properties. Bi2S3 possesses unique optical properties with a bandgap typically around 1.3 eV, making it suitable for visible light absorption. Its morphology can vary, often forming nanocrystals or QDs, which offer advantages as photocatalysts due to their high surface area. Achievements in using Bi2S3 QDs for photocatalysis include efficient solar-driven hydrogen generation [73]. However, certain limitations need to be addressed, namely the rapid recombination of electron–hole pairs. Performance can be improved by heterojunctions with other semiconductors, and photocatalytic applications can benefit from the combination of Bi2S3 QDs and g-CN [74]. Theoretical valence band and conduction band edge potentials suggest the formation of a Type II heterojunction, promoting charge separation. The synthesis of the Bi2S3 QDs/g-CN composite was executed through a well-structured two-step hydrothermal method. The process commenced with the preparation of g-CN, achieved by calcining melamine. The integrated composite was then formed by the synthesis moving on to the deposition of Bi2S3 QDs onto the surface of g-CN. Figure 9 illustrates the mechanism of the photocatalytic CO2 reduction of Bi2S3/g-CN nanocomposite. Bi2S3 QDs/g-CN composites underwent a methodical investigation of their photocatalytic ability, with a focus on how they responded to visible light irradiation [75]. This methodical approach was pivotal in assessing the potential of the composite in the desired CO2 reduction process. Variations in the amount of Bi2S3 QDs within the composite were introduced as experimental parameters. This variation aimed to optimize the photocatalytic performance, identifying the most efficacious composition. The novelty of this research lies in the introduction of Bi2S3 QDs into the g-CN matrix to create a Z-scheme composite. This innovative mechanism fosters efficient charge separation and mitigates electron–hole recombination, both of which are instrumental in elevating photocatalytic performance. In the course of the investigation, the Bi2S3 QDs/g-CN composites showcased a distinct enhancement in photocatalytic performance when juxtaposed with pristine g-CN [76]. Notably, the composite featuring 2% Bi2S3 QDs emerged as the most proficient catalyst, exhibiting the highest rate of CO production. Structural and optical alterations were observed upon the formation of the composite. The composite exhibited a more porous structure, which inherently facilitates the diffusion and adsorption of reactants. Additionally, the bandgap of the composite experienced a narrowing effect, rendering it more responsive to visible light wavelengths, a significant factor in its enhanced photocatalytic properties. The performance enhancement observed in the Bi2S3 QDs/g-CN composite was credited to the Z-mechanism established between Bi2S3 QDs and g-CN. This dynamic interaction played a pivotal role in promoting the efficient separation of photogenerated charge carriers and mitigating the recombination of electrons and holes.
4.5. NiS2 QDs
NiS2 is a semiconductor with an orthorhombic crystal structure, belonging to space group Pnma. It typically exhibits good crystallinity and structural properties. NiS2 has unique optical properties, characterized by a bandgap around 2.2 eV, enabling visible light absorption. Its morphology varies, often forming QDs with a high surface area. NiS2 QDs are advantageous as photocatalysts, showcasing achievements in hydrogen evolution and pollutant degradation [23]. Limitations such as limited visible light utilization can be overcome by heterojunctions with other semiconductors. Combining NiS2 QDs with g-CN enhances photocatalytic applications, forming a Type II heterojunction for efficient charge separation based on theoretical valence and conduction band edge potentials. In pursuit of enhancing the photocatalytic performance for carbon dioxide (CO2) reduction, the researchers embarked on a quest to augment the capabilities of g-CN by integrating 0D NiS2 QDs. The first step in the synthesis procedure was to create 0D NiS2 QDs using a hydrothermal technique. Following that, these QDs were carefully deposited onto 2D g-CN NSs by means of an easy in situ precipitation method [77]. Figure 10 shows the CO2 photoreduction mechanism using NiS2 QDs/g-CN heterostructures.
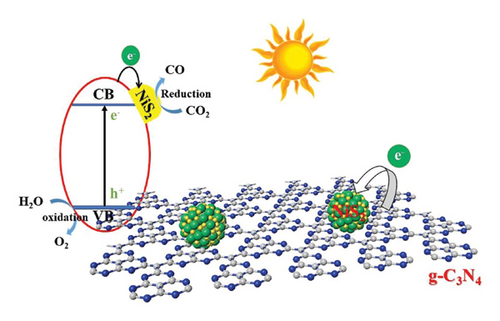
A thorough investigation was carried out to assess the newly formed composite’s photocatalytic CO2 reduction performance, especially in the presence of visible light. This methodical inquiry sought to shed light on the nanocomposite’s potential in the critical domain of CO2 reduction. The amount of NiS2 QDs put onto the g-CN substrate was one of the experimental parameters that the researchers changed in order to maximize the photocatalytic performance. The maximum efficiency was achieved when the weight ratio of NiS2 to g-CN was 2%. The novelty of this research endeavor resides in the inventive amalgamation of 0D NiS2 QDs onto 2D g-CN NS. This unique structural configuration played a pivotal role in augmenting charge separation and transfer dynamics, ultimately contributing to improved photocatalytic performance. The composite with a 2% weight ratio of NiS2 to g-CN had the highest rate of CO2 reduction, according to an analysis of the data. This rate was strikingly around 6.5 times higher than that of pure g-CN. Transmission electron microscopy (TEM) photos demonstrate that NiS2 QDs were successfully loaded onto g-CN NSs, resulting in morphological alterations. Furthermore, the bandgap of the composite was identified to be narrower than that of pure g-CN, rendering it considerably more responsive to visible light wavelengths, a pivotal factor in its enhanced photocatalytic performance. The mechanisms underlying the heightened performance of the composite can be ascribed to the synergistic interplay between g-CN and NiS2. This unique structural alliance fundamentally underpins efficient charge separation and transfer dynamics, thereby mitigating the recombination of photogenerated electron–hole pairs, a pivotal contributor to the observed enhancement in photocatalytic activity. In summation, the strategic modification of g-CN with 0D NiS2 QDs constitutes a significant advancement, dramatically enhancing its photocatalytic potential for CO2 reduction under visible light conditions. The composite, particularly the optimized version featuring a 2% weight ratio of NiS2 to g-CN, stands as a promising and potent avenue for the efficient reduction of CO2, holding significant implications for sustainable environmental remediation and energy conversion initiatives.
4.6. ZnIn2S4 QDs
ZnIn2S4 is a Type II semiconductor with a spinel crystal structure, commonly in space group Fd3m. It often exhibits excellent crystallinity and structural properties. Its optical properties are notable, featuring a bandgap of around 2.0–2.2 eV, allowing visible light absorption. Morphologically, ZnIn2S4 can take the form of QDs, offering a high surface area. These QDs serve as effective photocatalysts, with achievements in pollutant degradation and hydrogen production. Limitations like fast charge recombination can be addressed through heterojunctions with other semiconductors. Combining ZnIn2S4 QDs with g-CN enhances photocatalytic applications, forming a Type II heterojunction for efficient charge separation based on theoretical band edge potentials. In the realm of photocatalytic research, the primary objective of this study was to delve into the intriguing properties of ZnIn2S4/g-CN (ZIS/CN) composites, with a particular emphasis on their prowess in hydrogen production and tetracycline degradation. Figure 11 depicts the photocatalytic mechanism of degradation of tetracycline antibiotic in water using ZnIn2S4/g-CN nanocomposite.
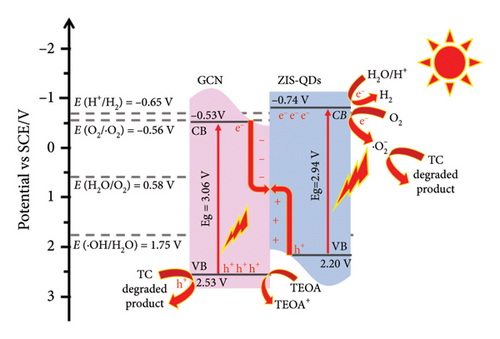
The synthesis process of these ZIS/CN composites was meticulously executed through an in situ growth approach, ensuring the precise integration of the constituent materials [78]. The novelty and innovation inherent in this research were notably underscored through the incorporation of nonmetallic polymer carbon nitride (CN) with inherent visible light responsiveness. This CN material, when seamlessly combined with ZnIn2S4 QDs, culminated in the creation of the ZIS/CN composite—a central subject of investigation in this study. Analysis of the results revealed a compelling enhancement in the photocatalytic activities exhibited by the ZIS/CN composite. The study introduced and emphasized the concept of an S-scheme within the composite structure, a key mechanism that effectively contributed to the augmentation of photocatalytic activities. While the results hinted at enhanced performance, specific details regarding changes in morphology and bandgap following the formation of the composite were not explicitly extracted from the provided snippets. Nevertheless, the positive impact of the S-scheme mechanism on photocatalytic activities was a focal point in the discussion. In conclusion, the ZIS/CN composite emerges as a promising avenue in the ongoing quest to develop highly efficient photocatalysts. Its demonstrated capabilities, particularly in the realms of hydrogen production and tetracycline degradation, hold great promise for addressing contemporary challenges in environmental and energy-related applications. The establishment of the S-scheme mechanism within this composite adds a layer of sophistication to its photocatalytic capabilities, making it a noteworthy contender in the realm of advanced materials for sustainable and environmentally conscious technology. The detailed analysis of recent literature on the photocatalytic degradation and H2 generation of metal sulfide QD-based g-CN nanocomposites was presented in Table 2.
| Photocatalyst | Bandgap | Pollutant | Dosage | Light source | Degradation efficiency | H2 generation (μmol.g−1.h−1) | Ref |
|---|---|---|---|---|---|---|---|
| CdS QDs | 2.45 eV | Cr | 10 ppm | Visible light | 46% in 150 min | [59] | |
| CdS QDs-g-CN-TiO2 | — | BPA | 10 ppm | Visible light | 95% in 120 min | 254 | [60] |
| SnS2 QDs/Ch | 2.5 eV | CV | 15 ppm | LED | 98% in 70 min | — | [15] |
| Bi2S3 QDs-BiNb | 2.02 eV | RhB | 5 ppm | Visible light | 99% in 90 min | — | [73] |
| ZnIn2S4 QDs-g-CN | 2.94 eV | TC | 40 ppm | Visible light | 55% in 120 min | 750 | [78] |
| g-CN | 2.75 eV | Cr | 10 ppm | Visible light | 25% in 150 min | — | [59] |
| Ag2S QDs–g-CN | 2.7 eV | — | — | Visible light | — | 471 | [36] |
| Ag2S QDs–g-CN-SnS2 | — | MO | 10 ppm | Visible light | 96% in 30 min | — | [66] |
| ZnIn2S4 QDs–g-CN-Bi2S3 | 1.21 eV | BPA | 10 ppm | Visible light | 98% in 210 min | — | [79] |
| ZnIn2S4 QDs–g-CN-Bi2S3 | 1.21 eV | — | — | Visible light | — | 663 | [79] |
| MoS2 QDs–g-CN-NCD’s | — | — | — | Visible light | — | 212 | [72] |
| NiS2 QDs–g-CN-AB | 2.72 eV | — | — | Visible light | — | 2434 | [23] |
| CdS NPs–g-CN | 2.24 eV | MB | 10 ppm | Visible light | 99% in 120 min | — | [58] |
| CdS NSs-g-CN | 2.24 eV | MB | 30 ppm | Visible light | 97% in 120 min | — | [53] |
| MoS2 NPs-g-CN | 2.26 eV | MB | 5 ppm | Sunlight | 95% in 80 min | — | [69] |
| MoS2 NPs-g-CN | 2.5 eV | MB | 5 ppm | Sunlight | 99% in 60 min | [70] | |
| SnS2 NPs-g-CN | 2.8 eV | MB | 10 ppm | Visible light | 96% in 180 min | — | [46] |
| SnS QDs-g-CN | — | MB | 20 ppm | LED | 99% in 60 min | — | [45] |
| ZnS NPs-g-CN | — | RhB | 10 ppm | Visible light | 99% in 60 min | — | [80] |
| Bi2S3 NPs-g-CN | — | RhB | 20 ppm | Visible light | 99% in 120 min | — | [76] |
| Bi2S3 NPs-g-CN | 2.75 eV | Cr | 20 ppm | Visible light | 97% in 180 min | — | [74] |
| NiS2 NPs-g-CN | 2.52 eV | TC | 20 ppm | Visible light | 65% in 120 min | 4025 | [43] |
4.7. ZnS QDs
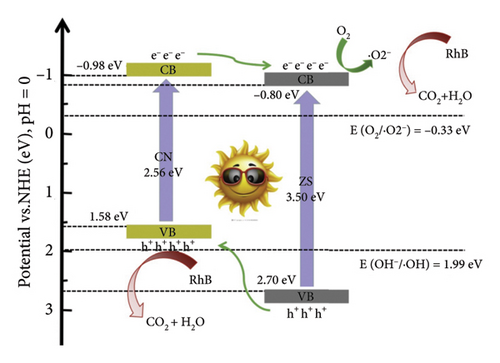
ZnS is a prominent II–VI semiconductor known for its zinc blend crystal structure and cubic symmetry, commonly in the space group F-43m. It exhibits good crystallinity and structural properties. The material possesses favorable optical properties with a bandgap around 3.6 eV, which corresponds to ultraviolet light absorption. Morphologically, ZnS can exist as QDs, providing a high surface area for photocatalytic applications. ZnS QDs have demonstrated advantages in photocatalysis, particularly in pollutant degradation and hydrogen production. To overcome limitations like rapid charge recombination, ZnS can be coupled with other semiconductors to form heterojunctions [80]. The charge separation and transfer in ZSCN–x during the photocatalytic degradation of RhB under full range (UV–vis) and only visible light irradiation is displayed in Figure 12. Combining ZnS QDs with g-CN enhances photocatalytic performance, creating a heterojunction based on theoretical band edge potentials [80] and graphitic carbon nitride (g-C3N4)-based photocatalysts [72]. While the specific aim of the researchers is not explicitly articulated in the extracted snippets, it can be inferred that their overarching objective revolved around the enhancement of photocatalytic performance for certain materials. This inference serves as a backdrop for understanding the subsequent elements of their study. The synthesis process employed to achieve their research goals involved the creation of a ZnS/Bi2S3/meso-g-CN composite heterojunction photocatalyst [79]. This intricate composite was meticulously prepared through a combination of hydrothermal and template calcination strategies, showcasing the researchers’ commitment to the precision of their experimental methods. The primary focus of their methodical inquiry was to assess the photocatalytic activity of the composite materials they had created. The breakdown of the organic contaminant BPA under visible light irradiation was the primary focus of this research. In their pursuit of comprehensive understanding, the researchers embarked on a dark reaction, observing the degradation rate of BPA for each catalyst over a 30-min duration. A key novelty introduced in their research was the ZnS/Bi2S3/meso-g-CN composite heterojunction photocatalyst, which demonstrated superior photocatalytic hydrogen (H2) production. This innovative concept signifies the potential of this composite in addressing environmental challenges and energy-related applications. The analysis of results and subsequent discussion unveiled that the ZnS/Bi2S3/meso-g-CN photocatalyst exhibited the highest degradation rate in the context of BPA degradation experiments. This enhancement in photocatalytic activity was attributed to the formation of a ternary heterogeneous structure within the composite. This intricate structure played a pivotal role in promoting effective charge separation and extending the photoresponse capabilities. Additionally, the mesoporous structure of the composite contributed by providing an increased number of surface-active sites, enhancing overall performance. However, specific details regarding changes in morphology and bandgap following the formation of the composite were not explicitly detailed in the extracted snippets. These aspects, if elucidated, could provide deeper insights into the underlying mechanisms of the photocatalytic processes. The researchers also presented a schematic diagram outlining the possible photogenerated charge transfer processes and the conceivable photocatalytic mechanisms governing the ZnS/Bi2S3/meso-g-CN heterojunction photocatalyst. These processes could include critical ones that are related to the creation of electron–hole pairs, the synthesis of hydrogen (H2), and the breakdown of BPA into carbon dioxide (CO2) and water (H2O). In conclusion, this study led to the development of a novel and highly efficient ZnS/Bi2S3/meso-g-CN composite heterojunction photocatalyst. Enhanced photocatalytic hydrogen production and the highest degradation rate in BPA degradation assays were two of this upgraded photocatalyst’s noteworthy features. The improved photocatalytic performance was determined to be caused by the complex ternary heterogeneous structure, the photothermal effect, and the mesoporous structure. These results demonstrate the composite material’s exciting potential in addressing contemporary problems related to renewable energy production and environmental sustainability. The other kind material available in the literature provides good activity [81, 82].
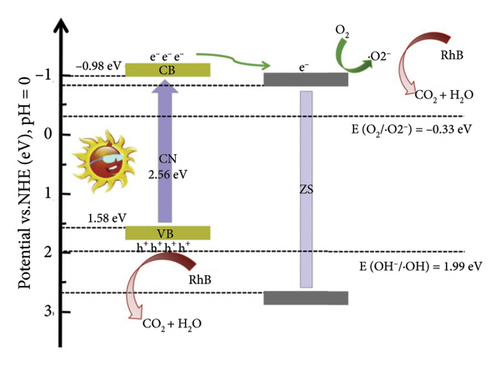
The interaction between these two materials significantly enhances the photocatalytic efficiency of the composites, primarily through improved charge carrier dynamics and broadened light absorption capabilities.
4.7.1. Enhanced Charge Separation
The integration of metal sulfide QDs with g-CN has been demonstrated to effectively extend the lifetime of photogenerated charge carriers. This is achieved through the formation of heterojunctions, which facilitate the spatial separation of electrons and holes. For example, in our study, we have reviewed the incorporation of CdS QDs with g-CN, where CdS acts as a photosensitizer. This arrangement helps in the efficient transfer of electrons from CdS to g-CN, thereby reducing electron–hole recombination and increasing the photocatalytic activity under visible light. Studies have shown that such heterojunctions can improve hydrogen evolution rates and degrade pollutants more effectively compared to single-component systems.
4.7.2. Extended Light Absorption
Metal sulfide QDs are known for their strong light absorption due to quantum confinement effects. When combined with g-CN, which primarily absorbs visible light, the resulting composite material can harness a broader spectrum of solar light. For instance, MoS2 QDs, when coupled with g-CN, extend the light absorption into the UV range, complementing the visible light absorption of g-CN. This expanded light absorption capability enables the activation of the photocatalyst under a wider range of light wavelengths, thus enhancing the solar utilization efficiency.
- •
Ag2S/g-CN composites: Recent research has highlighted the role of Ag2S QDs in improving the photocatalytic hydrogen evolution on g-CN NSs. The studies demonstrated that Ag2S QDs could effectively transfer electrons, enhancing the separation of charge carriers and increasing the hydrogen evolution rate significantly.
- •
ZnS and In2S3 codeposited on g-CN: Another study showed that the codeposition of ZnS and In2S3 QDs on g-CN leads to a dramatic improvement in the degradation efficiency of rhodamine B dye, attributed to the effective charge carrier separation and enhanced light absorption capabilities of the composite.
These examples illustrate the dynamic interactions between g-CN and metal sulfide QDs, which not only enhance photocatalytic activities but also open new avenues for designing efficient photocatalytic systems for environmental remediation and energy applications. We believe that these insights substantiate the potential of g-CN/metal sulfide QD composites in advancing the field of photocatalysis.
5. Conclusions
- (i)
Adaptable Photocatalytic Performers: In the field of photocatalysis, g-CN/metal sulfide QD nanocomposites have shown to be adaptable. By combining the special qualities of metal sulfide QDs and g-CN to improve charge separation and transfer, they have a synergistic impact that boosts photocatalytic activity.
- (ii)
Reducing Environmental Pollution: These nanocomposites’ photocatalytic uses offer great potential for reducing pollution in the environment. These substances provide long-term solutions for a healthier and more environmentally friendly environment, from the breakdown of organic contaminants to the conversion of CO2 into useful fuels.
- (iii)
Effective Energy Conversion: g-CN/metal sulfide QD nanocomposites have shown tremendous promise in the realm of energy conversion, going beyond environmental remediation. Their photoelectrochemical uses and capacity to capture solar radiation for the creation of hydrogen create new opportunities for renewable energy technology.
- (iv)
Special Heterojunctions: One of the main factors influencing improved photocatalytic activity is the emergence of heterojunctions between g-CN and metal sulfide QDs. To improve material qualities and increase efficiency, researchers need to keep delving deeper into the subtleties of these heterojunctions.
- (v)
Opportunities and Difficulties: Even though there has been a lot of improvement, issues with cost-effectiveness, stability, and scalability still exist. To overcome these obstacles, researchers ought to concentrate on creating scalable synthesis techniques and investigating cutting-edge composite structures.
6. Future Perspectives
- (i)
Tailored Material Design: Further studies should focus on the engineering and design of these nanocomposites, modifying their characteristics to suit certain uses. Through the adjustment of variables like surface area, shape, and bandgap, scientists can explore new avenues.
- (ii)
Scaling Up for Real-world Impact: Scientists need to concentrate on increasing the manufacturing of g-CN/metal sulfide QD nanocomposites in order to have a noticeable influence on tackling global issues. Large-scale, economical, and sustainable synthesis techniques are crucial.
- (iii)
Multidisciplinary Cooperation: It is essential for researchers to collaborate across disciplines, such as chemistry, materials science, physics, and engineering. Gaining a diverse range of skills can help us better understand and optimize these nanocomposites.
- (iv)
Beyond Laboratory Bench: It is critical to move beyond lab testing to practical implementations. Scholars ought to focus on converting their discoveries into workable solutions, encouraging creativity in sectors like solar fuel manufacturing, energy storage, and wastewater treatment.
- (v)
Effects on the Environment and Economy: It is crucial to consider the effects on the environment and economy of g-CN/metal sulfide QD nanocomposites. For these materials to be widely adopted, it will be important to evaluate their life cycle and sustainability.
In summary, there is great excitement and promise for the exploration of g-CN/metal sulfide QD nanocomposites in photocatalytic and photoelectrochemical applications. This study area’s interdisciplinary character, along with creative problem-solving and teamwork, has the potential to produce game-changing innovations that help create a more sustainable and clean future. It is encouraged for researchers to take the lead and push the limits of creativity and knowledge in this rapidly evolving sector.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Tejaswi Tanaji Salunkhe: conceptualization, methodology, writing – original draft and review and editing; Thirumala Rao Gurugubelli: conceptualization, methodology, writing – original draft and review and editing; Balaga Viswanadham: formal analysis, data curation, supervision, writing – review and editing; Bathula Babu: formal analysis, data curation, resources, and review and editing; T. V. M. Sreekanth: data curation, supervision, resources, and writing – review and editing; Kisoo Yoo: formal analysis, data curation, resources, and review and editing.
Funding
No funds were received.
Acknowledgments
The authors thank to GMR Institute of Technology, Rajam and SR University, Warangal, India.
Supporting Information
The graphical representation of present work is given in scheme 1. The fascinating world of g-CN–metal sulfide QD composites, including their synthesis, cooperative behavior, and heterojunction topologies such as Type I, Type II, Z-scheme, and S-scheme, and heterostructures enhance photocatalytic and photochemical efficiency.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




