Evaluation of Antiallergic Conjunctivitis Effects of Extract of Nelumbo nucifera Rhizome and Its Isolated Constituents Against Chemical-Induced Allergic Conjunctivitis in Experimental Rats
Abstract
Allergic conjunctivitis, an inflammation of the conjunctiva, is commonly treated with conventional drugs such as mast cell stabilizers, corticosteroids, antihistamines, NSAIDs, and others; these drugs associated lots of side effects including dryness, redness, and blurred vision. Nelumbo nucifera Gaertn., known as Indian lotus, is traditionally used for various ailments. This study aimed at assessing the antiallergic conjunctivitis effects of the hydroalcoholic extract of the rhizome of N. nucifera (HNN) and their isolated constituents (phytosphingosine and betulinic acid) in rats. Acute oral toxicity tests followed OECD Guideline No. 423, revealing no mortality up to 2000 mg/kg for HNN and 300 mg/kg for isolated constituents, indicating their safety. Rats were induced with allergic conjunctivitis and treated with varying doses of HNN extract and isolated constituents. The results showed a significant reduction in allergic signs, eye-scratching behavior, and eosinophil count in conjunctival tissues in a dose-dependent manner. This demonstrates the potential of HNN and its constituents in combating allergic conjunctivitis safely and effectively. Further research is warranted to explore their clinical applications.
1. Introduction
Allergic conjunctivitis manifests as inflammation of the conjunctiva, a delicate membrane enveloping the eye’s white portion and the inner eyelid surface. It arises from the immune system’s reaction to various allergens such as pollen, dust mites, pet dander, or specific molds [1]. Common symptoms encompass eye redness, itching, excessive tearing, swelling, and a sensation of burning in the eyes [2]. This condition can be seasonal, coinciding with periods of heightened allergen presence, or perennial, persisting throughout the year due to continuous exposure to indoor allergens. The main objectives of treating allergic conjunctivitis are to reduce and manage symptoms and enhance the standard of living [3]. This included lowering itching and minimizing redness, tears, conjunctival or eyelid edema, and other correlated conditions. Among individuals with long-term exposure to allergens and persistent illness, another goal of therapy is to break the pattern of inflammation and minimize it [1]. Some conventional drugs used in allergic conjunctivitis are mast cell stabilizers, corticosteroids, antihistamines, nonsteroidal anti-inflammatory drugs (NSAIDs), dual-action antiallergic drugs, antileukotrienes, anti-immunoglobulin-E (IgE), etc. [2]. These drugs are associated with various side effects such as dryness, redness, eye irritation, swelling, itching, glaucoma, cataract, blurred vision, pupil dilation increased intraocular pressure, gastrointestinal disturbance, and flu-like symptoms.
Medicinal plants are the courses of a few expected medications, and they hold better and more innocuous substitute for manufactured medicines [3]. Various parts of the plants, for example, leaf, root, stem, organic product, seed, and bark, are utilized to get several phytochemical constituents that produce specific pharmacological action [4–6]. Nelumbo nucifera Gaertn. is a plant found on the flood surface of water, related to Nelumbonaceae family. This plant is also called lotus [7]. It is also India’s floral emblem; the plant is widely distributed in central and northern India [8]. Various parts of the lotus plant such as rhizome, leaf, and flower as well as seed are generally utilized for the treatment of spermatorrhea, pharyngopathy, skin disorders, diarrhea, smallpox, coughing, hemoptysis, hematemesis, metrorrhagia, hematuria, flu, dysentery, hepatopathy, and hyperlipidemia, etc. It is effective as an anthelmintic, antiemetic, and diuretic, and in the therapy of strangury and dermatitis in the Ayurvedic medical system [9]. In renowned medication, it is utilized in the treatment of tissue irritation, malignant growth, leprosy, and skin disease, and as a toxic substance counteract [10].
Some biochemically active compounds that are used for therapeutic action are confined to the rhizome, leaf, kernel, and blossom, such as steroids, alkaloids, flavonoids, triterpenoids, polyphenols, and glycosides [11, 12]. Research on various parts of N. nucifera has shown some pharmacological action such as anticancer, antiviral, anti-ischemic, antioxidant, antidiarrheal, lipolytic, antiobesity, antipyretic, hypocholesterolemia, anti-inflammatory, hypoglycemic, antifungal, hepatoprotective, antibacterial, and diuretic actions [8]. Based on the literature survey, it was found that there is no study related to the conjunctivitis study. Therefore, the current study was planned to evaluate antiallergic conjunctivitis effects of the hydroalcoholic extract of N. nucifera (HNN) and its isolated constituents against chemical-induced allergic in experimental rats.
2. Materials and Methods
2.1. Drugs and Chemicals
The following reagents were indicated: egg albumin (SD Fine Chem, Mumbai), aluminum hydroxide (CDH Ltd, New Delhi), Bordetella pertussis-inactive microorganism suspension (Sigma-Aldrich), cetirizine hydrochloride (GSK Ltd, Baddi), sterile water for injection (Nirlife Healthcare, Mumbai), carboxy-methyl cellulose (SD Fine Chem, Mumbai), ether (CDH Ltd, New Delhi), and formaldehyde (CDH Ltd, New Delhi), provided from IFTM University Moradabad.
2.2. Identification, Collection, and Authentication of Plant Materials
The plant material, rhizome of N. nucifera, was taxonomically identified, gathered, and authenticated by the Ayurveda department Banaras Hindu University. The collected part of the plant was dried in the shade at normal temperature for 15 days. Then, the air-dried portion of the plant was reduced to a coarse powder [13].
2.3. Extraction of Plant Materials
The dried powder of the plant was passed through a 20-mesh sieve and then subjected to extraction with 70% ethanol (as the hydroalcoholic solvent gives the highest yield and is suitable for the extraction of phenols and flavonoids). The extraction (Maceration) was done with 70% ethanol in water at room temperature for 14 days to extract the plant material to be used. The solution was vaporized at lower pressure to get a semisolid material, which was then vacuumed and desiccated to find solid residues of HNN. The powdered extracts were kept in a sealed container until they were required [13].
2.4. Extractive Value
2.5. Loss on Drying
2.6. Total Ash Value
2.7. Preliminary Phytochemical Screening
The prepared extract of N. nucifera rhizome was treated with various analytical examinations for the determination of phytoconstituents present in extract, including carbohydrates, glycosides, alkaloids, protein, fat, amino acids, tannins, flavonoids, and steroids [14].
2.8. Isolation of Phytoconstituents
Column chromatography is one of the best techniques for separating and purifying all solids and liquids while doing a small-scale test. Column chromatography is widely used to isolate the component of a complex mixture. Column chromatography can also be used to estimate the number of different compounds in the combination.
A glass column 45 cm long, 2.5 cm diameter was used to separate constituents. A wet packing method was adopted for packing the column. A solution of stimulated silica gel was made in a solvent solution and poured into the column with a hollow glass cylinder. The column was then tapped with a rubber cork; 50 g of extract was taken with 100 g of silica gel for column chromatography (60–120 mesh) and mixed in a small quantity of solvent, making a homogenous slurry of this mixture by using a mortar. The mixture of the extract was fed very gradually through the column with the help of a glass chamber so as not to disturb the silica bed. The constituents were isolated using a suitable solvent system. The extract was thus placed in the column and allowed to be eluted with the solvent system of chloroform and methanol in different ratios. The fractions were collected from the column and the TLC was performed on the collected fractions; the fractions with similar TLC patterns were combined to yield a single fraction. Eight different fractions were gathered and dried [15].
2.9. Characterization of the Fractions F3 and F5
2.9.1. TLC Analysis
Each sample obtained from column chromatography was examined through TLC analysis. Each fraction was applied on a TLC plate and developed in a presaturated TLC development chamber with a solvent system of toluene: ethyl acetate and glacial acetic acid (5:4:1 v/v/v) at room temperature (25°C); the TLC plates were developed up to 80 mm. Following intensive drying, the spots on the prepared plates were seen using a short-wavelength UV light, and the images were captured for record purposes [16].
2.9.2. Spectroscopic Analysis
In spectroscopic analysis, mass spectroscopy (MS), Fourier transform-infrared spectroscopy (FT-IR), and nuclear magnetic resonance (NMR) analyses were executed to regulate and characterize the compounds. Depending on the comparable molecular weight, which was determined from the mass spectrum data, the extracted compounds were identified using the MS analysis. An MS was utilized, integrated with a Waters Corporation (MA, USA) ACQUITY UPLC™ system. This setup included a binary solvent delivery system, an autosampler, column management, and a photodiode array (PDA) detector. 1 mg/mL of sample was prepared in LCMS-graded solvent for all specimens, and acetonitrile (A: 85%) and water (B: 15%) were used as chromatography solvent flow all through a monolithic capillary silica establish C18 column (Waters Corporation’s (MA, USA) ACQUITY UPLC(R) BEH C18 1.7 μm, 2.1 × 100 mm). The sample’s injection volume was adjusted to 2 µL. The nebulizer gas and cone gas flow ratios were adjusted at 500 L/h and 50 L/h, respectively. The ion source for mass assessment was electrospray ionization (ESI), and the temperature during the mass spectrometer was set at around 120°C. In contrast, capillary voltage and cone voltage were fixed at 3.0 and 40 kV, respectively. For impact, argon was effective at a pressure of 5.5 × 10−5 torr. The achieved spectral data were clarified and then tentatively categorized [17].
Each compound’s spectrum study was performed utilizing a Bio-Rad FTS spectroscope, Win-IR. In summary, each sample was individually blended along with potassium bromide before being subjected to spectroscopic investigation in the 4000 to 400 cm−1 range [18].
NMR spectroscopic evaluation was carried out with a Bruker Avance 500 MHz NMR spectroscope utilizing the stated procedure. All sample was diluted in methanol-d4 (CD3OD), and tetramethylsilane was used as a reference standard. In summary, 5 mg of each sample was separately mixed in dimethyl sulfoxide (DMSO) as a reference solvent. The samples were subjected to spectroscopic examination using the NMR method [19, 20].
2.9.3. HPLC Analysis
The analysis was performed via the HPLC method to determine Fractions F3 and F5 qualitatively. The system and chromatographic conditions remained the same as per the standard protocol. A HPLC system equipped with the column Microsorb-MV C18 (150 × 4.6 mm; 5 μm) and then a PDA sensor was utilized to analyze the compounds. A mobile phase А (H2O): В (СН3СN) (Labscan) was used in the anisocratic mode, and the solvent ratio was taken as 75(А):25(В) v/v, and the flow rate was kept at 1 mL/min. At 210 nm, the compounds were identified. The compounds were identified at 210 nm by comparing their retention times with those of pure reference substances. The standard external technique was used to make the quantitative evaluation. The outcomes are shown as mean SD values from three separate observations [21].
2.10. Experimental Animal
Six-week-old (140–160 g) Wister rats were taken. The rats were kept in a climate-controlled room. Standard laboratory feed and water were supplied to the rats. All treatments with animals were carried out in compliance with the Animal Care and Use Committee. The investigations were conducted as per the standards provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India. All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Ethics Committee at IFTM University, Moradabad (837/PO/ReBiBt/S/04/CPCSEA) under Reference No. 2021/837ac/Ph.D./04.
2.11. Acute Oral Toxicity Study
Acute oral toxicity studies for HNN and their isolated constituents (phytosphingosine and betulinic acid) were conducted as per OECD guidelines No. 423 using albino Wistar rats. The rats were fasted and examined for the physical parameter and weighed, and then, the extract was administered at a dosage of 2000 mg/kg, while constituents were orally given to three rats at a dosage of 300 mg/kg at 48 h of intervals. After administration, the rats were carefully observed for 4 h for any clinical symptoms. Six hours after administration, the weight of the rats was recorded, and then, a clinical investigation was taken daily for the following 14 days [22, 23].
2.12. Antiallergic Conjunctivitis Activity
2.12.1. Grouping of Animals
-
Group I—sensitized control group receiving 1% CMC solution.
-
Group II—standard Group treated with Cetirizine hydrochloride (10 mg/kg)
-
Group III—treated with HNN at 100 mg/kg
-
Group IV—treated with HNN at 200 mg/kg
-
Group V—treated with HNN at 400 mg/kg
-
Group VI—treated with phytosphingosine at 50 mg/kg
-
Group VII—treated with betulinic acid at 50 mg/kg
2.12.2. Sensitization of Rats and Treatment
On the first day, all groups of rats were administered 0.6 mL of saline solution, containing 1010 killed B. pertussis cells, aluminum hydroxide (2 mg), and egg albumin (1 mg) by intraperitoneal injection. On the fifth day, we subcutaneously administered egg albumin (0.5 mg) in 1 mL saline solution at 10 places on the back side of rats. After that, the local sensitization was carried out daily from the 14th to the 42nd days with the administration of albumin from egg white in physiologic saline (10 mg/mL), 5 μL in each eye using micropipette [24].
For the evaluation of anticonjunctivitis activity, the actively sensitized rats were treated with their respective therapies from days 14–42. The sensitized control group was treated with 1% CMC solution at a dosage of 1 mL/day, p.o., and the test group was treated according to their respective group dissolved in 1% CMC solvent. The extracts were given at a dosage of 100 mg/kg, 200 mg/kg, and 400 mg/kg p.o, while their constituents, phytosphingosine and betulinic acid, were given at a dosage of 50 mg/kg p.o., and the standard group of the animal was treated with cetirizine hydrochloride at a dose of 10 mg/kg, p.o. After 1 h of dosing, local sensitization was given by implanting egg albumin in sterile saline (10 mg/mL) 5 μL into the bilateral eyes utilizing a micropipette. Then, allergic signs and eye-scratching behavior were observed.
2.12.3. Evaluation of Eye Scratching Behavior and Allergic Signs
Firstly, all animals were acclimatized in an animal cage (32 × 22 × 10 cm) for 10 min before investigation. After local sensitization, rats were kept in the animal cage. The majority of eye-scratching habits, including an undisrupted array of swift forelimb motions aimed at the ocular top layer, were recorded for 20 min, as well as allergic signs, such as hyperemia and edema of the conjunctiva, were measured utilizing a scoring system. Hyperemia was measured after 5 min, and edema was measured after 20 min of local sensitization (Table 1) [24].
| Score | Allergic signs | |
|---|---|---|
| Hyperemia | Edema | |
| 0 | Minimal or no signs | Minimal or no signs |
| 1 | Mild hyperemia in single-eye | Mild edema in single-eye |
| 2 | Mild hyperemia in bilateral eyes | Mild edema in bilateral eyes |
| 3 | High hyperemia in one eye and mild hyperemia in another eye | High edema in one eye and mild edema in the other eye |
| 4 | Extreme hyperemia in both eyes | Extreme edema in both eyes |
2.12.4. Histopathological Assessment
Twenty-four hours after the treatment on days 14, 21, 28, 35, and 42, one animal from all groups was sacrificed with intraperitoneal injection of ketamine (50 mg/kg) and decapitated, and each eye was removed surgically. Conjunctivas were excised, taken out, and kept for 2 days in 10% neutral buffered formalin. After that, the sample was fixed in paraffin and then 4-μm-thick section of conjunctiva was stained to determine the quantity of eosinophils present.
2.12.5. Statistical Analysis
All data are shown as mean ± S.E.M for the five rats. For eye-scratching behavior and allergic signs, a statistical study was executed by applying one-way ANOVA along with Dunnett’s test, and the Mann–Whitney U-test was implemented to evaluate allergy indications. A value below 0.05 was considered significant.
3. Results
3.1. Extractive Value
We used 1000 g of N. nucifera rhizome for hydroalcoholic extraction in 70% ethanol for 14 days, and the extract was found to be 61.4 g; therefore, the percentage extractive value was calculated as 6.14%.
3.2. Loss on Drying
We subjected 1 g of N. nucifera extract to dry in an oven at 105°C until its weight stabilized. The final weight of the extract was 0.974 g, resulting in a weight loss of 0.026 g, corresponding to a drying loss of 2.6%.
3.3. Total Ash Value
We used 2 g of extract of N. nucifera rhizome in a crucible and ignited until charcoal was eliminated and the final weight of ash was found to be 0.1 g; therefore, the percentage ash value was found to be 5%.
3.4. Primary Phytochemical Examination
Qualitative analysis of the HNN showed the presence of the following compounds: alkaloids, carbohydrates, tannins, flavonoids, proteins, fat, amino acids, saponins, triterpenoids, cardiac glycosides, and phenolic compounds.
3.5. Characterization of the Fractions F3 and F5
3.5.1. TLC Analysis
Many fractions have been isolated from the hydroalcoholic extract of N. nucifera. TLC analysis of all fractions was performed per the standard protocol. Each compound Rf was calculated and recorded. Each plate was developed in the same solvent system, and after development, each plate was visualized under 254 nm wavelength. The amount, percentage yield, and Rf values of different fractions are in Table 2.
| Quantity of extracts used (g) | Fraction | Quantity of fraction (mg) | % yield of fraction | Rf value |
|---|---|---|---|---|
| 50 | F1 | 120 | 0.24 | 0.254 |
| F2 | 130 | 0.26 | 0.249 | |
| F3 | 580 | 1.16 | 0.452 | |
| F4 | 110 | 0.22 | 0.531 | |
| F5 | 650 | 1.3 | 0.822 | |
| F6 | 90 | 0.18 | 0.885 | |
| F7 | 110 | 0.22 | 0.892 | |
| F8 | 70 | 0.14 | 0.980 |
During the column chromatography process, the solvent polarity was only increased when no separation occurred, which was confirmed by TLC, while the individual fraction pointed with a single spot of separated compound was collected and concentrated for further analysis. The chromatographic results reveal that the Rf values of Fractions F3 and F5 of N. nucifera were found to be 0.452 and 0.822, respectively, and were confirmed as single compound fractions (Figure 1). Other fractions of the extracts were obtained as a mixture of compounds based on Rf values and also found that the fractions have very low percentage yields such as Fractions F1, F2, F4, F6, F7, and F8. Therefore, it might contain more than one compound in a fraction, and their separation is very difficult. Therefore, Fractions F3 and F5 were processed for the characterization and evaluation of their antiallergic conjunctivitis activity.

3.5.2. Spectroscopic Analysis
3.5.2.1. Analysis for F3
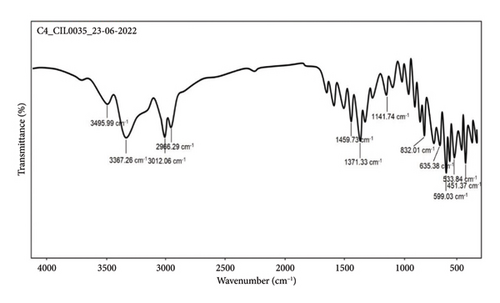
MS (ESI) m/z: 318.34 (M + 1) (Figure 2); (FT-IR, υmax, cm−1); 3495.99, 3367.26, 3012.06, and 2966.29 (–NH/NH2, –OH, CH2/CH3), 1459.73 and 1371.33 (C–O–C, C=O, and C–H vibrations), and 1141.74 and 832.01 (C–H and C–O vibrations) (Figure 3).
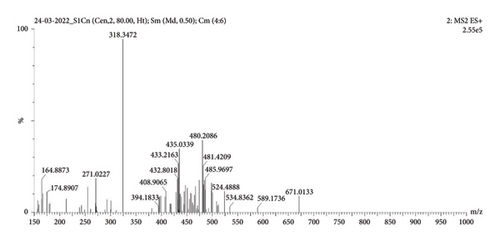
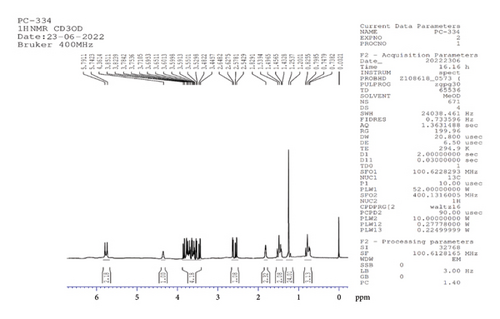
1H NMR (CD3OD, 500 MHz): δ 5.8, 4.4 (3H, d (J = 19.5 Hz), s, 3OH, at 3rd, 6th, 11th atom positions), 3.6 (4H, m, at 0th, 2nd, 8th positions of CH and CH2), 2.6, 1.8 (3H, m, CH at fifth position and NH2), 1.5, 1.2537, and 0.8 (29H, 12CH2 of aliphatic chain CH2 at the first position of atom and CH3 at 21st atom position) (Figure 4).
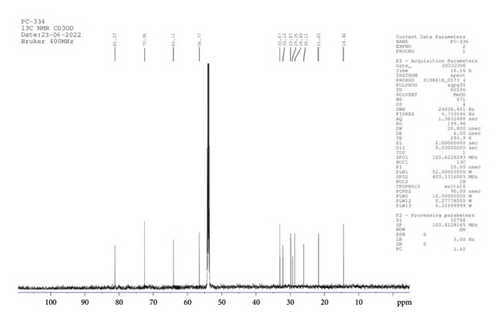
13C NMR (CD3OD, 400 MHz): δ 81.2, 72.3, 64.1, 56.8 (4C, at 0th, 2nd, 5th, and 8th atom positions), 32.7, 32.1, 29.9, 28.3, 28.9, 26.1, 21.6 (13C, 13CH2 of aliphatic ring), and 14.4 (1C, CH3) (Figure 5).
3.5.2.2. Analysis for F5
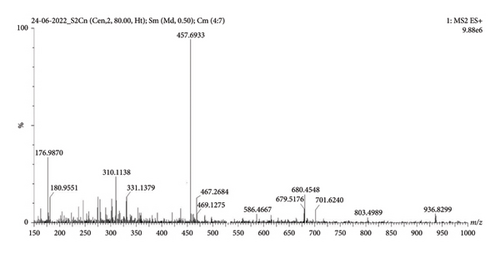
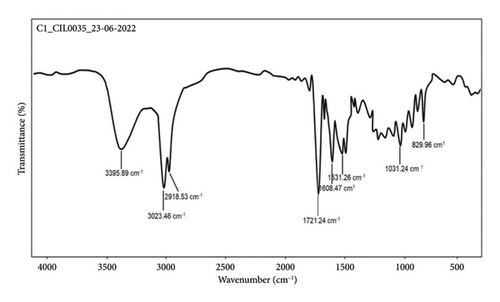
MS (ESI) m/z: 457.6933 (M + 1) (Figure 6); (FT-IR, υmax, cm−1); 3395.89, 3023.46, 2918.53 (-COOH, -OH, CH2/CH3), 1721.24 (C=O) 1608.47 and 1531.26 (C–O–C and C–H), and 1031.24 and 829.96 (aliphatic amines, aromatic C–H and C–Cl vibrations) (Figure 7).
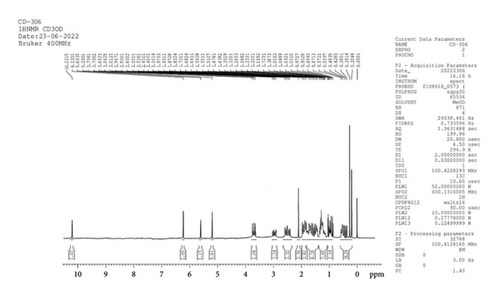
1H NMR (CD3OD, 400 MHz): δ 10.2, 6.2, 5.6, 5.2 (4H, s, –OH), 3.7 (1H, m, CH), 3.0, and 2.5 (3H, m, CH, CH2), 2.1 (2H, S, CH3), 1.9, 1.8, 1.3, 0.9, 0.4 (38H, 5m, CH2, and CH3) (Figure 8).
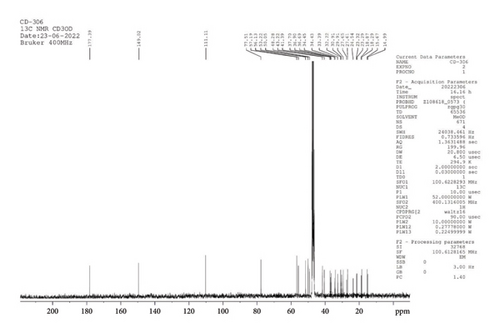
13C NMR (CD3OD, 400 MHz): δ 177.4, 149.0, 111.1, 77.5 (4C, CH, 27th, 33rd, 34th, and 1st position), 57.2, 56.1, 53.2, 50.1, 48.3, 43.2 (5C, CH, and CH2, at 24th, 3rd, 9th, 25th, 30th position), 41.4, 37.7, 36.9, 36.7, 34.5, 34.4, 33.4 (7C, CH, and CH2, 10th, 8th, 25th, 2nd, 5th, 28th, and 7th positions of the atom), 32.2, 30.9, 29.9, 27.7, 27.6, 24.5, 23.3, 23.3, 18.9, 18.3, 15.7, 15.0 (14C, CH2, CH3 at 23rd, 29th, 22nd, 0th, 19th, 16th, 13th, 35th, 16th, 6th, 21st, and 17th positions of the atom) (Figure 9).
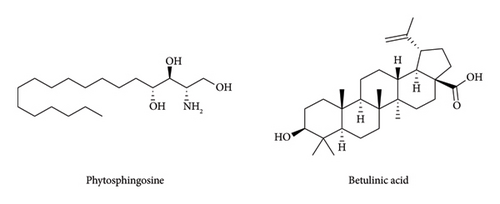
In spectroscopic analysis, MS, FT-IR, and NMR (1H and 13C NMR) analyses were performed, and the result revealed that the F3 fraction predominantly contains phytosphingosine, while the F5 fraction mainly contains betulinic acid (Figure 10). However, minor impurities were also detected within both fractions, as purification was not performed.
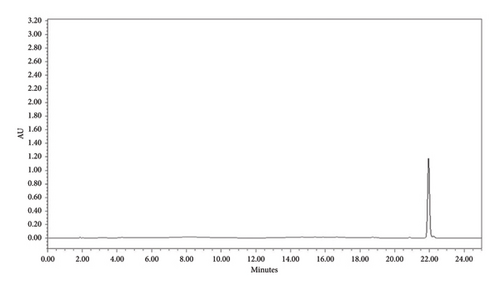
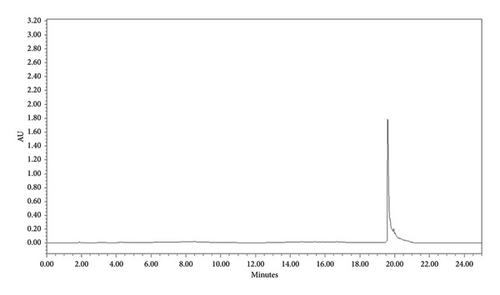
3.5.3. HPLC Analysis
HPLC analysis was performed to determine the purity of each compound. This analysis was done based on the intensity of the peak. The investigation showed that the compounds F3 (phytosphingosine) and F5 (betulinic acid) were found to be more than 90% pure. The retention time for both compounds was found to be 21.991 min (Figure 11) and 19.784 min (Figure 12), respectively. Based on the peak intensity, both compounds exhibited low peak intensity.
3.6. Acute Oral Toxicity Study
As per OECD Test guideline 423, an acute oral toxicity investigation was conducted to determine the efficacy of a hydroalcoholic extract of N. nucifera and its constituents. The animals were monitored for 14 days (postadministration), with specific emphasis on the initial 4 hours following treatment. All animals were seen to be just minimally sedated during the initial hour of treatment and to be normal and active after 2 hours. No other signs of toxicity were identified over the observation period, and each animal survived 14 days after being given an HNN and its constituents (phytosphingosine and betulinic acid).
Based on the findings, it was determined that the extract was safe up to 2000 mg/kg, and LD50 was higher than 2000 mg/kg. To test the efficacy of the HNN in animal studies, the dosage was estimated as 200, 100, and 400 mg/kg, representing 1/10, 1/20, and 1/5 of 2000 mg/kg. Phytosphingosine and betulinic acid were safe up to 300 mg/kg, and LD50 was higher than 300 mg/kg. As a result, the dosage was estimated at 50 mg/kg to investigate its efficacy in animal studies.
3.7. Anticonjunctivitis Activity
3.7.1. Evaluation of Eye Scratching Behavior and Allergic Signs
There was a considerable change in eye scratching and allergy signs following the antigen challenge. Scratching of the eyes was detected instantaneously after external antigen installation and continued for 20 min. The percentage of eye-scratching behavior was considerably elevated by repetitive topical usage of antigen up to the 18th day. Then, a substantial reduction in eye-scratching behavior was found from the 19th to the 42nd day for the treated group compared to the control group, with the effect being dose-dependent (Table 3). Comparable results were reported with allergy signs similarly sustained throughout local sensitization and had a potential effect from days 19 to 42 (Table 4).
| Group | Eye-scratching behavior score on days | ||||
|---|---|---|---|---|---|
| 14th | 21st | 28th | 35th | 42nd | |
| Group I | 12.1 ± 0.3 | 13.0 ± 0.4 | 13.5 ± 0.3 | 14.5 ± 0.5 | 14.0 ± 0.0 |
| Group II | 12.2 ± 0.3 | 10.2 ± 0.2 ∗ | 8.6 ± 0.6 ∗∗∗ | 7.0 ± 0.5 ∗∗∗ | 5.0 ± 0.0 ∗∗∗ |
| Group III | 12.4 ± 0.2 | 12.0 ± 0.3 ∗ | 11.3 ± 0.3 ∗ | 9.2 ± 0.5 ∗∗ | 8.0 ± 0.0 ∗∗ |
| Group IV | 12.2 ± 0.3 | 11.5 ± 0.3 ∗ | 9.2 ± 0.2 ∗∗ | 7.8 ± 0.2 ∗∗∗ | 6.0 ± 0.0 ∗∗∗ |
| Group V | 12.8 ± 0.5 | 11.2 ± 0.3 ∗ | 8.6 ± 0.4 ∗∗∗ | 7.2 ± 0.0 ∗∗∗ | 5.0 ± 0.0 ∗∗∗ |
| Group VI | 13.2 ± 0.3 | 11.8 ± 0.4 ∗ | 8.2 ± 0.3 ∗∗∗ | 6.2 ± 0.2 ∗∗∗ | 5.0 ± 0.0 ∗∗∗ |
| Group VII | 12.6 ± 0.4 | 11.5 ± 0.2 ∗ | 8.8 ± 0.4 ∗∗∗ | 7.3 ± 0.2 ∗∗∗ | 5.0 ± 0.0 ∗∗∗ |
- Note: Values are represented as n = mean ± SEM; statistical analysis was performed using one-way ANOVA followed by Dunnet’s test. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 against Group I. Group I received 1% CMC solution and work as control, Group II received cetirizine hydrochloride (10 mg/kg), Group III received HNN (100 mg/kg), Group IV received HNN (200 mg/kg), Group V received HNN (400 mg/kg), Group VI received phytosphingosine (50 mg/kg), and Group VII received betulinic acid (50 mg/kg) orally. The upright bar indicated animal mean ± SEM (n = 5) for eye-scratching score.
| Group | Allergic signs score on days | ||||
|---|---|---|---|---|---|
| 14th | 21st | 28th | 35th | 42nd | |
| Group I | 4.7 ± 0.3 | 5.1 ± 0.0 | 6.2 ± 0.3 | 6.9 ± 0.0 | 7.8 ± 0.0 |
| Group II | 5.1 ± 0.5 | 4.6 ± 0.5 | 3.4 ± 0.6 ∗∗∗ | 2.9 ± 0.5 ∗∗∗ | 2.1 ± 0.0 ∗∗∗ |
| Group III | 6.2 ± 0.3 | 5.0 ± 0.3 | 4.8 ± 0.2 ∗∗ | 4.1 ± 0.2 ∗∗∗ | 3.6 ± 0.0 ∗∗∗ |
| Group IV | 5.8 ± 0.3 | 5.3 ± 0.3 | 4.2 ± 0.2 ∗∗ | 3.4 ± 0.3 ∗∗∗ | 2.9 ± 0.0 ∗∗∗ |
| Group V | 5.2 ± 0.2 | 3.4 ± 0.3 ∗ | 3.2 ± 0.4 ∗∗∗ | 2.6 ± 0.2 ∗∗∗ | 2.2 ± 0.0 ∗∗∗ |
| Group VI | 5.8 ± 0.2 | 4.2 ± 0.4 ∗ | 3.3 ± 0.3 ∗∗∗ | 2.5 ± 0.2 ∗∗∗ | 2.1 ± 0.0 ∗∗∗ |
| Group VII | 6.2 ± 0.3 | 4.4 ± 0.3 ∗ | 3.5 ± 0.2 ∗∗∗ | 2.6 ± 0.2 ∗∗∗ | 2.3 ± 0.0 ∗∗∗ |
- Note: Values are represented as = mean ± SEM; statistical analysis was performed using one-way ANOVA followed by Dunnet’s test. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001against Group I. Group I received 1% CMC solution and work as control, Group II received cetirizine hydrochloride (10 mg/kg), Group III received HNN (100 mg/kg), Group IV received HNN (200 mg/kg), Group V received HNN (400 mg/kg), Group VI received phytosphingosine (50 mg/kg), and Group VII received betulinic acid (50 mg/kg) orally. The upright bar indicated the animal mean ± SEM (n = 5) for allergic sign scores.
The constituents (phytosphingosine and betulinic acid) and cetirizine hydrochloride exhibited a slightly better impact and a considerable reduction in eye-scratching activity and allergy signs from the 16th day of study.
3.7.2. Histopathological Assessment
The histopathological assessment showed a significant marker of mononuclear infiltration in conjunctival tissue for the control group receiving 1% CMC. Treatment with HNN showed a significant reduction in mononuclear infiltrations in dose-dependent manner. The constituents (phytosphingosine and betulinic acid) and cetirizine hydrochloride greatly reduced the infiltration.
The number of eosinophils in the conjunctival mucosa of rats sensitized by antigen is shown in histopathological photomicrograph. The quantity of conjunctival eosinophils in the control group increases from Day 14 to Day 42. In comparison with the control group, the number of conjunctival eosinophils dropped continuously in the HNN-treated groups, and the effect was found to be dose-dependent. The constituents (phytosphingosine and betulinic acid) and cetirizine-treated groups showed comparable results.
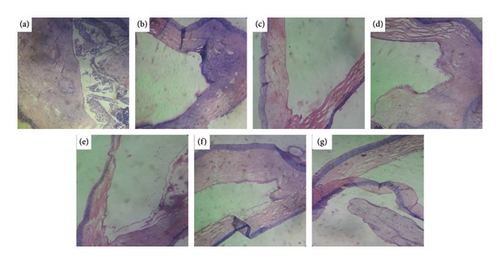
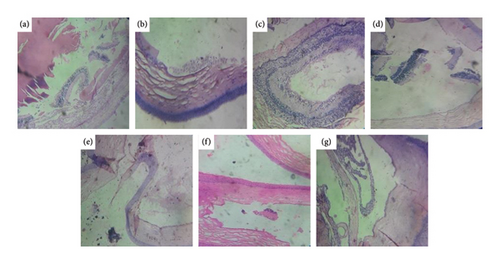
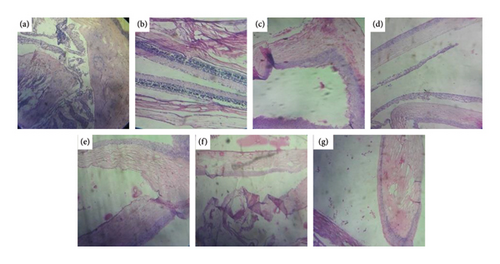
Comparison of photomicrographs of the conjunctival tissues of different groups for the evaluation of the presence of eosinophil in different groups of the rats on different challenging days is shown in Figures 13, 14, 15, 16, and 17.
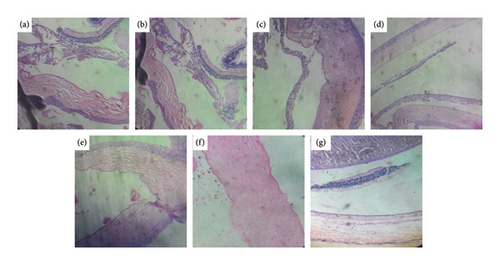
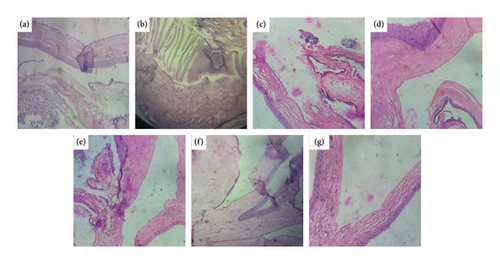
Comparison of histopathological photomicrograph taken on the 14th day of the study (Figure 13).
Comparison of histopathological photomicrograph taken on the 21st day of the study (Figure 14).
Comparison of histopathological photomicrograph taken on the 28th day of the study (Figure 15).
Comparison of histopathological photomicrograph taken on the 35th day of the study (Figure 16).
Comparison of histopathological photomicrograph taken on the 42nd day of the study (Figure 17).
4. Discussion
Plant medicines have been effective in healthcare and safe; hence, self-medication is a frequent practice [25]. Consequently, there is a scarcity of information on these products’ toxicological nature and side effects. Along these lines, an acute toxicity study is required not exclusively to recognize the further scope of dosages in animal research yet in addition to clarify the other symptoms produced by the test compounds being examined. However, it is essential and practical for determining the medication’s therapeutic efficacy [26].
According to OECD guideline 423, an acute toxicity investigation was done with the 14 days of the observation period, and no morbidity or mortality was observed in HNN and their constituent (phytosphingosine and betulinic acid)-treated rats [23]. Also, the results showed no adverse effect up to 2000 mg/kg for HNN and 300 mg/kg for constituents, indicating that LD50 was more than 2000 and 300 mg/kg, respectively.
The earlier investigation revealed that the marked eye-scratching activity was observed after continuous topical administration of antigen [27]. It is also said that eye-scratching behavior was spotted in guinea pigs with presensitized antigens [28]. Eye-scratching behavior in rats seems to be more accurate than in guinea pigs and mice for assessing irritation in allergic conjunctivitis. This idea was validated by the presence of hyperemia and edema, with eye-scratching behavior [29]. It is, thus, possible that the itching of the eyes seen in sensitized animals is primarily due to histamine produced by an antigen–antibody response.
The model of allergic conjunctivitis induced by ovalbumin in rats has been used for screening antiallergic agents [30]. This model of ocular allergic disease is typically related to Type 1 hypersensitivity reactions. Allergic conjunctivitis is a biphasic allergic reaction, early by degranulation of mast cells and later by cell infiltration, mainly eosinophils [1]. The HNN and their constituents (phytosphingosine and betulinic acid) reduced the allergen-specific IgE effects, indicating a mechanistic deviation of activity from antihistaminic and mast cell stabilizing agents.
Its mechanism is supposed to be the suppression of the FcεRI receptor, as a study described that the kaempferol isolated from N. nucifera extract downregulated the FcεRI receptor and established the mast cell, and suppression of IgE, interleukin-4 (IL-4), and leukotriene B-4 (LTB-4) production as a study described that lotus root extract decreases the allergic symptoms by suppressing the production of IL-4, Ig-E, and LTB4 [31, 32]. The treatment with HNN and their constituents significantly reduced allergen-specific immunoglobulins. This presupposes that these drugs may have immunosuppressive effects. In one study, the antiallergic effects of Moringa oleifera leaf extract and its isolated constituents on conjunctivitis were investigated, and significant responses were observed [33].
In this model, we investigate the eosinophil number in the conjunctiva and eye-scratching activity [34]. In this research, we demonstrated that histamine greatly increases allergic signs and symptoms through local sensitization and discover eosinophils’ presence. As a result, it is assumed that this model of recurrent topical antigen treatment on the ocular surface represents a chronic allergic conjunctivitis model.
HNN produced inhibition in allergic signs and eye-scratching behavior significantly; therefore, we found that HNN had a potent inhibition activity on clinical allergic conjunctivitis caused by antigen in rats in a dose-dependent manner, while phytosphingosine and betulinic acid produced a similar effect with 400 mg/kg HNN. This antiallergic activity of the extract and its constituent in allergic conjunctivitis is primarily because of its antihistaminic and mast cell stabilizing activity. The standard drug, cetirizine (10 mg/kg, p.o.), diminished all allergic indications. Cetirizine has been shown to have H1 antagonistic action [35]. Mast cells are widely present in the conjunctiva, while eosinophils are released from the conjunctiva during conjunctivitis. Mast cells and eosinophils possess histamine and have a strong affinity for IgE receptors expressed on their exterior [36]. H1 antagonists completely prevented antigen-induced ocular scratching behavior, as revealed by this study. As a result, we proposed that histamine produced by mast cells and eosinophils was responsible for ocular itchiness caused by an antigen–antibody response.
5. Conclusion
During the acute oral toxicity study of the HNN and its constituents, no mortality was reported in any animals up to the dose level of 2000 mg/kg for extract and 300 mg/kg for constituents, indicating their practically nontoxic. Oral administration of the HNN significantly decreased allergic signs and eye-scratching behavior, and markedly decreased eosinophil into the conjunctiva tissues. The constituents (phytosphingosine and betulinic acid) produced a slightly rapid and better effect in managing allergic signs and eye-scratching behavior while decreasing the eosinophil similar to the 400 mg/kg HNN. Hence, the present study showed that the HNN and their constituents (phytosphingosine and betulinic acid) produced a significant anticonjunctivitis activity against allergic conjunctivitis and are entirely safe for treatment.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Phool Chandra: conceptualization, validation, investigation, and writing–original draft preparation. Arvind Kumar Patel: conceptualization, methodology, validation, investigation, and writing–original draft preparation. Neetu Sachan: validation, writing–original draft preparation, resources, writing–review and editing, visualization, and supervision. Atul Kabra: software, data curation and resources, and writing–review and editing. Neha Singh: methodology and data curation. Abdulrahman Alshammari: resources and writing–review and editing. Norah A. Albekairi: resources, writing–review and editing. H. C. Ananda Murthy: writing–original draft preparation, resources, and writing–review and editing.
Funding
The authors are thankful to the researchers supporting the project under No. RSP2025R491, King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors are thankful to the researchers supporting the project under No. RSP2025R491, King Saud University, Riyadh, Saudi Arabia. The authors also want to express warm regards and sincere thanks to the honorable vice-chancellor of the university for providing the necessary facilities in the laboratories.
Open Research
Data Availability Statement
Data can be obtained through a written request to the corresponding author.




