Fabrication of Magnetite Nanoparticles With Hydrophobic Imidazolium Ionic Liquid for the Efficient Role in Oil Spill Collection
Abstract
As one of the main environmental obstacles, oil spill causes serious consequences on the environment, fauna, and flora. The traditional methods for cleaning up oil spills were found time-consuming, expensive, and ineffective. Magnetite nanoparticles have attracted great attention as alternatives to the conventional methods as a result of the substantial efficiency, quick controlling, and remedying of oil spills. In the present work, trisubstituted imidazole was prepared by one-pot reaction followed by alkylation with tetradecyl bromide to obtain hydrophobic 4,5-diphenyl-1,3-ditetradecyl-2-(2-(tetradecyloxy)phenyl)-1H-imidazole-3-ium bromide, tetradecylimidazolium ionic liquid (TTIML). After that, magnetite nanoparticles were prepared and coated with TTIML in order to control the particle size and modify the magnetite surface to be hydrophobic enough to spread in the oil spill and consequently cleaning the oil by the application of an external magnet. The structures of magnetite nanoparticles coated with newly synthesized ionic liquid (TTIML-Fe3O4) was confirmed using FTIR, XRD, TEM, and DLS. Only about 10 wt% of IL was sufficient to obtain monodispersed Fe3O4 nanoparticles as confirmed by TGA thermogram. The oil spill collecting efficiency of Fe3O4 NPs reached 95.5 ± 2.9% at magnetite to oil ratios (1 : 25) which indicate its application.
1. Introduction
The contamination of shoreline and offshore waters with petroleum crude oil spill during oil drilling and transportation has harmful impacts on human health, aquatic life, and flora [1]. It affects badly the growth of the indigenous creatures living in water bodies, disrupting offshore and onshore ecosystems. On that account, developing modern technologies to remove the spilled oil on water and to protect marine life and the environment is a crucial issue [2]. Several techniques have been introduced for recovering oil from water, including (a) booms, (b) skimmers, (c) in situ burnings, (d) dispersants, (e) bioremediation, (f) sorbents, and (g) magnetic nanocomposites [3–5]. Booms act as mechanical barriers to prevent oil spillage from further spreading, while skimmers are mechanical equipment that physically remove oil from the water surface. Another physical method for oil removal is the in situ burning technique by flamethrowers hung under helicopters. Toxic gases resulting from oil burning is the main drawback of this method as it affects marine and avian life in the ocean and above it [5].
The dispersants, basically chemical detergents, are utilized to spread oil on a larger sea area to allow air and light to be in contact with the seawater [6, 7]. Although this method is effective, it is harmful to coral reefs and fish due to the toxic chemicals used and also poisonous oil components that are dispersed in great distances [8]. The bioremediation technique, as environment friendly, can be utilized as the ex situ or in situ approach, but it leads to the loss of spilled oil [9]. Sorbent materials recover oil from the water surface by adsorbing and/or absorbing of oil spillage which is found to be simple and relatively cost-effective [10, 11]. Secondary pollution, high cost, low efficiency, and long process time are some of the drawbacks of conventional methods, so developing more efficient and economical techniques is an urgent need.
Nanomaterials have caught the attention of researchers to be applied for spilled oil clean-up because of their exceptional physical and chemical properties due to their high surface area and nanoscale size comparing with their bulk counterparts [12, 13]. Magnetic nanoparticles have unique characteristics over other nanoparticles because of their biocompatibility and low toxicity and high response to external magnets [14, 15].
Modification of the magnetite surface with different organic compounds provides unique chance to engineer its interfacial characteristics while retaining its basic ones which can direct its application [16]. Covering of the magnetite surface with different shells can occur directly using various chelating molecules or by covering with the silica layer which in turn facilitate the attachment of different molecules on its surface by the modification on silanol groups but with some risk of aggregation. Culita et al. coated the magnetite surface with amino silane which facilitates surface modification with vanillin, formylsalicylic acid through the Schiff base reaction [17]. Furthermore, cysteine-functionalized Fe3O4@SiO2 nanoparticles were synthesized and applied as efficient nanoadsorbants for Pb(II) from aqueous solutions [18].
One of the most important fields that functionalized magnetite nanoparticles can be applied as solid catalysts is the sustainable and cleaner biodiesel production because of their facile separation by an external magnetic field, recyclability, and high performance [19]. Xie and Wan fabricated core–shell structured magnetite-MOF using the layer-by-layer assembly technique followed by its functionalization with ionic liquids to form Fe3O4@HKUST-1-ABILs. This catalyst was applied as efficient magnetically recyclable solid for catalytic conversion of soybean oil into biodiesel [20]. In addition, magnetite nanoparticles were coated with imidazolium IL-functionalized silica to prepare the corresponding silica composites, and lipase was immobilized on the composite surface. This composite was efficiently applied as a magnetically separable biocatalyst for the interesterification of palm stearin and rice bran oil [21].
In our recent work, we have addressed the removal of oil spill as one of the most deleterious environmental problems using magnetite nanoparticles. To modify magnetite nanoparticle surfaces to be compatible with oil spills, new hydrophobic imidazolium ionic liquid was prepared via a facile method to control the size and hydrophobicity of magnetite nanoparticles (MNPs). This hydrophobicity facilitates the interaction of MNPs with the spilled oil layers above water bodies, and as a result, it cleaned the oil contamination using strong magnets [22, 23]. Firstly, the Debus–Radziszewski reaction was used to prepare imidazole in the one pot reaction from a benzil, 2-hydroxy benzaldehyde and ammonia. Secondly, the prepared imidazole derivative was interacted with tetradecyl bromide by the fusion reaction to prepare hydrophobic room temperature ionic liquid which was used as the capping agent for magnetite preparation. The surface characteristics and particle size distribution of MNPs were controlled using the prepared hydrophobic ionic liquid. The synthesized monodispersed hydrophobic MNPs were applied as the oil spill collector with a high efficiency, 96.3 ± 1.5% at low magnetite to oil ratio (1 : 10). Moreover, MNPs were recycled five times without showing significant loss in its efficiency.
2. Experimental
2.1. Materials
Salicylaldehyde, hydrated ferric chloride (FeCl3.6H2O), hydrated ferrous chloride (FeCl2.4H2O), and ammonium hydroxide (NH4OH) were supplied by Sigma-Aldrich, while ethanol (99.9%) was purchased from (Sinpharm Chemical Reagent Co.). 1-bromotetradecane was purchased from Tokyo Chemical Industry Co. (TCI). Sea water was brought from Arabian Gulf at Dammam coast, and Arabian heavy crude oil was obtained from Riyadh refinery unit (Aramco Co.) with specifications stated in our former work [24],.
| Function group | ν (cm−1) |
|---|---|
| Aromatic -CH | 3056 |
| Aliphatic -CH | 2925, 2853 |
| C=N | 1603 |
| C=C | 1493 |
| Function group | δH |
|---|---|
| CH3 | 0.82 |
| CH2 | 1.17–1.26 |
| N-CH2, +N-CH2 | 3.76–3.80 |
| OCH2 | 4.01 |
| Ar-H | 7.09–7.51 |
| Function group | δC |
|---|---|
| CH3 | 13.92 |
| CH2 | 22.51, 28.80, 29.08, 29.19, 29.36, 29.47, 31.74 |
| N-CH2, +N-CH2 | 46.36 |
| OCH2 | 68.25 |
| Ar-H and Cq-Ar | 112.07, 125.22, 128.13, 129.09, 129.96, 130.73, 131.82, 131.87 |
| N-Cq-N and Cq-O | 145.63, 156.75 |
2.2. Synthesis Procedure
- a.
Synthesis of 2-(4,5-diphenyl-1H-imidazole-2-yl)phenol (1):
-
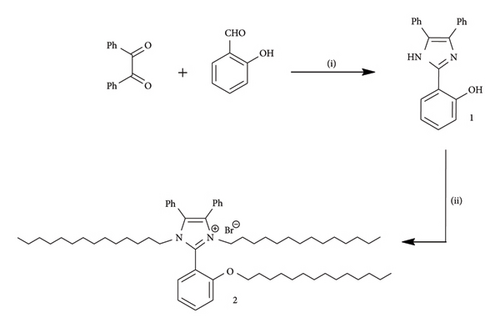
A solution of benzil (3.94g, 0.0188 mol) in acetic acid (30 mL), ammonium acetate (7.47 g, 0.097mol), and salicylaldehyde (2.29g, 0.0188 mol) were refluxed for 10 h. The hot reaction mixture was filtrated off, and the filtrate was concentrated under reduced pressure and then allowed to stand to attain room temperature. Then, the solid product obtained by adding water (150 mL) and washing by ammonia yield the title product. The product was recrystallized from ethanol to yield trisubstituted imidazole (93%) [25].
- b.
Synthesis of 4,5-diphenyl-1,3-ditetradecyl-2-(2-(tetradecyloxy)phenyl)-1H-imidazole-3-ium bromide (2):
-
A mixture of compound 1 (0.01 mol) and tetradecyl bromide (0.03 mol) was fused in an oil bath at 180°C for 5 h. After filtration of the residue triturated with diethylether, the filtrate was concentrated to evaporate the solvent and washed by petroleum ether. Then, tetradecylimidazoluim ionic liquid (TTIMIL) (2) was obtained as a brown viscous liquid at r.t. yield (81%); IR (KBr) (Table 1): ν/cm−1: 3056 (CH-Ar), 2925, 2853 (CH-aliphatic), 1603 (C=N), 1493 (C=C); 1H-NMR (400 MHz, CDCl3) (Table 2) δH: 0.82 (t, 9H, 3CH3), 1.17–1.26 (m, 72H, 36CH2), 3.76–3.80 (m, 4H, N-CH2, +N-CH2), 4.01 (t, 2H, OCH2), 7.09–7.51 (m, 14H, Ar-H); 13C-NMR (75 MHz, CDCl3) (Table 3) δC: 13.92, 22.51, 28.80, 29.08, 29.19, 29.36, 29.47, 31.74 (3CH3, 36CH2), 46.36 (2 N-CH2), 68.25 (O-CH2), 112.07, 125.22, 128.13, 129.09, 129.96, 130.73, 131.82, 131.87 (14CH-Ar & 3Cq-Ar), 145.63, 156.75 (N-Cq-N, & Cq-O).
- c.
Preparation of magnetite nanoparticles cCapped with IL
-
Coprecipitation of ferric chloride hexahydrate (FeCl3.6H2O, 5.4 g) and ferrous chloride tetrahydrate (FeCl2.4H2O, 2.0 g) was used to prepare magnetite nanoparticles as reported in our previous work [26]. An external magnet was used to collect the formed MNPs followed by washing several times with H2O. In order to modify the surface of MNPs with the prepared IL, MNPs were dispersed in 50 mL of ethanol using a probe sonicator for 10 min, and then, IL solution was added (1 g dissolved in 10 mL of ethanol) under mechanical stirring for 2 h at 60°C. The formed Fe3O4/IL was collected using an external magnet, washed with ethanol to remove excess IL, and then dried in a vacuum oven at 60°C (Scheme 1).
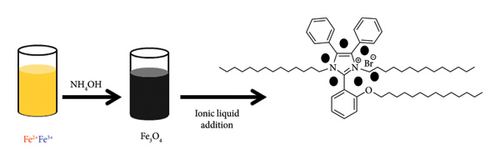
2.3. Characterization Methods of IL and Fe3O4/IL
1HNMR and 13CNMR (Bruker AVANCE DRX-400 MHz NMR spectrometer) with CDCl3 as a solvent were used to characterize the prepared IL. FT-IR (Nicolet FT-IR spectrophotometer) was used to analyze IL and Fe3O4-IL by grinding and mixing an appropriate amount of the sample with KBr in order to fully disperse the samples in the potassium bromide, and then, a tablet was formed by pressing the sample, which after that placed in an infrared spectrometer for detection. Thermogravimetric analysis of IL and Fe3O4-IL was carried out under a nitrogen atmosphere at a heating rate of 10°C per minute using TGA and TDG (Shimadzu DTG-60M). The dynamic light scattering technique (DLS) (Zetasizer Nano ZS Malvern) was used to characterize the particle size of Fe3O4/IL in the colloidal ethanol solution. X-ray diffraction (XRD) (BDX-3300 diffractometer) was employed to determine the crystalline structure of Fe3O4/IL. The contact angle was measured using the drop shape analyzer (DSA-100, Krüss GmbH, Hamburg, Germany). The magnetic characteristics of Fe3O4/IL were determined using vibrating-sample magnetometry (VSM) with a LDJ9600 magnetometer (LDJ Electronics, MI, USA).
2.3.1. Transmission Electron Microscopy (TEM) Analysis of Nanoparticle Dispersions
The size and morphology of the magnetite nanoparticles were examined by TEM(JEOL JEM2100F). First, a colloidal solution of magnetite nanoparticles in the ethanol solvent was prepared with a concentration of 1000 ppm, and then, a drop of the dispersion was placed on a carbon-coated copper TEM grid and air-dried at room temperature.
2.3.2. Statistical Analysis
The application of magnetite nanoparticles as the oil spell collector was conducted in triplicate, and data were presented as the mean ± standard error.
2.4. Efficiency of Fe3O4/IL as the Oil Spill Collector
Magnetite nanoparticle powder (TTIML-Fe3O4) was spread on the oil spot placed on the surface of sea water (250 mL of sea water in a 500− mL beaker) with gentle stirring using a glass rod. An external magnetic (Nd–Fe–B, 4300 Gauss) was applied to collect the oil/magnetite mixture in order to determine the efficiency of magnetite nanoparticles to spread and adsorb oil spill on their surface. Magnetite to Arabian oil ratios ranged from 1 : 10 to 1 : 50 wt% to determine the least ration with the highest removal efficiency.
3. Results and Discussion
Synthesis of 2-(4,5-diphenyl-1H-imidazole-2-yl)phenol (1) accessed by the heating reaction of benzil with salicylaldehyde and ammonium acetate in acetic acid afforded the desired product 1. Further, alkylation of the aforementioned imidazole with tetradecyl bromide yielded TTIMIL (2) as depicted in Scheme 2.
3.1. Characterization of TTIML and Fe3O4/TTIML
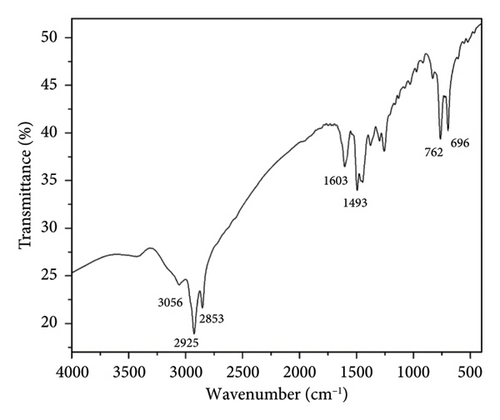
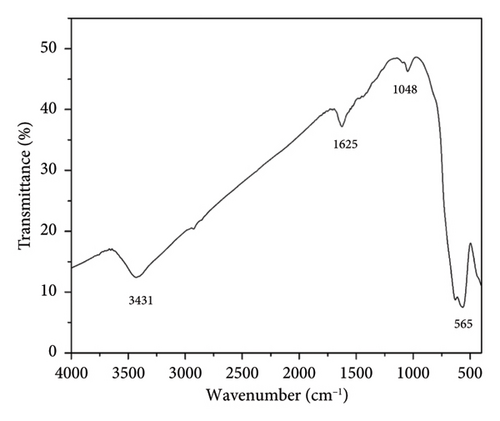
The chemical structures of TTIML and TTIML-Fe3O4 were investigated utilizing FT-IR spectra as displayed in Figures 1(a), 1(b), respectively. The FT-IR spectrum of TTIMIL revealed the presence of the stretching absorption bands at ν 3056, 2925, 2853, 1603, and 1493 cm−1 due to the aromatic -CH, aliphatic –CH, C=N and C=C groups, respectively (Figure 1(a)). Moreover, the appearance of the strong band at 565 cm−1 (Figure 1(b)) is related to the stretching vibrations of Fe-O to indicate the formation of MNPs [27, 28]. The shifting of the stretching absorption band of C=N from 1603 cm−1 (TTIML spectra) to 1625 cm−1 (TTIML-Fe3O4 spectra) is due to the chemisorption of IL molecules on the magnetite surface. In addition, the 1H-NMR and 13C-NMR spectra were utilized to confirm the formation of TTIML. The former disclosed the presence of two triplet signals at δH = 0.82 and 4.01 ppm due to the methyl –CH3 and methylene –OCH2 protons of tetradecyl group, respectively. In addition, it exhibited three multiplets; two multiplets at δH = 1.17–1.26 and 3.76–3.80 ppm due to the methylene –CH2-, -N-CH2, -N+-CH2- protons of the tetradecyl group and one multiplet at δH = 7.09–7.51 ppm for aromatic protons of phenyl groups (Figure 2).

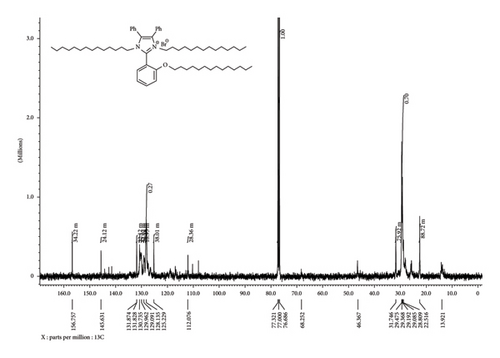
The 1H-NMR spectrum disclosed the presence of two triplet signals at δH = 0.82 and 4.01 ppm due to the methyl –CH3 and methylene –OCH2 protons of the tetradecyl group, respectively. In addition, it exhibited three multiplets; two multiplets at δH = 1.17–1.26 and 3.76–3.80 ppm due to the methylene –CH2-, -N-CH2, -N+-CH2- protons of the tetradecyl group and one multiplet at δH = 7.09–7.51 ppm for aromatic protons of phenyl groups (Figure 2). The 13C-NMR spectrum displayed four signals at δC = 13.92, 22.51, 31.74, 46.70, and 68.27 ppm due to the sp3 carbons of methyl –CH3, methylene –CH2, -N-CH2, -N+-CH2-, and –OCH2 carbons, and eight spectral lines were exhibited at δC = 112.07, 125.22, 128.13, 129.09, 129.96, 130.73, 131.82, and 131.87 ppm due to the sp2 carbons. In addition, two spectral lines were revealed at δC = 145.63 and 156.75 ppm corresponding to N-Cq-N and Cq-O carbons (Figure 3).
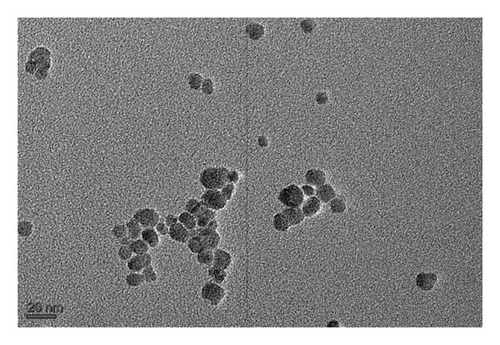
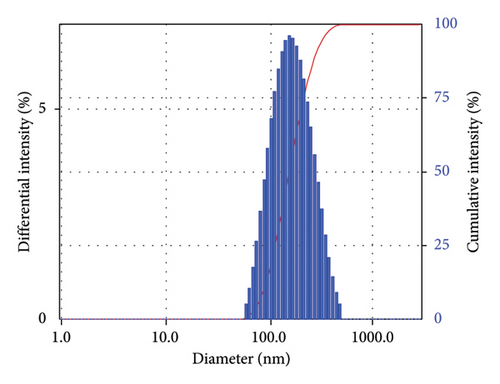
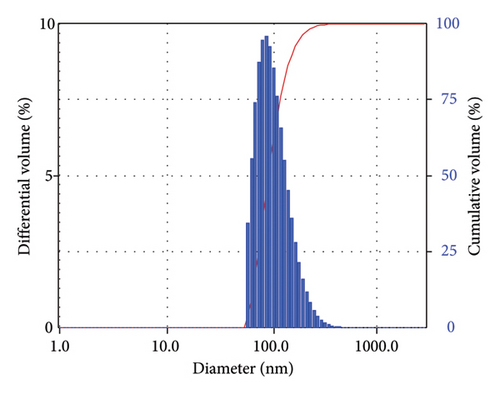
The morphology of TTIML-Fe3O4 was analyzed by TEM (JEOL JEM-2100 F). The TEM micrograph (Figure 4) showed a spherical shape of the Fe3O4 nanoparticles with good monodispersity and a mean diameter of 5.52 nm. It can also be seen that there are slight aggregates, which may be formed as a result of incomplete coating of individual particles [29]. Moreover, the DLS was used to provide a measure of the size of TTIML-Fe3O4 particles from the scattered light in an ethanol solution. Figures 5(a), 5(b) show intensity and volume distributions of TTIML-Fe3O4, respectively. The cumulative results for the diameter (d) and polydispersity index (PI) were 201.1 nm and 0.16, respectively, that indicate the ability of TTIML to form monodispersed magnetite nanoparticles [30]. The increment in the diameter of TTIML-Fe3O4 NPs comparing with that determined from TEM micrograph is regarding to MNPs-solvent interaction that result in the formation of some agglomerations [31].
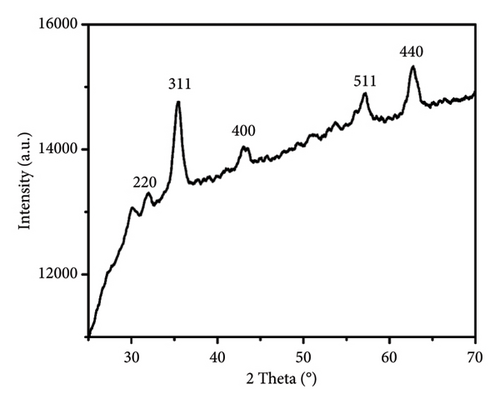
Powder XRD technique is utilized to study the natures of the material as crystalline or amorphous [32]. XRD of TTIML-Fe3O4 (Figure 6) highlights the formation of magnetite as the major crystalline phase due to the appearance of its characteristic peaks at 2 theta, 30.14°, 35.91°, 43.21°, 57.27°, and 62.51° relating to planes 220, 311, 400, 511, and 440, respectively. As shown from Figure 6, the diffraction peaks’ positions are well matched with the standard XRD data for bulk magnetite without the contaminations with any other oxides [33].
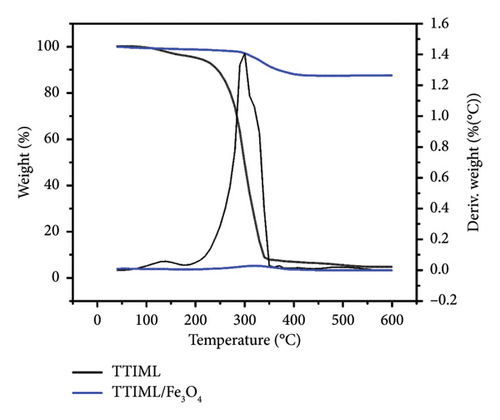
TGA and TDG thermograms (Figure 7) were used to investigate the thermal degradation steps of the prepared ionic liquid (TTIML) and to determine its percentage in TTIML-Fe3O4 [34, 35]. As noticed from Figure 7, the thermal degradation of TTIML occurred in two steps. The first step is attributed to the evaporation of physically attached water to the TTIML that took place between 100°C and 160°C. The main step is the second one, which reflected the degradation of the organic structure of TTIML, and took place at a temperature range from 200°C to 350°C [36]. There was no noticeable weight loss in TTIML-Fe3O4 below 100°C as a result of the high hydrophobicity of the prepared IL-coated magnetite nanoparticles [37]. The percentage of TTIML coating on the magnetite surface was determined from the remaining weight percentage above 610°C, and it was around 10% which meant that 10% of TTIML was enough to prepare magnetite nanoparticles with hydrophobic properties.
3.2. Oil Collecting Efficiency of TTIML-Fe3O4 NPs
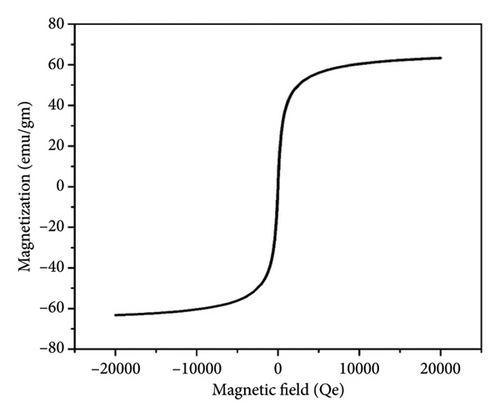
The main factors that control the efficiency of MNPs as oil spill collectors are high hydrophobicity, magnetism, and monodispersity [37, 38]. The hydrophobicity of magnetite surfaces enables the particles to disperse and interact with oil spills in order to get the highest collecting capacity. Contact angle measurements can be applied to detect whether the prepared MNPs have hydrophilic or hydrophobic surfaces. The value of the contact angle of the water droplet with a surface gives an indication of whether this surface is hydrophilic or hydrophobic. Surfaces with the contact angle (θ) < 90° are hydrophilic, whereas those with θ > 90° are hydrophobic [39]. The contact angle of a compressed magnetite disk with seawater drop was found to be 133° (Figure 8) to substantiate the hydrophobicity of the prepared MNPs. The monodispersed particles with a uniform particle size afford better chance for particles to cover the higher surface area of oil spill. Additionally, monodisperse particles interact efficiently with crude oil and form uniform oil/magnetite composite which can be collected easily using an external magnet [37]. The monodispersibility and the low particle size of TTIML-Fe3O4 colloidal solution in the ethanol solvent were confirmed by DLS studies (Figure 5) which showed that the particle size and the polydispersity index were 201.1 nm and 0.16, respectively. The high hydrophobicity, low particle size, and mono-dispersibility of TTIML-Fe3O4 allow magnetite nanoparticles to disperse easily in oil spill in order to remove it easily using an external magnet. The remediation of oil spillages using MNPs mainly relies on the magnetic properties of the prepared hybrid material as we apply an external magnet to collect the oil spill/magnetite mixture [40–42]. Therefore, the magnetization values of TTIML-Fe3O4 were determined, as shown in Figure 9, to be about 63 emu/g. The high magnetization values of TTIML-Fe3O4 implied its capability to achieve the target.
In our study, oil spill collecting efficiency experiments were conducted utilizing a range of MNPs to oil ratios from 1:4 to 1:50 wt% to identify the best economic ratio, and the efficacies of MNPs for oil removal were listed in Table 4. TTIML-Fe3O4 showed higher removal activities even at low magnetite ratios (1 : 25) when comparing with those obtained in previous studies [41–43]. For example, R. Malhas et al. [41] applied magnetite nanoparticles with different particle sizes, Magnetite A, 35 μm, Magnetite B, 5 μm, and Magnetite C, 15 nm for crude oil remediation. The highest efficiency was for Magnetite C, 15 nm that reach 96.3% at the 1 : 4 magnetite to crude oil ratio (Table 4). Our prepared MNPs reached higher efficiency 98.8 ± 1 at the same magnetite to crude oil ratio. Moreover, Abdullah and Al-Lohedan [42] used sodium alginate derivatives, OA-ALG, and DA-ALG to prepare magnetite nanoparticles, OA-MNPs, and DA-MNPs to apply for oil spill cleanup. Their efficiencies are listed in Table 4. It was obvious that our materials have more efficiencies at almost all magnetite to oil ratios. The high efficiency of TTIML-Fe3O4 could be attributed to the low particle size, high hydrophobicity, and high magnetic characteristic that were ensured by TEM and DLS, contact angle, and magnetization studies, respectively.
| MNPs/efficiency (%) | Oil spill collecting efficiency using different MNPs/crude oil wt% | |||||
|---|---|---|---|---|---|---|
| 1 : 2 | 1 : 4 | 1 : 10 | 1 : 25 | 1 : 50 | Reference | |
| TTIML-Fe3O4 | 98 ± 0.5 | 95.3 ± 3 | 95.5 ± 2.9 | 87 ± 3.3 | Current study | |
| Magnetite A | 67.62 | 65.61 | [41] | |||
| Magnetite B | 88.29 | 86.97 | [41] | |||
| Magnetite C | 98.1 | 96.3 | [41] | |||
| OA-MNPs | 93 | 87 | 78 | [42] | ||
| DA-MNPs | 87 | 75 | 67 | [42] | ||
| APC-MNPs/plant extract | 78 | 74 | 70 | [43] | ||
| APH-MNPs/plant extract | 90 | 88 | 83 | [43] | ||
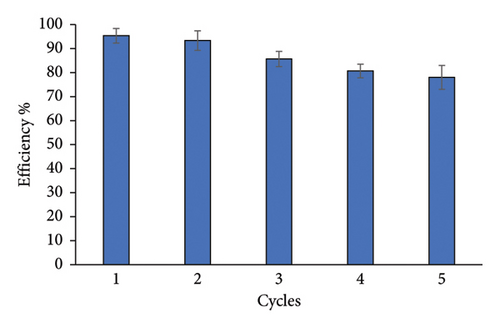
The reusability of TTIML-Fe3O4 was tested to analyze its recapture abilities. The reuse experiments were carried out for five cycles using the ratio of magnetite to oil (1 : 10 wt%), and MNPs were regenerated by washing several times with chloroform. MNPs showed high performance even after five cycles, 83%, as shown in Figure 10 to indicate the economical application of the synthesized MNPs to treat the crisis of oil spill.
4. Conclusion
This work covered the preparation of trisubstituted imidazole by the one pot Debus Radazuski reaction of benzil with salicylic acid and ammonium acetate in acetic acid. After that, hydrophobic trialkyl imidazolium ionic liquid (TTIML) was prepared by the alkylation of the aforementioned imidazole by tetradecyl bromide via the fusion reaction. The synthesized imidazolium IL (TTIML) was successfully utilized as capping agents to Fe3O4 by the postsynthesis method. Various characterization techniques such as FTIR, XRD, TEM, DLS, TGA, and DSA were applied to characterize TTIML-Fe3O4. The polydispersity index (PI) and particle size were 0.16 and 201.1 nm, respectively, to verify the monodispersibility of the prepared MNPs. The contact angle of a droplet of sea water on magnetite thin films was 133° that meant the prepared material is hydrophobic enough for application as the oil spill collector. The high magnetization value (63 emu/g), high dispersibility, low particle size, and high hydrophobicity of TTIML-Fe3O4 enhance the efficiency of MNPs as the oil spill collector, which at a magnetite to oil ratio of (1:25) reached 95.5 ± 2.9%.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Abdelrahman O. Ezzat: conceptualization, methodology, investigation, and writing – review and editing. Mohd Sajid Ali: reviewing and editing. Noorah A. Faqihi: editing. Hamad A. Al-Lohedan: supervision and resources.
Funding
The authors appreciate the funding through the Deanship of Scientific Research Chairs; Research Chair of Surfactants, King Saud University.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University, for funding through Vice Deanship of Scientific Research Chairs; Research Chair of Surfactants.
Open Research
Data Availability Statement
The data used to support the findings of this study are available on request from the corresponding author.




