Advances in Adsorbent Materials for Pharmaceutical Pollutant Removal: A Review of Occurrence, Fate, and State-of-the-Art Remediation
Abstract
The rise in population growth that necessitates an increase in animal husbandry activities has led to overconsumption, improper use, and disposal of pharmaceuticals such as antibiotics leading to environmental pollution. In developing countries where we have inefficient and outdated wastewater treatment facilities that are unable to effectively remove these pollutants from domestic wastewater, the effect is the contamination of groundwater supply used for domestic purposes, necessitating the development of advanced techniques. The adoption of adsorption-based technologies with the use of adsorbents such as carbon-based nanomaterials, biochar, and functionalized nanomaterials has proven to be effective in removing these contaminants from aqueous solutions. For instance, a 3D boron-doped graphene composite has been reported to effectively remove amitriptyline from water with a maximum adsorption capacity of adsorption capacity of 737.4 mg/g. Magnetic starch has proven to be effective in removing diclofenac from aqueous solutions, with a reported maximum adsorption capacity of 620.51 mg/g. However, current studies have a limited understanding of the adsorption process, adsorption mechanisms, and universality of the adsorbents used. Future studies should focus on developing cost-effective and sustainable adsorbents as well as exploring integrated approaches and technologies that combine adsorption with other technologies and techniques for effective removal of pharmaceutical contaminants, ensuring cleaner and safer water resources. To this effect, this review highlights the impact of the overconsumption of pharmaceutical compounds and the different adsorbents thereof that have been incorporated in wastewater treatment for the removal of pharmaceutical compounds from water using the adsorption method. It discusses in detail the increase in adoption of the use of various nanocomposites in the removal of these pollutants from surface waters and the removal efficiencies.
1. Introduction
The sustainability of life on Earth requires high-quality water for domestic purposes, economic activities, and the survival of other organisms [1]. Water has several properties such as its ability to store heat and regulate the Earth’s temperature, in its ice form, it is a habitat for aquatic organisms during winter, and it is a universal solvent. However, the rapid increase in population leading to water shortages, the limited and uneven geographical distribution of freshwater resources, and the growing contamination of freshwater sources have become a significant hindrance to economic and human development [2]. The global overconsumption of pharmaceutical drugs for diagnosing, preventing, and treating diseases in humans and animals has led to their emergence as growing environmental contaminants. Figure 1 represents the IQVIA (IMS Health, Quintiles, VIA) Institute global historical and projected use of pharmaceuticals by region, 2018–2028, defined daily doses (DDD) in billions.
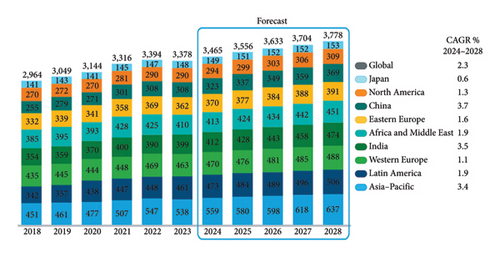
As represented in Figure 1, the annual trend report of the global usage of pharmaceuticals from IQVIA Institute has pointed out that the global trend in the consumption of pharmaceuticals from 2018 to 2024 has increased by 17% (501 billion DDD) as depicted in Figure 1. For every reported region, both for the historical usage and estimated usage, there is an increase in DDD in billions. Over the past five years (2018–2023), the global usage of pharmaceuticals grew by 14% and a further 12% increment is expected through 2028. From Figure 1, China, India, and Japan that are part of Asia–Pacific have been reported separately. The highest estimated volume growth over the next 5 years is expected in China, India, and Asia–Pacific, and all are > 3% compound annual growth (CAGR). The lowest estimated growth over the next 5 years is expected in Japan (0.6% CAGR). Pharmaceutical usage in Latin America and Asia–Pacific will be higher than other regions over the next 5 years. While pharmaceuticals are administered to animals and humans in controlled amounts to treat diseases, significant concentrations of ingested pharmaceuticals are excreted from the human body and animals and find their way into the aquatic ecosystems via sewage discharge [3]. The introduction of these pharmaceutical contaminants into the waterways remains unregulated, posing challenges for monitoring their toxicity to aquatic organisms and the environment. This necessitates the removal of these contaminants before releasing wastewater effluent into the environment [4]. Processes and methods such as reverse osmosis, adsorption, membrane separation, advanced oxidation, and photocatalytic degradation have proven effective in removing these contaminants from aqueous solutions [5]. The adsorption process has proven effective over conventional treatment methods due to its simplicity, affordability, and reusability of adsorbents which enhances the adsorption process by adsorbing specific contaminants selectively [6]. For instance, activated carbon (AC) is the most effective adsorbent for both organic and inorganic contaminants due to its unique physicochemical properties such as high porosity, high surface area, and hydrophobicity properties [7].
A lot of review articles reporting pharmaceutical contamination of water sources have been published [2, 8–15]. For instance, a review by Felis et al. [16] focused on the prevalence of antimicrobial pharmaceuticals in aquatic environments and the ecotoxicity as well as the associated antibiotic resistance phenomenon that is primarily caused by the inefficiency of wastewater treatment plants (WWTPs) to completely remove these pharmaceutical compounds during treatment. After examining the potential threats of these pharmaceuticals on human health and the environment, this review builds on the review of Felis et al. by addressing advanced methods such as adsorption and materials capable of removing these pharmaceuticals efficiently to reduce their distribution in aquatic environments. Mpatani et al. [17] discussed different treatment techniques for the removal of trimethoprim in aqueous media, adsorption being one of them. This review aims to expand on the review of Mpatani et al. by discussing various adsorbents that have been reported effective in removing trimethoprim and other pharmaceutical compounds from aquatic environments.
2. Occurrence of Pharmaceuticals in the Environment
Traditional water treatment technologies partially eliminate pharmaceutical contaminants, posing a significant risk to marine organisms and public health [18]. The prevalence of these chemicals in the marine ecosystem, coupled with the widespread use of pharmaceuticals globally and the introduction of new ones, has contributed to their persistence and their potent metabolites. The occurrence of pharmaceuticals in the environment varies across regions (Figure 1), making global comparisons of concentrations meaningless due to potential discrepancies in targeted chemicals across different regions [19]. Figure 2 illustrates the global occurrence of pharmaceuticals and personal care products (PPCPs) in surface water [20].
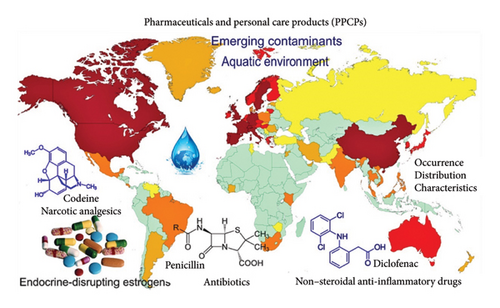
Thus, pharmaceuticals detected in a particular area may not be present in other areas where they are not widely used [19]. Most pharmaceuticals have been detected in various aquatic environmental compartments including influents, sewage and hospital effluents, surface waters, groundwater, drinking water, and river sediments [11, 18, 21]. Groundwater, often used as drinking water, may contain pharmaceutical contaminants that enter through infiltration or bank filtration from surface water or soil leaching. Although reliable toxicological studies on the long-term consumption of drinking water with low concentrations of selected pharmaceuticals are not available, scientists continue to discuss the continuous impact of these substances on humans and animals [16]. The most reported prioritized pharmaceutical pollutants are antibiotics followed by lipid regulators, nonsteroidal anti-inflammatory drugs (NSAIDs), hormones, antidepressants, and other PPCPs. For antibiotics, sulfamethoxazole has the highest weighted average risk quotient (WARQ) value of 21.3 followed by diclofenac (DIC). Even though most occurring pharmaceutical pollutants in aquatic environments have been reported to have high-risk levels, some PPCPs have been reported to be highly toxic irrespective of their low occurrence levels in aquatic environments [22]. In Kenya, Africa, doxycycline, amoxicillin, sulfamethoxazole, trimethoprim, ciprofloxacin (CIP), and norfloxacin have been detected in wastewater, surface waters, and river sediments at concentrations ranging from 0.1 to 56.6 μgL−1 [21]. In Asia, ampicillin and amoxicillin have been reported to have high concentrations reaching 354 and 101 μgL−1, respectively, in surface waters in Sri Lanka [2]. Pharmaceuticals such as venlafaxine, azithromycin, and other endocrine-disrupting compounds have been detected in coastal areas of various European countries including Portugal, Spain, Italy, Netherlands, and Norway [23]. Fluoxetine, norfluoxetine, and paroxetine have been detected in aquatic environments in North America with concentrations ranging from 0.58 to 1.08 ng/g [24]. In Antarctica, the concentration of pharmaceuticals in lake waters has been determined to range from < 0.66 to 70 ng/L [25].
3. Common Exposure Routes of Pharmaceuticals and Their Detection in Aquatic Environment
3.1. Exposure Routes of Pharmaceuticals to Aquatic Environment
Pharmaceutical industries produce healthcare commodities, vitamins, vaccines, hormones, proteins, cell cultures, antibiotics, and personal care products essential for human health management. In the process, pharmaceutical effluent is generated and contains a mixture of active pharmaceutical ingredients, by-products, heavy metals, chemicals, microorganisms, and cell culture associated with the production process and is discharged in aquatic ecosystems, i.e., WWTPs, a major sink for pharmaceutical effluent [26]. Figure 3 illustrates the production release of human and veterinary pharmaceuticals into the environment [27].
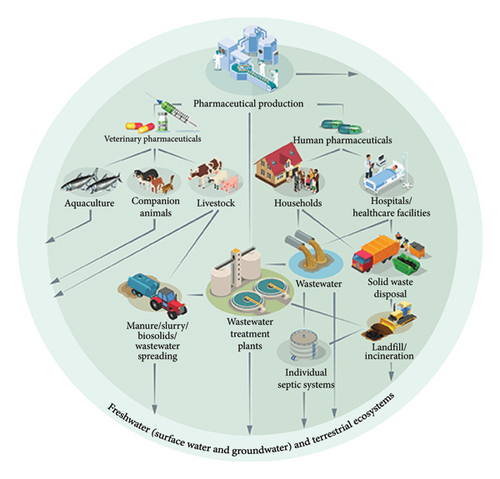
The main sources of pharmaceutical contaminants include direct discharge of raw and treated wastewater from hospital, municipal, and industrial WWTPs; sewage sludge; sewer leakage or overflow; animal manure; metabolized and unmetabolized pharmaceuticals in human feces and urine; surface runoff; and use of municipal wastewater onto lands [28]. Improper disposal of pharmaceutical products such as antibiotics, antiseptics, steroids, and hormones in the environment increases the spread of persistent pharmaceutical micropollutants that penetrate surface and groundwater, posing significant risks to humans and aquatic life [29]. Figure 4 depicts the sources and pathways of pharmaceutical contaminants into the environment [30].
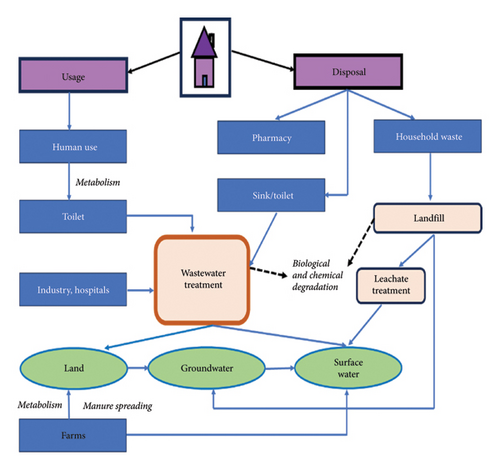
3.2. Detection of Pharmaceuticals in Aquatic Environment
Pharmaceuticals and their metabolites have been detected at low concentrations (ng/L to μg/L) in aquatic ecosystems such as surface water, groundwater, seawater, and drinking water [21]. A vital step in assessing the impact of pharmaceutical compounds and their metabolites in aquatic environments is establishing and validating methods and techniques for their extraction and determination in marine environmental samples. One of the traditional techniques used for the identification of pharmaceuticals in marine environmental samples is the enzyme-linked immunosorbent assay (ELISA) technique which screens pharmaceutical compounds with similar structures in a sample [31]. Since it is difficult to differentiate similar structures using the ELISA technique, chromatographic techniques such as liquid chromatography [32] and supercritical fluid chromatography [33] coupled with tandem mass spectrometry have been developed for easier identification and determination of pharmaceutical compounds in aquatic environmental matrices [34].
Low concentrations of pharmaceutical compounds in environmental matrices make direct analysis using chromatographic techniques challenging, prompting the use of preconcentration techniques of the samples such as solid phase extraction (SPE) which not only reduces matrix effects but also isolates the target compounds effectively [35]. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a highly effective method for the rapid analysis of pharmaceuticals in aquatic sample matrices. LC-MS/MS offers high sensitivity, selectivity, and robustness, enabling the detection of polar organic compounds at parts per billion (ppb) levels. Additionally, the use of electrospray ionization and multiple reaction monitoring modes enhances effective identification, confirmation, and quantification of pharmaceutical compounds [36].
4. Fate and Toxicity of Pharmaceutical Compounds
The flow of pharmaceutical contaminants into water systems occurs through various sources, including wastewater treatment effluent, direct drug disposal into the environment, human and animal waste, hospital effluent, veterinary and agricultural practices, and industrial discharges [37]. Pharmaceuticals’ fate in the environment is influenced by their natural degradability and physicochemical properties like hydrophobicity index, solubility, and chemical structure, which are significantly influenced by local factors or microenvironmental parameters like hydrolysis, photolysis, microbial degradation, or biodegradation [33].
Contamination of water resources with pharmaceutically active compounds, antiepileptics, and opiates poses serious health risks, including neurophysiological, genotoxicity, and carcinogenicity [22]. Antidepressants, benzodiazepines, and antihistamines can affect fish behavior, fitness, food-web properties, and ecosystem functioning. Long-term exposure to these drugs can lead to species trait changes, such as male fish feminizing due to the exposure to estrogen in aquatic ecosystems, and reduced fish fertility and reproductive functions due to exposure to dutasteride drugs [38, 39]. Pharmaceutical exposure has severe reproductive effects on aquatic biota, affecting the early life stages of various organisms [40]. Irrigation with wastewater can lead to a higher accumulation of pharmaceuticals in soil than in groundwater, with a high leaching capacity of pharmaceuticals. These drugs negatively impact soil microbial activities and can enter the food chain through sewage treatment plants and livestock waste fertilization [2].
The bioactivity and persistence of most of the pharmaceutical compounds and their metabolites pose a significant challenge for conventional physical, biological, and chemical treatment methods [37]. Thus, the wastewater effluent contaminated with pharmaceuticals contaminates groundwater, surface water, seawater, and drinking water, posing severe risks to public and animal health [41]. Even at low concentrations (ng/L or μg/L levels) in aquatic systems, pharmaceutical compounds and their metabolites pose significant risks to the environment and human and animal health [33, 42]. Therefore, developing effective wastewater treatment technologies is crucial in their removal from surface waters contaminated with these pollutants [43].
To date, we have a wide array of wastewater treatment technologies that are available, including reverse osmosis, biological treatment, AC filtration, UV radiation, electrochemical degradation, microfiltration, phytoremediation, and advanced oxidation processes (AOPs), which have been successfully utilized in varying degrees in the removal of pharmaceutical from wastewater [44, 45]. Tahir et al. reviewed the removal of PPCPs using various metal–organic framework–derived adsorbents such as magnetic MOF-nanocomposites among other MOF nanocomposites [46]. The use of granular AC, powdered AC, and AC-based adsorbents in the removal of various pharmaceutical pollutants has been discussed by Yi Yang et al. [47] and Yunlong Leo et al. [48].
Considering the raised subjects, this review builds upon the aforementioned reviews by providing an overview of the current state of the adsorption process and different adsorbents used to remove pharmaceutical compounds and their metabolites from water ecosystems. The gaps in the literature, suggestions, and future studies of various adsorbents and adsorption processes are also discussed.
5. Treatment Techniques for Remediation of Pharmaceuticals in the Aquatic Environment
In most WWTPs, conventional methods of treating wastewater from pharmaceuticals are still in use and are based on two stages of treatment: physical and chemical treatment [38]. Conventional wastewater treatment techniques aim to remove pathogens, reduce color and turbidity, and control odor and taste. Nevertheless, these methods have low pharmaceutical removal efficiencies, necessitating the use of advanced wastewater treatment techniques and methods [49]. Techniques used to remove pharmaceutical residues in WWTPs include membrane filtration techniques, adsorption methods, AOPs, and combined technologies [50].
One of the wastewater treatment methods is adsorption. The adsorption process involves adhering atoms, ions, or molecules from gases or liquids onto a solid surface [51]. Adsorption has gained a lot of interest in the removal of organic pollutants due to its simplicity, high efficiency, cost-effectiveness, and the diversity of adsorbents such as metal oxides, zeolites, carbonaceous materials, and organic–inorganic hybrid materials [30]. It involves the removal of pollutants from a liquid or gas phase and reducing their concentration on a solid surface, until the amount of the impurities in the fluid phase is in equilibrium with the solutes’ concentration at the surface. An adsorbate is the species adsorbed on a surface while an adsorbent is the medium on which the adsorbate is adsorbed. The stages of the adsorption process are bulk diffusion; film diffusion; intraparticle diffusion; and adsorption on the solid surface. Adsorption efficiency is influenced by several factors such as temperature, pH, contact time, adsorbent dose, agitation rate, pollutant concentration, ionic strength, and nature of the adsorbent, including its surface area, particle size, pore structure, and the presence of functional groups on the surface [52]. The adsorption treatment method has proved to be efficient in treating waster compared to newer methods such as electrochemical methods, AOPs, membrane technologies, and catalysis methods due to its simple design and ease of operation, cost-effectiveness, universality of the adsorbents, no or limited by-products, and high chances of reusing the adsorbent [53]. Adsorption is a versatile method that removes both organic and inorganic pollutants in the presence of biological matter in various fluid media including wastewater and releases less hazardous by-products in comparison with other treatment methods [11].
Several factors, including the chemical structure, concentration, solubility, charge, ecotoxicity, and bioactivity of pharmaceutical compounds, impact the removal rate and removal efficiency of pharmaceutical contaminants in WWTPs. These properties affect the physical and chemical interactions between the pharmaceutical contaminants, the wastewater, and the treatment process, influencing their removal efficiency. Thus, it is crucial to take into account the properties of these pharmaceutical compounds to develop effective and sustainable wastewater treatment technologies that can efficiently remove these contaminants from the wastewater before being discharged into the environment [41, 54].
5.1. Adsorption Mechanisms
The interactions between the adsorbent and the pharmaceutical compounds are influenced by the physiochemical properties of both the adsorbent and the pharmaceutical compounds [55]. Interaction mechanisms between the pharmaceuticals and adsorbents include the following.
5.1.1. Hydrophobic (Including Weak van der Waals) and Electron Donor–Acceptor Interactions
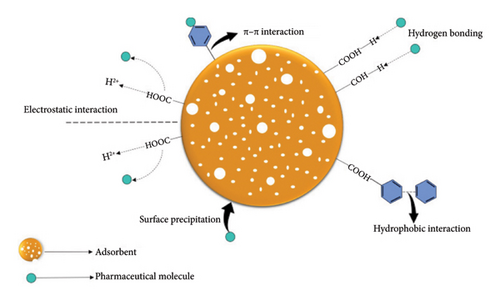
These interactions involves the sorption of hydrophobic and nonionic molecules which are facilitated through hydrogen bonding with the hydroxyl groups present on the surface of the molecules [56].
5.1.2. Ion Exchange and Electrostatic Interaction
One of the interaction mechanisms between the adsorbent and the pharmaceutical compounds is ion exchange between the pharmaceutical compounds and the protons on oxygen-containing functional groups such as hydroxyl groups and carboxyl groups. Electrostatic interactions are dependent on pH, whereby at a high pH when the isoelectric point of the adsorbent surface reduces, the electrostatic interaction between the adsorbent and the ions increases [57].
5.1.3. Surface Complexation
Surface complexation involves the interactions between pharmaceutical compounds with multiatom structures containing distinct metal-functional groups on the adsorbent’s surface [58].
Figure 5 illustrates the interaction mechanisms between pharmaceuticals and the adsorbent [59].
5.2. Adsorption Isotherm and Kinetic Models
To better understand the batch adsorption process, we employ the use of adsorption isotherms to quantitatively compare different adsorbent materials and optimize their use by observing the maximum adsorption capacity as a function of the experimental conditions [60]. The adsorption isotherm models are used to determine the equilibrium performance at a constant temperature [61] and the various mechanisms of interactions such as hydrogen bonding, electrostatic interactions, hydrophobic interactions, and surface complexation between the adsorbent and the adsorbate [62]. Adsorption kinetic studies provide information on the rate of adsorption which is fundamental in terms of the contact time required to remove the maximum amount of the adsorbate [60]. Commonly used mathematical kinetic models are pseudo-first-order (PFO) and pseudo-second-order (PSO) models [63]. Adsorption isotherms and kinetic models are depicted in Table 1.
| Nonlinear equation | Parameters | References | |
|---|---|---|---|
| Kinetic models | |||
| Pseudo-first order (PFO) |
|
[64] | |
| Pseudo-second order (PSO) | k2 (g/mg min) is the rate constant for the PSO model | [61] | |
| Elovich | qt = (1/β)ln(1 + αβt) |
|
[65] |
| Weber–Morris (WM) intraparticle diffusion | qt = kit1/2 + Ci |
|
[66] |
| Boyd |
|
|
[67] |
| Isotherm models | |||
| Langmuir | qe = (qmaxKLCe)/(1 + KLCe) |
|
[68] |
| Freundlich |
|
[69] | |
| Temkin | qe = ((RT)/bT)ln(ATCe) |
|
[70] |
| Sip’s |
|
[71] | |
| Dubinin–Radushkevich (DR) model |
|
|
[72] |
| Van’t Hoff equation |
|
|
[73] |
5.3. Adsorbents for Pharmaceutical Compounds
Application of traditional adsorbents has limitations such as slow adsorption kinetics which limits their efficiency, pH dependence of the wastewater that can fluctuate, poor heat and mass transfer performance, disposal of used adsorbents which cannot be regenerated adding to adverse environmental impacts [74, 75], and low desorption efficiency especially in highly polluted environments. Thus, to overcome these limitations, research and development of newer, cost-effective, and efficient adsorbents for large-scale applications have been conducted [1, 76]. Recently, a wide range of materials have gained interest in the removal of pharmaceutical contaminants from aquatic ecosystems, such as metal-oxide nanoparticles [77], AC [70], chitosan-based adsorbents [64], biogenic adsorbents [52], biochar [78], composites [65], agricultural residues [79], and MOFs [80]. Removal of PCs such as trimethoprim [81] and carbamazepine [82] has been reported.
5.3.1. Biochar
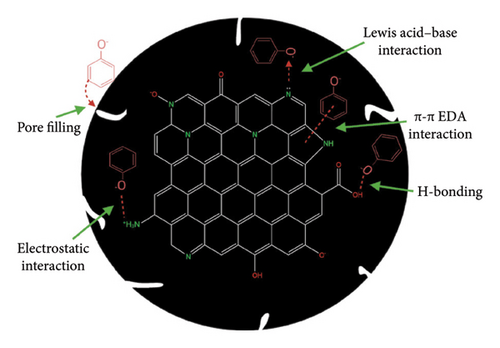
Biochar is an economically reliable and functional material extracted by carbonizing biomass such as organic feedstocks from crops, forestry residues, sewage sludge, algal biomass, and poultry manure in the absence of oxygen [83]. Properties such as high specific surface area, cost of regeneration, porous structure, surface functional groups, high mineral content, and microstructure play a crucial role in its effectiveness in removing emerging environmental contaminants, soil remediation, greenhouse gas reduction, and energy production [84]. Biochar has been used in the adsorption of pharmaceutical contaminants from wastewater with an average removal rate of 14.2%–65.5% [85] with varying adsorption capacities of CIP qmax (75.97 mg/g), DIC qmax (40.99 mg/g) [86], and norfloxacin qmax (166.48 mg/g) [87]. Various adsorption mechanisms of organic contaminants on N-doped biochar as shown in Figure 6 include the following. The micropores and mesopores on biochar surfaces determine the pore-filling mechanism. In addition to functional groups, porous biochar surfaces, with larger pore sizes, perform better in the adsorption of polar and nonpolar organic pollutants; hydrogen bonding is a dipole–dipole attraction that occurs between the hydrogen donor and acceptor functional groups present on the surface of the N-doped biochar that binds pollutants through hydrogen bonding; π–π electron donor and acceptor (EDA) interactions are crucial for the adsorption of planar aromatic pollutants on graphene-like biochar surfaces. Uneven charge sharing resulting from incomplete graphitization forms π electron-rich and deficient regions that donate or accept electrons from aromatic organic pollutants through π–π EDA interactions which are increased by N-doping on biochar, as nitrogen functional groups withdraw electrons from the graphene layer, creating positive surroundings for π-electron-acceptors and π-electron-enriched aromatic rings; Lewis acid–base interactions have been reported between N-doped biochar and organic pollutants, with changes in N-functionality content and binding energies after adsorption; electrostatic interactions between organic pollutants and biochar surfaces attract opposite charges, similar to heavy metal removal, and is dependent on solution pH [88].
5.3.2. Metallic Nanomaterials
The use of nanomaterials in removing pollutants such as pharmaceutical contaminants from wastewater has great potential to improve water purification and decontamination efficiency [89]. Metal oxide nanoparticles like SiO2, ZnO, TiO2, and WO3 have been widely recognized for their potential in environmental remediation [90]. Many metal oxide nanoparticles have been used to remove pharmaceuticals from aqueous solutions [77]. Adsorption of trimethoprim from aqueous solutions using ZnO NPs at optimal conditions of NP dosage 0.2 g/100 mL, 110 min, pH 5, 25°C, and an initial concentration of 50 ppm yielded a maximum adsorption capacity of 118.782 mg/g and maximum removal of 84.6% [91]. Bimetallic NPs are unique metal components with chemical stability, reactivity, and size-dependent electrical and optical properties, influenced by composition, shape, and size distribution. Bimetallic NPs have a higher pollutant removal ability compared to monometallic nanoparticles. Their structural features, including multishell, core-shell segregated, intermetallic, and heterogeneous structures, depend on combining metal nature (relative bond strength, atomic dimensions, charge transfer, and electronic and magnetic effects) [92]. Bimetallic nanoparticles have been found effective in the removal of pharmaceuticals [93]. Fe–Cu supported on alginate-limestone has been reported to remove CIP and levofloxacin (LEV) from wastewater. Optimal conditions for the removal of CIP were CIP 20 ppm, 45 min, pH 6, and 30°C with a maximum removal efficiency of 97.3% while those for LEV were LEV 10 ppm, 40 min, pH 7, and 30°C, with a maximum removal efficiency of 100% [94].
5.3.3. Chitosan and Chitin-Based Adsorbents
Chitosan is a polysaccharide that contains two types of monomers, one containing an acetamido group and the other one containing an amino group. Chitosan is obtained from chitin, which is obtained from natural sources such as shrimp residuals, exoskeletons of insects and fungal cell walls, crab and lobster, fungal mycelia, and green algae. Diacylation of chitin through enzymatic and alkaline methods produces chitosan. Chitin is differentiated from chitosan in the substitution of the acetamide group. Some properties of chitosan such as its polycationic character in acid media, its ability to form hydrogen bonds, van der Walls and electrostatic interactions, higher degree of diacylation (DD), crystallinity, molecular weight, solubility, increased surface area, and decreased particle size make it an effective adsorbent. Chitosan is modified with other additives such as cellulose, starch, alginate, clays, carbon-based materials, metal–organic frameworks, and other polymeric materials to improve mechanical properties and thermal stability and increase the surface area for chitosan [60]. For instance, mud-crab chitosan has been reported to adsorb rifampicin (RIF), streptomycin (STM), and ibuprofen with maximum adsorption capacity of 66.91, 11.00, and 24.21 mg/g, respectively [64]. Magnetic quaternary chitosan-based nanosorbents are effective for magnetically assisted removal of DIC, naproxen, and ketoprofen from water with optimum conditions of pH 5, 30 min, and NSAID initial concentration of 100 mg/L with maximum adsorption capacity of 188.5, 438.1, and 221.5 mg/g, respectively [95].
5.3.4. Agro-Waste–Based Adsorbents
Sustainable water and wastewater management involves the use of green adsorbents as alternatives to synthetic adsorbents. Agricultural waste is a source of green adsorbents that have enhanced efficiency, sustainable production cost, and high regeneration capacity. Agro-wastes are solid residues generated from agricultural activities such as crops and livestock production. The major component of agricultural waste is lignocellulosic material which consists of cellulose, hemicellulose, lignin, pectin, proteins, lipids, starch, and simple sugars. Agro-waste–based adsorbents are used in the form of raw biomass or chemical or physical forms. In most cases, the biomass is converted to AC through pyrolysis; AC has a high surface area which increases its uptake or adsorption capacity and high porosity which increases the adsorption sites of the AC. The production of AC from agricultural waste is a two-phase process: the carbonization phase which involves the development of the microcrystalline structure of the AC and the activation phase which can be either physical or chemical activation. AC produced from vegetable origins and peach stones has been reported to exhibit a maximum adsorption capacity of 242 and 335 mg/g, respectively. The production of biochar from biomass involves the pyrolysis of naturally available biomass and plant residuals such as wood and fruit peels in the absence of oxygen. It has been reported that biochar derived from mung bean husk displayed a 99.16% removal efficiency for ibuprofen with a maximum adsorption capacity of 59.76 mg/g [96]. Hemp seed nanocomposites have been used to remove ibuprofen at optimum conditions of initial concentration of the ibuprofen of 100 mg/L, 30 min, and pH 8 with a maximum adsorption capacity of 21.50 mg/g [97].
5.3.5. AC
AC, a porous material made from organic and industrial waste, is widely used in wastewater treatment for the adsorption of water pollutants, despite its high cost. Its pores are categorized as micropores, mesopores, and macropores, resulting in a high surface area [98]. The adsorption capacity of AC is influenced by its porous structure and surface area, as well as surface functional groups. These include hydrogen atoms and heteroatoms like oxygen, nitrogen, chlorine, and sulfur. The proportion of these atoms on the adsorbent’s surface can be altered post-activation. Oxygen groups, primarily resulting from oxidation, and nitrogenous groups depend on the raw material’s nature. Functional groups can facilitate bonding by attracting positively charged ions like metal ions [99]. Adsorption of pharmaceuticals is influenced by physicochemical characteristics, water matrix, and dissolved organic matter. The dissolved organic matter can limit adsorption by blocking the pores and adsorption sites on the surface of the AC. Pharmaceuticals can also interact with dissolved oxygen and dissolved organic matter on the PAC surface affecting the adsorption efficiency of the AC. A study on the removal of trimethoprim, sulfamethoxazole, and DIC from water using powdered AC showed that the removal of trimethoprim was the most effective due to its high hydrophobicity and the charge, while sulfamethoxazole was the least effective. The adsorption efficiencies of sulfamethoxazole, DIC, and trimethoprim were 46%, 88%, and 96%, respectively [54]. The removal of ibuprofen using groundnut shell activated carbon (GNSAC) at optimum conditions of 0.62 g/L adsorbent dosage, 30°C, and 30 min contact time achieved a removal efficiency of 68.53%. The maximum adsorption capacity was 40.82 mg/g [77].
5.3.6. Carbon Nanotubes (CNTs)
CNTs are highly versatile and easily modified materials for decontaminating pharmaceutical contaminants in aquatic environments due to their high porosity, large contact surface area, and small particle size [100]. CNTs can be functionalized to remove contaminants through hydrophobic, electrostatic, π-π interactions, and hydrogen bond adsorption mechanisms [101]. The adsorption capacity of CNTs varies between single-walled and multiwalled nanotubes, with single-walled nanotubes exhibiting higher removal rates for ibuprofen, and multiwalled ones exhibiting high removal rates for amoxicillin. The high cost and toxicity of CNTs limit their applications in wastewater treatment [102, 103]. The maximum adsorption capacities of amoxicillin, CIP, and sulfamethoxazole from aqueous solution using chitosan-CNT hydrogel beads at optimal conditions of pH 7, contact time of 24 h, adsorbent dosage of 1.5 g/L, and initial adsorbate concentration of 40 mg/L have been reported to be 23.1, 23.7, and 25.17 mg/g, respectively [104].
5.3.7. Cellulose-Based Adsorbents
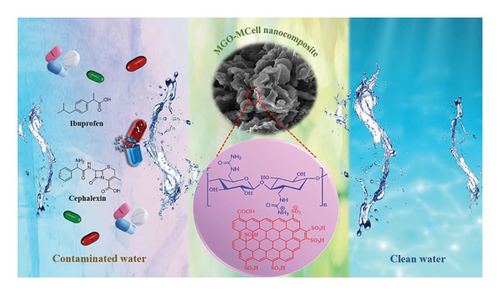
Over the past few years, nanocellulose and its derivatives have drawn attention as promising bio-based materials for water treatment applications due to their biodegradability, high adsorption efficiency, natural abundance, cost-effectiveness, and biocompatibility. The abundance of hydroxyl functional groups on the surfaces of cellulose nanocrystals (CNCs) and cellulose nanofibrils (CNFs) enables a broad range of surface modifications which results in the successful production of nanocomposites with tunable characteristics. Nanocelluloses, particularly their chemically modified hydrogels or aerogels, have been extensively researched as effective adsorbents for oil, heavy metals, dyes, and some pharmaceuticals [105]. The main drawbacks of using cellulose-based adsorbents include the generation of large amounts of sludge, its poor moisture resistance, low regeneration, limited mechanical strength, and chemical stability [106]. Lignin NPs and cationic nanoparticles are susceptible to colloidal suspension in water limiting their regeneration; thus, they are cross-linked with other materials to develop cellulose-based microporous materials used in the removal of pharmaceutical pollutants [107]. The removal of CIP and LEV using Fe clay-cellulose acrylamide beads at optimum conditions of 10 mg/L initial drug concentration, pH 5, 15 mg/L adsorbent dosage, 25°C, and 180 min contact time achieved maximum adsorption capacity of 57.84 mg/g and 38.01 mg/g, respectively [108]. As shown in Figure 7, modified graphene oxide/modified cellulose (MGO-Mcell) nanocomposite has been used to remove 91% of 20 ppm of ibuprofen and 82% of 40 ppm of cephalexin from aqueous solutions under optimal conditions of contact time 90 min, pH 7, and at room temperature with a maximum adsorption capacity of 30.21 mg/g and ∼99.01 mg/g, respectively [109].
5.3.8. Composite Nanoparticles
Composite nanoparticles are nanomaterials with composite structures that are made of two or more nanomaterials with sizes ranging from 1 to 100 nm with a mutual contact interface between them. Composite nanoparticles enhance the performance and properties of single components. Based on their structural properties, composite nanoparticles are categorized into three groups: simple hybrid; core or shell-structured; and multifunctional quantum dots [110]. The characteristics of the composites are different from the characteristics of each component used to produce the nanocomposite. Various nanocomposites such as Fe3O4@Zr-MOF adsorbent for tetracycline (qmax of 942.12 mg/g) [80], Fe3O4@MIL-101(Cr) adsorbent for metoprolol (qmax of 28.3 mg/g) [111], and 3D-BGO adsorbent for amitriptyline [65] have been identified to be effective in the adsorption of various pharmaceutical compounds. Other nanocomposites such as CN-based magnetic composites, Fe3O4 NPs magnetic composites, ZIF/zeolite composites, ZIF-67 composite, plant-based NP composites, Ag NP-based composites, biochar composites, Fe2O3/carbide nanofiber, CoFe2O4 NPs, and silk nanofibril-MOF composites have been used in the removal of various pollutants including heavy metals, antibiotics, dyes, and organic pollutants from an aquatic environment. For instance, the removal of ibuprofen using TiO₂-GNSAC at optimum conditions of 0.5 g/L adosrbent dosage, 30°C, and 50 min contact time achieved a removal efficiency of 81.78% and a maximum adsorption capacity of 55.56 mg/g [77]. Biocomposites of chitosan inserted into acid-activated calcium bentonite have been reported to adsorb CIP effectively in fresh and saline water at optimal conditions of initial concentration of 50 ppm, pH 5.5, adsorbent dose of 1.067 g/L, and room temperature (25 ± 2°C), with a maximum removal efficiency and adsorption capacity of 99.2% and 50 mg/g, respectively [112].
Various composites have been used to remove pharmaceutical contaminants from aquatic systems as illustrated in Table 2.
| Adsorbent | Pharmaceutical contaminants (PCs) | Removal efficiency (%) | Maximum adsorption capacity, qmax (mg/g) | Kinetics adsorption model | Isotherm adsorption model | Reference |
|---|---|---|---|---|---|---|
| Fe3O4@MIL-101 (Cr) | Acebutolol | 94.1–97.1 | 30.9 | Pseudo-second-order | Langmuir | [111] |
| Metoprolol | 28.3 | |||||
| 3D boron-doped graphene composite (3D-BGO) | Amitriptyline | 89.31 | 737.4 | Pseudo-second-order | Langmuir | [65] |
| Magnetic starch (MAST) | Diclofenac sodium | — | 620.51 | Pseudo-second-order | Langmuir | [113] |
| Activated carbon of Chlorella vulgaris (ACCV)/Fe3O4 mc | Tetracycline (TTC) | 90.47 | 26.18 | Pseudo-second-order | Langmuir | [114] |
| Cellulosic sisal fiber/polypyrrole-polyaniline NPs | Ibuprofen | 88 | 19.45 | Pseudo-second-order | Langmuir | [115] |
| Activated carbon (AC)/TiO2 composite | Diclofenac (DCF) | — | 153.8 | — | Langmuir | [116] |
| Carbamazepine (CBZ) | 105.3 | |||||
| Sulfamethoxazole (SMX) | 125.0 | |||||
| Gum Arabic-magnetic nanoparticles (GA-MNPs) | Ciprofloxacin | 96.30 | 54.6449 | Pseudo-second-order | Freundlich | [117] |
| NiFe2O4/activated carbon magnetic composite | Ibuprofen | 86.46 | 261.35 | Pseudo-second-order | Sip’s model | [118] |
| Ketoprofen | 97.75 | |||||
| Magnetic poly (St-AMPS) nanoparticles | Ceftriaxone sodium (CFX) | — | 150.602 | Pseudo-second-order | Langmuir | [119] |
| Diclofenac sodium (DCF) | 47.824 | |||||
| Atenolol (ATN) | 119.904 | |||||
| Activated carbon-chitosan-graphene oxide (GO) | Acetaminophen (ACP) | — | 13.7 | Pseudo-second-order | Langmuir | [120] |
| Carbamazepine (CBZ) | 11.2 | |||||
6. Conclusion and Future Perspective in Adsorption of Pharmaceutical Wastes on Adsorbents
Global population growth and animal husbandry have led to a rise in pharmaceutical consumption and pharmaceutical waste accumulation in aquatic ecosystems. Conventional methods encounter challenges to completely remove these contaminants, leaving significant pharmaceutical residues. The future of the adsorption of pharmaceuticals using cellulose-based adsorbents requires functionalization and modification techniques such as the grafting of cellulose with other nanomaterials to enhance its adsorption capacity and selectivity for pharmaceutical compounds. Designing nanocomposites by combining various nanomaterials such as CNTs, metallic nanoparticles, biochar, AC, and chitosan for the selective removal of specific pharmaceuticals improves removal efficiency and reduces the effects of the by-products. Future research is necessary to optimize chemical activation and pyrolysis conditions in the production of biochar and AC to maximize their adsorption properties. Recommendations for improving the adsorption of pharmaceutical adsorption from wastewater include optimizing adsorption processes by integrating them with other methods such as AOPs or membrane filtration to enhance removal efficiency. Developing effective methods for adsorbent regeneration that extend their lifecycle and recyclability. Additionally, surface functionalization of adsorbents can enhance selectivity and specificity toward pharmaceutical compounds. It is also essential to evaluate the efficiency and economic feasibility of developing adsorbents and adsorption systems. Moreover, it is neccessary to scale up laboratory studies to real-world applications and assess the economic feassibilty at large-scale performance. There is limited research on the stability and performance of reused adsorbents under varying experimental and environmental conditions and comprehensive life cycle assessments of recycling and disposing of adsorbents. More studies are needed to understand the molecular mechanisms of adsorption of various pharmaceutical compounds, which can help in the development of effective adsorbents. To address existing research gaps, further studies should focus on conducting pilot-scale studies to evaluate the performance of adsorbents in industrial applications. These studies should assess long-term stability, regeneration capacity, cost-benefit analysis, and environmental impacts associated with the production, usage, recyclability, and disposal of adsorbents. Additionally, advanced analytical tools such as modelling and simulation should be employed to study molecular mechanisms of adsorption processes.
In conclusion, pharmaceuticals are crucial for human and animal health but their misuse and improper disposal can pose significant risks to aquatic and bio-ecosystems. The contaminants and their metabolites are complex and resistant to conventional wastewater treatment methods. Adsorption is a low-cost alternative for removing pharmaceuticals, and composite adsorbents are a promising prospect due to their enhanced nanomaterial properties and ease of modification. The use of nanotechnology in wastewater treatment in the development of adsorbents such as biochar, AC, nanocomposites, metallic nanoparticles, cellulose-based adsorbents, and chitosan adsorbents is effective in enhancing adsorption processes. Nanocomposites are cost-effective adsorbents with excellent pharmaceutical adsorption capacity, leading to removal rates of up to 86%–97%. The PSO model is best suited for understanding the interaction kinetics of the adsorbate with various composite adsorbents. The Langmuir isotherm model is frequently used to describe the adsorption equilibrium.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




