Design, Synthesis, and In Vitro and In Silico Biological Exploration of Novel Pyridine-Embedded 1,3,4-Oxadiazole Hybrids as Potential Antimicrobial Agents
Abstract
Antibiotic resistance represents a significant public health challenge in the current century. The β-lactam antibiotics, together with carbapenems, are inactivated by zinc-dependent bacterial enzymes called metallo-β-lactamases (MBLs). Presently there are no clinically permitted MBL inhibitors, and to produce such drugs, it is indispensable to comprehend their inhibitory action. We investigated an efficient synthesis of pyridine-embedded 1,3,4-oxadiazole hybrids (3a-c) and their antimicrobial activity against different microbial strains. The compounds were characterized by spectral techniques (viz., IR, NMR, and mass). The in vitro antibacterial and antifungal activity was also performed; the compounds (3a-c) displayed excellent antimicrobial activity. The in silico docking studies were evaluated with proteins New Delhi Metallo-Beta-lactamase-1 (NDM-1) and Mycobacterium tuberculosis enoyl reductase (INHA). All the compounds demonstrated a significant binding affinity for the docked proteins. Additionally, molecular dynamics were disclosed for compounds (4a-c).
1. Introduction
Antibiotics revolutionized healthcare in the mid-20th century and continue to serve as a cornerstone of contemporary medicine. Among the various classes of antibiotics, β-lactams are the most extensively utilized, with broad-spectrum penicillins and cephalosporins representing 55% of antibiotic consumption in 2010 [1]. The β-lactam antibiotics are categorized into four distinct subfamilies: penicillins, cephalosporins, monobactams, and carbapenems. The latter group was specifically designed to address infections caused by multidrug-resistant Gram-negative opportunistic pathogens and is utilized solely within hospital environments, often as a last-resort treatment option [2]. In the context of Gram-negative bacteria, the predominant mechanism of resistance to β-lactams is facilitated by the production of β-lactamases (BLs), which are enzymes capable of hydrolyzing the amide bond within the azetidinone (β-lactam) ring [3–5].
The β-lactamase enzymes serve as a common resistance mechanism against the degradation of β-lactam antibiotics, leading to the inactivation of these antimicrobial agents. Bacteria synthesize β-lactamase enzymes (BLs), which represent a prevalent strategy for resisting the effects of β-lactam antibiotics, ultimately rendering the antibiotic ineffective [6]. BLs are classified into four molecular classes (A, B, C, and D) based on their amino acid sequences [7].
Enzymes classified under the A, C, and D main categories are referred to as serine-β-lactamases (SBLs) because of the presence of a structural serine residue. This residue engages with the carbonyl carbon of the β-lactam ring, facilitating the ring-opening process. The BLs of classes A, C, and D demonstrate efficacy against various antibiotics, including penicillins, cephalosporins, and monobactams [8]. Metallo-β-lactamase (MBL) belongs to class B and is notable by the subsistence of a divalent zinc ion.
Many cephalosporins, penicillins, and carbapenems are inactivated by these enzymes through the action of hydroxyl groups associated with zinc [9]. The dependence on zinc ions and the identity of their sequences categorize MBLs into three subclasses: B1, B2, and B3. It has been unwavering that the B1 subclass, which includes IMP-1, VIM-2, and New Delhi Metallo-Beta-lactamase 1 (NDM-1), is the mainly clinically noteworthy [10, 11]. The capacity of NDM-1 to effectively hydrolyze nearly all β-lactam antibiotics represents a significant global health risk [12, 13]. This resistance gene is produced by certain bacteria. Various carbapenemases, primarily produced by the extensive bacterial family Enterobacteriaceae, are capable of degrading penicillins, cephalosporins, and, as a final option, carbapenems.
Among heterocyclic molecules, 1,3,4-oxadiazoles are well known for their biological activities. They possess a broad spectrum of biological properties, viz., antifungal, antibacterial, anti-TB [14–17], anticancer [18], antioxidant [19], antiviral [20], antileishmanial [21], anti-inflammatory [22], antidiabetic [23], anticonvulsant [24], and analgesic activities. Further, the anti-TB drug isoniazid and its derivatives showed various biological activities such as anti-TB, antibacterial, antifungal [25–28], inhibitors of urease and inflammatory markers [29], and anticancer activity [30].
Conversely, 1,3,4-oxadiazoles are well known and extensively investigated by investigators owing to their many imperative biological and chemical properties [31]. The capability of these heterocyclic compounds to undergo diverse chemical reactions has made them crucial for molecule arrangement because of their privileged structure, which has massive biological potential [32]. For example, the compounds containing the 1,3,4-oxadiazole core, presently utilized in the medical field include an antiretroviral drug, raltegravir, and an anticancer therapeutic mediator zibotentan [33, 34].

Given the abovementioned literature work and in continuation of our recent work on heterocyclic molecules such as indole [35–39], quinoline [40–45], 1,3-oxazine [46], coumarin [47, 48], and other heterocycles [49, 50], in the form of reviews, we elaborated on the synthesis and pharmacological activities. We herein report the design, synthesis, spectral, biological evaluation, and molecular modeling studies of novel compounds (3a-c) and (4a-c) by making use of hydroxy/amino benzoic acids (Scheme 1).
2. Materials and Methods
2.1. Measurements
IR spectral data were recorded as KBr discs on a PerkinElmer-Spectrum RX-IFTIR. FT NMR Spectrometer model Avance-II is used for 1H NMR (at 400 MHz) and 13C NMR (at 100 MHz) measurements using DMSO as solvent and TMS as reference standard. A mass spectrometer equipped with an ESI source having mass a range of 4000 amu in quadruple and 20,000 amu in Tof is used for ESI-mass spectral study (Waters Micromass Q-Tof Micro). The reaction is monitored by TLC. For the elemental analysis, the Flash EA1112 series is used.
2.2. Experimental
The chemicals utilized in the experiments were obtained from certified suppliers and used in their original form without any further refinement. The physical properties of compounds 3(a-c) were comparable with the literature values [51, 52].
2.2.1. General Procedure for the Synthesis of Compounds (3a-c)
- •
4-(Carboxymethoxy)benzoic acid (3a): Colorless crystals; yield: 78%; m.p. 245°C. IR (KBr) (in cm−1): 1662, 1654 (stretch, C=O, acid), 1528, 1456, (C=C, Aromatic) 1158, 1150 (C-O). 1H NMR (400 MHz, DMSO-d6) δ = 12.22, 12.09 (br, COOH), 8.12 (d, 2H, Ar-H), 7.23 (d, 2H, Ar-H), and 4.85 (s, 2H, -CH2). 13C NMR (100 MHz, DMSO-d6) δ = 169.7, 168.4, 163.4, 130.8, 122.5, 114.8, and 63.8. MS (ESI): m/z (%): M●+ 196 (42); Anal. Calcd for C9H8O5: C, 55.11; H, 4.11%. Found: C, 55.14; H, 4.08%.
- •
2-(Carboxymethoxy)benzoic acid (3b): Off-white crystals; yield: 85%; m.p. 189°C. IR (KBr) (in cm−1): 1649, 1638 (stretch, C=O, acid), 1512, 1445, (C=C, Aromatic) 1156, 1142 (C-O). 1H NMR (400 MHz, DMSO-d6) δ = 12.18, 12.04 (br, COOH), 6.82–7.85 (m, 4H, Ar-H), 4.76 (s, 2H, -CH2). 13C NMR (400 MHz, DMSO-d6) δ = 12.28 (br, COOH), 10.82(br, NH), 6.71–8.92 (m, 7H, Ar-H). 13C NMR (100 MHz, DMSO-d6) δ = 168.2, 165.2, 157.1, 132.4, 130.9, 119.2, 116.1, 113.4 and 66.4. MS (ESI): m/z (%): M●+ 196 (38); Anal. Calcd for C9H8O5: C, 55.11; H, 4.11%. Found: C, 55.08; H, 4.14%.
- •
4-((Carboxymethyl)amino)benzoic acid (3c): Light brown color crystals; yield: 84%; m.p. 234°C. IR (KBr) (in cm−1): 3342 (-NH), 1666, 1658 (stretch, C=O, acid), 1532, 1461, (C=C, Aromatic) 1162, 1154 (C-O). 1H NMR (400 MHz, DMSO-d6) δ = 12.08, 11.79 (br, COOH), 10.62 (s, 1H, -NH), 8.09 (d, 2H, Ar-H), 7.03 (d, 2H, Ar-H), and 4.38 (s, 2H, -CH2). 13C NMR (100 MHz, DMSO-d6) δ = 172.4, 169.5, 152.2, 131.4, 119.3, 112.4, and 45.4. MS (ESI): m/z (%): M●+ 195 (63); Anal. Calcd for C9H9NO4: C, 55.39; H, 4.65; N, 7.18%. Found: C, 55.32; H, 4.69; N, 7.21%.
2.2.2. General Procedure for the Synthesis of Compounds (4a-c)
- •
2-(Pyridin-4-yl)-5-(4-((5-(pyridin-4-yl)-1,3,4-oxadiazol-2-yl)methoxy)phenyl)-1,3,4-oxadiazole (4a): Brown crystals; yield: 74%; m.p. 186°C. IR (KBr) (in cm−1): 1621, 1612, 1602, 1596 (-C=N-), 1168, 1156, 1146 (C-O-C). 1H NMR (400 MHz, DMSO-d6) δ = 6.69–8.92 (m, 12H, Ar-H) and 4.35 (s, 2H, -CH2). 13C NMR (100 MHz, DMSO-d6) δ = 164.1, 162.8, 160.8, 152.6, 150.2 (2), 149.2 (3), 144.2, 139.2, 127.6, 126.5(2), 124.2(2), 121.5(2), 114.8(2), and 73.4. MS (ESI): m/z (%): M●+ 398 (62); Anal. Calcd for C21H14N6O3: C, 63.31; H, 3.54; N, 21.10%. Found: C, 63.28; H, 3.52; N, 21.16%.
- •
2-(Pyridin-4-yl)-5-(2-((5-(pyridin-4-yl)-1,3,4-oxadiazol-2-yl)methoxy)phenyl)-1,3,4-oxadiazole (4b): Light brown crystals; yield: 75%; m.p. 294°C. IR (KBr) (in cm−1): 1619, 1604, 1598, 1586 (-C=N-), 1162, 1148, 1142 (C-O-C). 1H NMR (400 MHz, DMSO-d6) δ = 6.81–8.86 (m, 12H, Ar-H) and 4.26 (s, 2H, -CH2). 13C NMR (100 MHz, DMSO-d6) δ = 165.8 (2), 163.8, 163.2, 158.6, 150.2 (4), 144.3 (2), 131.5, 130.2, 122.6, 121.8 (4), 117.2, 113.4, and 72.8. MS (ESI): m/z (%): M●+ 398 (45); Anal. Calcd for C21H14N6O3: C, 63.31; H, 3.54; N, 21.10%. Found: C, 63.36; H, 3.58; N, 21.06%.
- •
4-(5-(Pyridin-4-yl)-1,3,4-oxadiazol-2-yl)-N-((5-(pyridin-4-yl)-1,3,4-oxadiazol-2-yl)methyl)anili-ne (4c): Dark brown crystals; yield: 69%; m.p. 215°C (decompose). IR (KBr) (in cm−1): 3256 (stretch, NH), 16,121 1613, 1604, 1596 (-C=N-), 1166, 1156, 1150 (C-O-C). 1H NMR (400 MHz, DMSO-d6) δ = 10.46 (br, NH), 6.82–8.43 (m, 12H, Ar-H) and 4.33 (s, 2H, -CH2). 13C NMR (100 MHz, DMSO-d6) δ = 165.2 (3), 163.8, 150.8 (4), 149.6, 144.5 (2), 129.2 (2), 122.2 (4), 115.2, 114.3 (2) and 51.8. MS (ESI): m/z (%): M●+ 397 (45); Anal. Calcd for C21H15N7O2: C, 63.47; H, 3.80; N, 24.67%. Found: C, 63.42; H, 3.83; N, 24.71%.
2.3. Druglikeness Profile
The data presented in Supporting Information File S5 represent the expected druglikeness profiles and ADMET characteristics of newly prepared compounds [53–55].
2.4. Biological Evaluation
2.4.1. Antimicrobial Activity
The antimicrobial activity of compounds (3a-c) and (4a-c) was evaluated by Mueller–Hinton (disc) and potato dextrose agar (well diffusion) methods. The activity follows CLSI’s international suggestion [56, 57]. The details of the study are provided in the Supporting Information File S6.
2.4.2. Anti-TB Activity
The investigation into anti-TB activity employs a previously established method [58, 59] involving the dispersion culture technique utilizing Kirchner’s media supplemented with Tween-80 to target the M. tuberculosis (ATCC 25177) strain. Test samples were formulated at concentrations of 100, 50, 25, 12.5, 6.25, 3.12, and 1.56 μg/mL in DMF.
2.5. Molecular Modeling Studies
2.5.1. Molecular Docking
2.5.1.1. Protein Preparation
Crystal structure of M. tuberculosis enoyl reductase (INHA) complexed with 1-cyclohexyl-n-(3,5-dichlorophenyl)-5-oxopyrrolidine-3-carboxamide (PDB ID: 4TZK), and structure NDM-1 (PDB ID: 3ZR9) was downloaded in the .pdb format. The bound ligands (3s)-1-cyclohexyl-N-(3,5-dichlorophenyl)-5-oxopyrrolidine-3-carboxamide (641) of 4TZK and nicotinamide adenine dinucleotide (NAD) and isoniazid were considered as a reference for 4TZK and ertapenem as a reference for 3ZR9. The proteins were converted into pdbqt using the PyRX0.9 [60–62].
2.5.1.2. Ligand Preparation
To validate the docking tool and to obtain the interaction score, the co-crystallized ligands were redocked. The successful scoring function, as defined by the validation technique described in the literature, is the one where the RMSD of the best-docked conformation is less than 2 Å from the experimental one [60–62].
2.5.2. Molecular Dynamics Simulation
In this research, an MD simulation lasting 50 ns was performed to evaluate the stability of the APO-Protein and protein-ligand complexes, employing RMSD and RMSF plots for the analysis [63, 64]. MD simulations were conducted for both proteins, taking into account the APO form as well as the protein-ligand complexes that were identified following the docking process, utilizing Desmond Maestro v11.3 [61, 62]. The details of the study are provided in the Supporting Information File S7.
3. Results and Discussion
3.1. Chemistry
The synthesis of hydroxy/amino benzoic acid–derived novel compounds 3 (a-c) and 4 (a-c) is illustrated in Scheme 1. The compounds (carboxymethyl)hydroxy/amino benzoic acid (3a-c) were prepared by the reaction of substituted hydroxy/amino benzoic acid 1(a-c) and chloroacetic acid in the presence of the aq. NaOH. The reaction was monitored by TLC (ethyl acetate: methanol). The products are obtained in comparative yield. Compounds 3(a-c) were analyzed by IR, NMR (1H and 13C), and mass spectral techniques. The IR spectrum of compound 3a displays characteristic stretching peaks at 1662 cm−1, 1654 cm−1 (two carboxylic acid groups), 1528 cm−1, 1456 cm−1 (two aromatic functions, -C=C-), and 1158 cm−1 and 1150 cm−1 (two -C-O-C- functions of oxadiazole). The 1H NMR spectrum of compound 3a showed peaks δ 12.22, 12.09 (two -COOH), 8.12, 7.23, (eight protons of two aromatic groups), and 4.85 (-CH2), representing the formation of arylacetic acid. The mass spectrum of compound 3a confirmed the molecular ion peak at M●+ at 196 (42%). The above spectral data confirm the formation of compound 3a from p-hydroxy benzoic acid 2a and chloroacetic acid.
The compounds 3(a-c) were used for further cyclization reaction by treatment with isoniazid and POCl3 gave cyclized products 4(a-c), in good yield. The structures of these compounds were analyzed by IR, 1H NMR, and mass spectra. The compound 4a in its IR spectrum displayed peaks at 1621, 1612, 1602, 1596, 1168, 1156, and 1146 cm−1 which are due to -C=N- and oxadiazole -COC- functions, respectively. The 1H NMR spectrum of compound 4a exhibited singlet and multiplet at δ 6.69–8.82 and δ 4.35 due to twelve aromatic protons and one –CH2 function, respectively. Compound 4a presented a molecular ion peak recorded at M●+ 398 (62%), which is equivalent to its molecular weight.
3.2. Antimicrobial Activity
3.2.1. Antibacterial and Antifungal Activity
The newly synthesized compounds 4a and 4c exhibited excellent antibacterial activity with a MIC value of 3.125 μg/mL against B. subtilis, which is more potent than the reference drug. Also, other compounds showed moderate to good activity against B. subtilis. The compounds 4a and 4c exhibited high inhibitory performance against S. aureus, with a MIC of 6.25 and 3.125 μg/mL, respectively, and under the same condition, the reference drug showed activity with a MIC of 6.25 μg/mL. The remaining compounds showed moderate antibacterial activity against S. aureus. In the antibacterial activity against E. coli, the compounds 4a and 4c displayed admirable activities with MIC of 3.125 and 6.25 μg/mL, respectively. The rest of the compounds demonstrated comparable activity against the tested pathogen. The compounds 4a and 4c have shown outstanding antibacterial activity against S. typhi with MIC of 3.125 μg/mL, which is better than the tested standard drug. The remaining compounds revealed a medium performance against the tested pathogen S. typhi.
The synthesized compounds 4a and 4c displayed excellent antifungal activity against C. albicans with MIC of 6.25 and 3.125 μg/mL, respectively, equal to the reference drug fluconazole. The remaining compounds illustrated less to medium activity. Regarding the antifungal activity against C. oxysporum, compounds 4b and 4c exhibited more potent activity than the standard drug with MIC of 3.125 μg/mL. The rest of the compounds exhibited good to comparable activity against the tested organism. Compounds 4a and 4c performed an excellent antifungal activity with MIC of 3.125 and 6.25 μg/mL against A. flavus. The remaining compounds exhibited less to good activity against A. flavus. Finally, compounds 4a and 4c displayed hopeful activity against A. niger with MIC of 6.25 and 3.125 μg/mL as compared to the reference drug (MIC of 6.25 μg/mL). The rest of the compounds showed moderate activity. The results of antibacterial and antifungal activities are tabulated in Table 1.
| Compounds | Antibacterial activity in MIC (μg·mL−1) | Antifungal activity in MIC (μg·mL−1) | Anti-tuberculosis activity in MIC (μg·mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | E. coli | S. typhi | C. albicans | C. oxysporum | A. flavus | A. niger | M. tuberculosis | |
| 3a | 12.5 | 12.5 | 25 | 12.5 | 12.5 | 12.5 | 25 | 12.5 | 6.25 |
| 3b | 25 | 25 | 25 | 12.5 | 25 | 25 | 25 | 25 | 12.5 |
| 3c | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 6.25 |
| 4a | 3.125 | 6.25 | 3.125 | 3.125 | 6.25 | 3.125 | 3.125 | 6.25 | 3.125 |
| 4b | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 12.5 | 12.5 | 12.5 | 6.25 |
| 4c | 3.125 | 3.125 | 6.25 | 3.125 | 3.125 | 3.125 | 6.25 | 3.125 | 3.125 |
| Gentamycin | 6.25 | 6.25 | 6.25 | 6.25 | - | - | - | - | - |
| Fluconazole | — | — | — | — | 6.25 | 6.25 | 6.25 | 6.25 | - |
| Ciprofloxacin | — | — | — | — | — | — | — | — | 3.125 |
3.2.2. Anti-TB Assay
The anti-TB activity for the novel compounds is depicted in Table 1. Among the synthesized compounds, 4a and 4c showed outstanding activity with a MIC of 3.125 μg/mL. Also, the standard drug ciprofloxacin showed inhibitory activity at the same concentration against M. tuberculosis bacteria (MTB). The remaining compounds showed good to moderate inhibition effects against the tested pathogen.
3.3. Molecular Modeling Studies
3.3.1. Molecular Docking Studies
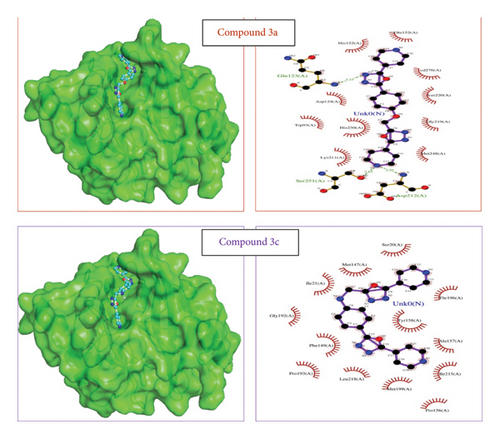
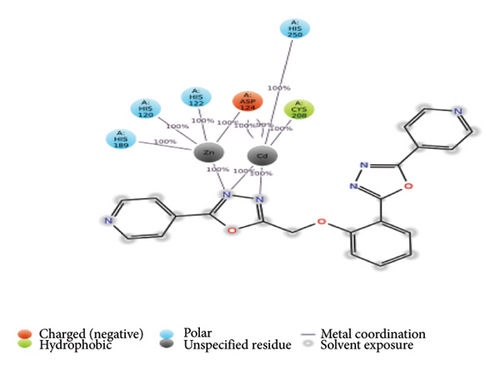
The ligands 3(a-c) and 4(a-c) along with the reference ligands ertapenem for 3ZR9, isoniazid, (3S)-1-cyclohexyl-n-(3,5-dichlorophenyl)-5-oxopyrrolidine-3-carboxamide (641), and NAD for 4TZK were docked to the proteins. For the validation of the docking tool, the docked conformation of reference ligands was analyzed with the 2D interaction plots available in PDBSum. It is observed that the conformations were matching, similar hydrogen bonds were observed, and the vina score for these reference ligands is considered for further analysis. The reference ligand ertapenem (PDB 3ZR9) formed two hydrogen bonds with Asn220 and Ala74. For the protein 4TZK, reference ligand isoniazid formed three hydrogen bonds with Gly14, Thr39, and Leu63. The lowest vina score was observed for compounds 3(a-c) (Figure 1). For the protein 3ZR9, the key metal ion cadmium at the active site along with Asp124, Cys208, and His250 forms an active site for the ligand to interact [65–67]. From the docking results, it can be observed that ligands 4a, 4b, and 4c form two hydrogen bonds with Asp212 and Ser251 and hydrophobic interactions with other active site residues Asp124 and His250 including cadmium ion with a strong vina score of −8.6, −9.3, and −8.6, respectively (Table 2). These ligand complexes were further considered for the molecular dynamics simulation.
| Sl no. | Ligands | 3ZR9 (vina score) | 4TZK (vina score) |
|---|---|---|---|
| 1 | 3a | −5.4 | −7.2 |
| 2 | 3b | −5.8 | −7.3 |
| 3 | 3c | −5.5 | −7.1 |
| 4 | 4a | −8.6 | −10.6 |
| 5 | 4b | −9.3 | −9.2 |
| 6 | 4c | −8.6 | −10.7 |
| 7 | Ertapenem | −8.1 | |
| 8 | Isoniazid | −5.9 | |
| 9 | (3S)-1-Cyclohexyl-n-(3,5-dichlorophenyl)-5-oxopyrrolidine-3-carboxamide (641) | −8.2 | |
| 10 | NAD | −9.8 |
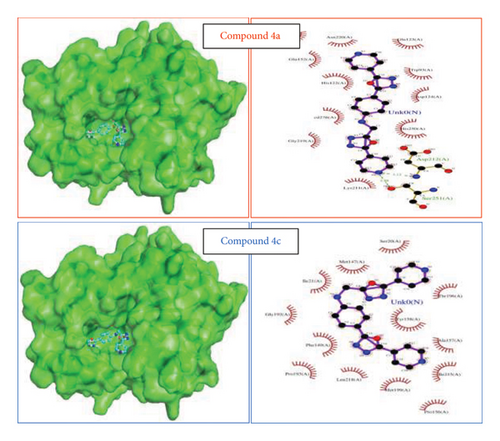
“The reference isoniazid, 641 and NAD binds the protein 4TZK with forming three hydrogen bonds (Gly14, Thr39, and Leu63), one hydrogen (Gly96), eight hydrogen bonds (Ser20, Ile21, Ala22, Asp64, Val65, Ser94, and Ile194), respectively [68, 69]. Though ligands 3a, 3b, and 3c showed a prospective vina score, the lowest score was observed for 4a and 4c. Ligands showed one hydrogen bond with Ser94 for compound 4a and the hydrophobic interaction for 4c at the active site of the protein with the vina score of −10.6 and −10.7, respectively (Table 2). These ligand complexes were further considered for the molecular dynamics simulation (Figure 2). Results and vina scores of the remaining compounds are provided in the Supporting Information File S8.
3.3.2. Molecular Dynamics Simulation
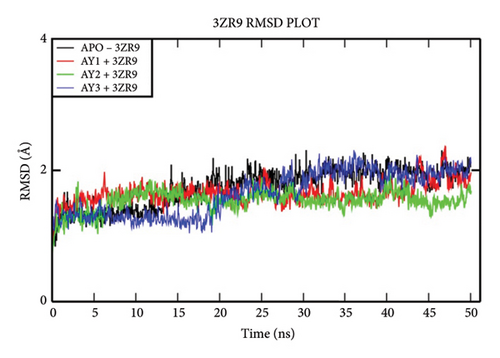
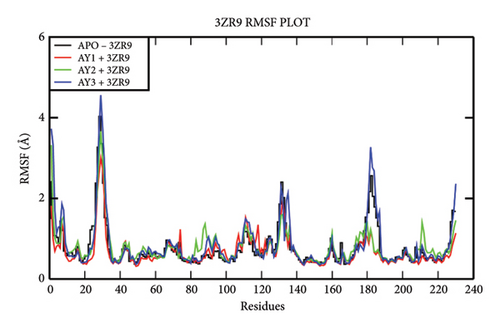
The overall RMSD analysis of the APO protein of NDM-1 (PDB: 3ZR9) shows a stable interaction. The results of RMSD of the APO show 1.73 ± 0.28Å, complex with Compound 4a as 1.65 ± 0.17 Å, complex with Compound 4b as 1.41±0.16Å and complex with Compound 4c as 1.30 ± 0.33Å as in Figure 3(a). From the results of the RMSF plot, it can be observed that a fluctuation around residues 20-40 which is a loop region for the ligand complexes is compared to the APO form in Figure 3(b). From the ligand atom interaction with the protein residue, it can be observed that a strong ionic bond form between cadmium (Cd) and zinc (Zn) ions of Compound 4b with Asp124, Cys208, His250, His189, His120, and His122. AY1 does not form any strong interaction with any amino acids and AY3 formed a hydrogen, pi-pi stacking bond with His250 as represented in Figure 3(c).
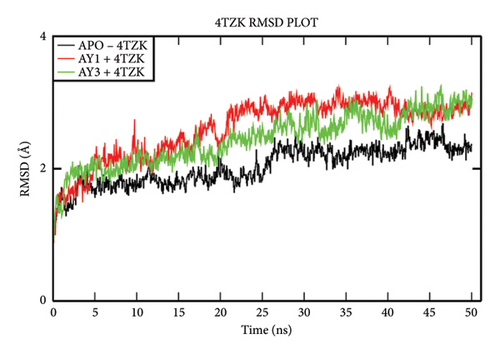
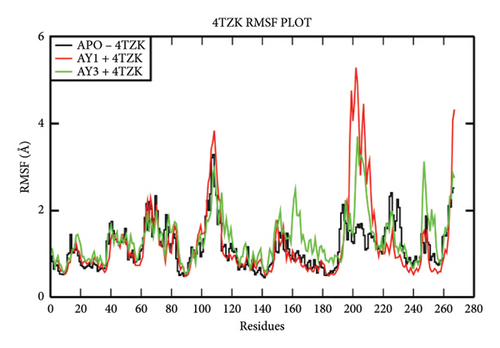
The overall RMSD analysis of M. tuberculosis enoyl reductase (INHA) and APO form showed an average of 2.04 ± 0.27Å, Compound 4a with 2.60 ± 0.45Å, and Compound 4c with 2.44 ± 0.38Å. All the ligands maintained stability in the interaction with the protein throughout the simulation time. The RMSF plot depicts the fluctuation around 100–120 and 200–210 positions. From the ligand atom interaction with the protein residue, it can be observed that the ligand AY1 formed a strong hydrophobic interaction with Phe149 along with Pro193, Tyr158, and Ile194 which are in the NAD binding pocket. AY3 formed hydrophobic interaction with Met147, Ile194, Tyr158, and Met 103. Both the ligands formed a hydrophobic interaction with Tyr158 which is a catalytic residue (Figures 4(a), 4(b), and 4(c)).
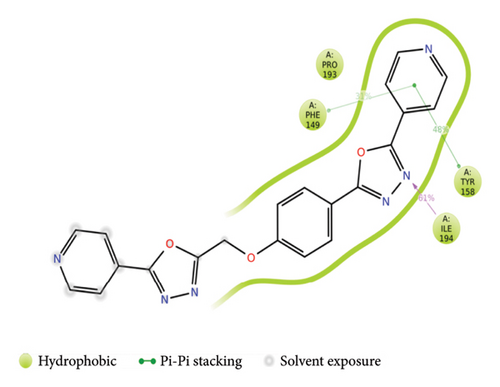
3.4. SAR Studies
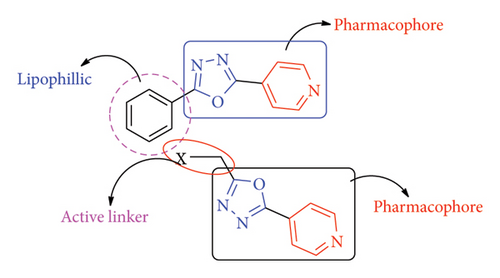
In common, newly synthesized compounds 3(a-c) and 4(a-c) confirmed equivalent antimicrobial activity as compared to standard drugs. The microbial inhibition of synthesized compounds exhibited that the derivatives 3a, 3c, 4a, and 4c had promised activity against tested pathogens, which was nearly comparable to the tested standard drug. The compounds with p-substitution showed excellent inhibition activity against tested pathogens as compared to o-substitution. The literature survey suggested that the methylene bridge is optimum for bioactivity (Figure 5). Further, the compounds having amino groups showed better activity as compared to ether derivatives.
4. Conclusion
In conclusion, the compounds (3a-c) and (4a-c) were synthesized from hydroxy/amino benzoic acids with chloroacetic acid followed by a reaction with isoniazid, respectively. These reactions are straightforward, practical, and, to date, innovative.
These aforesaid compounds (3a-c) and (4a-c) were evaluated for their antimicrobial activity against various microbial pathogens by the MIC method. The antimicrobial potential of prepared compounds exhibited that the derivatives 3a, 3c, 4a, and 4c had promised activity against tested pathogens, which was nearly comparable to the tested standard drug. These compounds showed activity with MIC of 3.125 μg/mL, and under the same condition, the standard drug exhibited MIC of 6.25 μg/mL. In docking studies, the compounds 4a and 4c exhibited good binding affinity toward the docked enzymes. Further, in dynamics studies, these compounds showed excellent binding properties.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors are grateful to the Principal, Vijaya College, Bengaluru, and Guru Nanak First Grade College, Bidar, for providing laboratory facilities. The authors are thankful to the Punarayu Labs, Bengaluru, and SAIF, Punjab University, for spectral analysis. The authors are also grateful to Skanda Life Sciences Pvt. Ltd., Bengaluru, for biological studies.
Supporting Information
Supporting Information File S1: 1H NMR spectrum of compound 3a. Supporting Information File S2: 13C NMR spectrum of compound 3a. Supporting Information File S3: 1H NMR spectrum of compound 4a. Supporting Information File S4: 13C NMR spectrum of compound 4a. Supporting Information File S5: Physiochemical parameters of compounds 3(a-c) and 4(a-c) predicted by Swiss ADME. Supporting Information File S6: Procedure for antimicrobial activity. Supporting Information File S7: Details of molecular modeling studies-protein preparation, ligand preparation, and molecular dynamics simulation. Supporting Information File S8: Results of vina scores by molecular docking studies.
Open Research
Data Availability Statement
The data and materials presented in this manuscript can be made available as per the editorial policy of the journal.




