Amino-Oligosaccharide as a Plant Immune Inducer to Manage Stem Canker Disease Caused by Neoscytalidium dimidiatum in Dragon Fruit (Hylocereus spp.)
Abstract
The dragon fruit (Hylocereus spp.) industry is often impacted by plant diseases, especially the stem canker disease caused by Neoscytalidium dimidiatum. The present study demonstrated that a 5% amino-oligosaccharide solution exhibited a lower in vitro antifungal activity (19.60%) against N. dimidiatum, while displaying significant in vivo efficacy (83.80%) in controlling stem canker disease in dragon fruit during field trials. Meanwhile, we found that, compared to the control, amino-oligosaccharide significantly augmented the total phenolic, lignin, and flavonoid contents as well as effectively enhanced the activities of antioxidant enzymes in dragon fruit. Furthermore, the nontargeted metabolomics analysis revealed that amino-oligosaccharide treatment significantly impacted flavonoids content and predominantly influenced key Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways associated with starch and sucrose metabolism (ko00500), proximal tubule bicarbonate reclamation (ko04964), glutamatergic synapse (ko04724), glutathione metabolism (ko00480), and protein digestion and absorption (ko04974) in dragon fruit. These findings underscore the substantial potential of amino-oligosaccharide as a plant immune inducer in effectively suppressing stem canker disease on dragon fruit.
1. Introduction
The dragon fruit (Hylocereus spp.), a member of the cactus family, is native to Central and South America. It has now been cultivated in various subtropical and tropical regions worldwide [1]. This fruit holds significant importance due to its nutritional, ornamental, coloring, medicinal, industrial, and high consumption values [2]. In recent years, as the cultivation area of dragon fruit has rapidly expanded, there has been a gradual exacerbation of pest and disease issues [3–5]. The prevalence of canker disease caused by Neoscytalidium dimidiatum in dragon fruit stems significantly impacts both the yield and quality of the fruits, while also posing a substantial threat and presenting tremendous challenges for prevention and control efforts [6, 7]. Based on research findings and statistical data, the occurrence of canker disease has been documented in all dragon fruit cultivation areas in Guizhou Province [8]. Currently, the control and prevention of stem canker disease in dragon fruit heavily rely on off-season pruning as well as the application of synthetic and plant-based fungicides [9, 10]. However, their extensive and excessive utilization has led to environmental pollution and an escalating risk of residues to human and animal health.
Amino-oligosaccharide, an efficient and environmentally friendly biological inducer primarily obtained from chitin in marine biological shells through deacetylation and enzymatic degradation, represents a novel plant immune inducer characterized by its remarkable efficacy, low toxicity, and environmental friendliness [11]. Amino-oligosaccharides have increasingly become a focal point for intensive scientific research, as an expanding body of practical and clinical studies underscores their significant health-promoting benefits. These effects manifest through a broad spectrum of bioactive properties, such as antibacterial, antioxidant, anti-inflammatory, and antitumor activities [12]. Meanwhile, in the context of plant protection, previous studies have consistently demonstrated that amino-oligosaccharides not only promote plant growth but also effectively induce defense mechanisms by triggering reactive oxygen species (ROS) accumulation in crop cells, such as those of tobacco and strawberries, and modulating MAPK signal transduction pathways in crops like tobacco and rice [13–17]. In recent years, amino-oligosaccharide has been extensively utilized for the management of various plant diseases including papaya ringspot virus, CMV disease in Passiflora spp. (passion fruit), Peronospora parasitica on Chinese cabbage, wheat powdery mildew, potato virus Y disease, kiwifruit soft rot, among others [11, 18–22]. However, there is currently no literature available regarding the utilization of amino-oligosaccharide for controlling stem canker disease caused by N. dimidiatum in dragon fruit.
The field of metabolomics encompasses a comprehensive area of research that focuses on the analysis of metabolites, facilitating intricate phenotypic characterization through the integration of intricate metabolic profiles [23]. The versatile nature of the metabolomics approach makes it widely applicable in biomarker discovery and etiological investigation, toxicological mechanism elucidation, as well as quality control applications in food engineering [24–26]. Nontargeted metabolomics, also known as metabolite fingerprinting using liquid chromatography–mass spectrometry (LC-MS), gas chromatography–mass spectrometry (GC-MS), and other nontargeted technologies, employs exploratory data analysis to identify significant metabolites based on their distinctive characteristics [27]. Enrichment analysis of metabolic pathways is commonly employed to investigate metabolic differentials by identifying differential metabolites [28, 29]. Hence, it is well suited for the holistic investigation of comprehensive information differential samples.
This study aimed to determine the in vitro antifungal activity and in vivo control efficacy of amino-oligosaccharide against stem canker disease in dragon fruit, as well as assess its impacts on the nutritional quality and antioxidant enzyme activity of dragon fruit. Furthermore, nontargeted metabolomics analysis was employed to investigate the interaction between amino-oligosaccharide and stem canker disease in dragon fruit. These findings provide a theoretical foundation for enhancing resistance induction techniques against canker disease in dragon fruit using amino-oligosaccharide, thereby promoting sustainable dragon fruit production.
2. Materials and Methods
2.1. In Vitro Antifungal Activity Test
The in vitro antifungal activities of 45% difenoconazole suspension concentrate (Hangzhou Udragon Chemical Co., Ltd., Hangzhou, China; labeled as X-1), 40% myclobutanil suspension concentrate (Shenzhen Noposion Agrochemicals Co., Ltd., Shenzhen, China; labeled as X-2), 430 g/L tebuconazole suspension concentrate (Shenzhen Noposion Agrochemicals Co., Ltd., Shenzhen, China; labeled as X-3), and 5% amino-oligosaccharide aqueous solution (Hainan Zhengye Zhongnong High-Tech Co. Ltd., Hainan, China; labeled as X-4) against N. dimidiatum, the main pathogen isolated from the stem canker disease in dragon fruit, were measured by the cross-crossing method [30].
2.2. Field Trials Test
| Disease level | Lesion condition |
|---|---|
| 0 | No lesion |
| 1 | The area of sporadic disease lesions on the stem surface accounts for less than 5% of the entire seedling |
| 3 | The area of sporadic disease lesions on the stem surface accounts for 6%–10% of the entire seedling |
| 5 | The area of sporadic disease lesions on the stem surface accounts for 11%–25% of the entire seedling |
| 7 | The area of sporadic disease lesions on the stem surface accounts for 26%–50% of the entire seedling |
| 9 | The area of sporadic disease lesions on the stem surface accounts for more than 50% of the entire seedling |
2.3. Determination of Nutritional Quality
To investigate the impact of X-4 on the nutritional quality of dragon fruit, we utilized well-established analytical methods to quantify the levels of total phenols, lignin, and flavonoids in dragon fruit samples collected at 1, 3, 5, 10, and 15 days post-application under field conditions [32, 33].
In brief, 0.1 g of dragon fruit sample was added to 2 mL of a mixture of methanol and water (60/40, v/v), mixed for 2 min, incubated for 30 min at 60°C, and centrifuged at 4000 rpm for 10 min. Then, 0.2 mL of the supernatant was mixed with 1.0 mL of Folin-Ciocalteu reagent. After 2 min, 2.8 mL of saturated sodium carbonate solution was added to the mixture and vortexed for 1 min, followed by incubation for 2 h at room temperature. The absorbance was measured at 760 nm using gallic acid as a reference standard. The total phenolics content in each dragon fruit sample was expressed as mg gallic acid equivalents per gram of the sample.
Middle portions of the third internode from plants with similar growth were ground to a fine powder in a mortar; liquid nitrogen was then added to the powder, and 0.5 g of this was weighed into a 10 mL centrifuge tube. Chlorophyll was extracted with 95% ethanol; n-hexane:ethanol (2:1, v/v) was used to extract lipids. The remaining precipitate was added to 2.5 mL of 25% bromoacetyl glacial acetic acid; the reaction was maintained at 70°C for 30 min. The samples were cooled to room temperature in cold water, 0.5 mL of each sample was transferred to a 50 mL centrifuge tube, and 0.9 mL of 2 mol/L NaOH and 5 mL of acetic acid were added. Then, 0.1 mL of 7.5 mol/L hydroxylamine hydrochloride was added to terminate the reaction. Acetic acid was added to adjust the volume up to 15 mL. Absorbance at 280 nm was measured using a spectrophotometer (Shimadzu UV-2450, Japan). Lignin content is expressed as the absorbance at 280 nm of the solution per unit mass of fresh sample.
For the measurement of total flavonoids in each dragon fruit sample, 0.1 g of plant extract (10 mg/mL) was mixed with 28 μL of NaNO2 (50 g/L) and kept for 5 min. After that, 28 μL of AlCl3 (100 g/L) was added and the mixture was allowed to react for 6 min. Then, 120 μL of 1 mol NaOH was added to the mixture, and the absorbance was immediately measured at 510 nm. The flavonoids content was calculated as mg/g dry weight.
2.4. Determination of Antioxidant Enzyme Activity
The dragon fruit plants were fully sprayed with X-4 (50 mg/L). The spraying standard was such that water droplets hung on the branches of the dragon fruit but did not fall. Three plants were treated repeatedly in each group, while sterile distilled water was used as the CK group. Then, they were placed in a climate incubator with a temperature of 28°C, 12 h of alternating light, and a humidity of 80%. Samples were taken respectively before the spraying of X-4 and on the 1st, 3rd, 5th, 10th, and 15th days after the spraying. The activities of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), polyphenol oxidase (PPO), and peroxidase (POD) in dragon fruit were assessed using commercially available enzyme assay reagent kits provided by Suzhou Geruisi Biotechnology Co., Ltd. (Suzhou, China) [33, 34].
2.5. High-Throughput Nontargeted Metabolomics Study
After 15 days of spraying, the stems of dragon fruit treated with X-4 and sterile distilled water were collected. Nontargeted metabolomics analysis was performed by Jiangsu Sanshu Biotechnology Co., Ltd. (Jiangsu, China). The metabolites were analyzed using ultrahigh-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) employing a Vanquish UHPLC system equipped with an Orbitrap Q Exactive TM HF-X mass spectrometer (Thermo Fisher, Bremen, Germany).
Briefly, the samples (50–100 mg) were accurately weighed into the centrifuge tube, added 1 mL of precooling extraction solution (water–acetonitrile–isopropyl alcohol mixed solution, 1:1:1, v/v/v), homogenized for 60 s, extracted for 30 min by low-temperature ultrasonication, centrifuged for 10 min with 12,000 rpm at 4°C, set still for 1 h to precipitate protein at −20°C, centrifuged for 10 min with 12,000 rpm at 4°C, the supernatant solution was dried in vacuum, added 0.2 mL of 30% acetonitrile solution, homogenized and centrifuged for 15 min at 14,000 rpm and 4°C, and the supernatant was taken for computer detection.
The extracted sample was analyzed using an UPLC-Orbitrap-MS system (UPLC, Vanquish; MS, HFX). The analytical conditions were as follows: UPLC: column, Waters HSS T3 (100 × 2.1 mm, 1.8 μm); column temperature, 40°C; flow rate, 0.3 mL/min; injection volume, 2 μL; solvent system, phase A were Milli-Q water (0.1% formic acid), phase B were acetonitrile (0.1% formic acid); gradient program, 0 min phase A/phase B (100:0, v/v), 1 min phase A/phase B (100:0, v/v), 12 min phase A/phase B (5:95, v/v), 13 min phase A/phase B (5:95, v/v), 13.1 min phase A/phase B (100:0, v/v), 17 min phase A/phase B (100:0, v/v).
HRMS data were recorded on a Q Exactive HFX Hybrid Quadrupole Orbitrap mass spectrometer equipped with a heated ESI source (Thermo Fisher Scientific) utilizing the Full-ms-ddMS2 acquisition methods. The ESI source parameters were set as follows: sheath gas pressure, 40 arb; aux gas pressure, 10 arb; spray voltage, +3000 V/−2800 V; temperature, 350°C; and ion transport tube temperature, 320°C. The scanning range of the primary mass spectrometry was (scan m/z range) 70–1050 Da, with a primary resolution of 70,000 and secondary resolution of 17,500.
2.6. Data Analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation and enrichment analysis identified metabolites were annotated using KEGG Compound database (https://www.kegg.jp/kegg/compound/); annotated metabolites were then mapped to KEGG Pathway database (https://www.kegg.jp/kegg/pathway.html). The supervised multivariate data analysis methods, namely, principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA), were employed to identify and extract metabolites with significant statistical relevance. Variable importance in projection (VIP) values for each metabolite were obtained through the OPLS-DA model. Model validity was assessed based on R2 (cumulative) and Q2 (cumulative) values. Statistical significance of metabolites was determined using t-tests, while fold change calculations were performed to assess differential expression. Metabolites meeting the criteria of VIP > 1, p value < 0.05, and fold change FC ≥ 2 or FC ≤ 0.5 were considered as differentially expressed metabolites. All experiments were conducted in triplicate, and statistical analyses including one-way ANOVA with Duncan’s multiple test and Student’s t-test were performed using IBM SPSS Statistics 26 software.
3. Results and Discussion
3.1. In Vitro Antifungal Activity and In Vivo Control Efficiency Test
Amino-oligosaccharide (X-4) represents a novel class of biologically active agents capable of modulating plant metabolism, augmenting plant immune responses, and enhancing disease resistance and stress tolerance mechanisms in plants [9]. Reports indicated that X-4 exhibits remarkable bioactivity against a wide range of plant pathogens, encompassing fungi, viruses, and bacteria [35–38]. However, Table 2 shows that X-4 reveals lower in vitro antifungal activity against N. dimidiatum, with the inhibition rate of 19.60% at 50 μg/mL, than those of X-1 (92.89%), X-2 (98.67%), and X-3 (93.24%), respectively. Meanwhile, the results presented in Table 3 demonstrate that, following a 15-day spraying period, the control efficiency of amino-oligosaccharide against stem canker disease in dragon fruit reached an impressive level of 83.80%, which was lower than both X-2 and X-3 while even outperforming that of X-1. These findings highlight the significant potential of amino-oligosaccharide to effectively suppress the occurrence of stem canker disease during the growth phase of dragon fruit.
| Treatment | Hypha diameter (cm) | Inhibition rate (%)∗ |
|---|---|---|
| X-1 | 0.77 ± 0.02 | 93.24 ± 0.73b |
| X-2 | 0.78 ± 0.03 | 92.89 ± 1.52b |
| X-3 | 0.55 ± 0.02 | 98.67 ± 0.42a |
| X-4 | 3.72 ± 0.65 | 19.60 ± 1.10c |
| CK | 4.50 ± 0.98 |
- ∗The inhibition rates of different treatment groups were indicated by distinct lowercase letters, demonstrating significant differences compared to the CK group (p < 0.05).
| Treatments | Disease index (%) | Control efficiency (%)∗ |
|---|---|---|
| X-1 | 6.50 ± 0.62b | 76.60 ± 1.25c |
| X-2 | 3.83 ± 0.21d | 86.21 ± 0.67a |
| X-3 | 3.70 ± 0.62d | 86.69 ± 0.49a |
| X-4 | 4.50 ± 0.25c | 83.80 ± 0.64b |
| CK | 27.78 ± 1.26a | — |
- ∗The disease index and control efficiency of different treatment groups were indicated by distinct lowercase letters, demonstrating significant differences compared to the CK group (p < 0.05).
3.2. Results of Total Phenolic, Lignin, and Flavonoid Contents
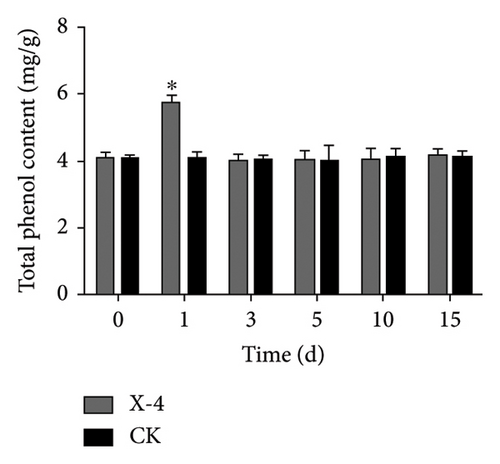
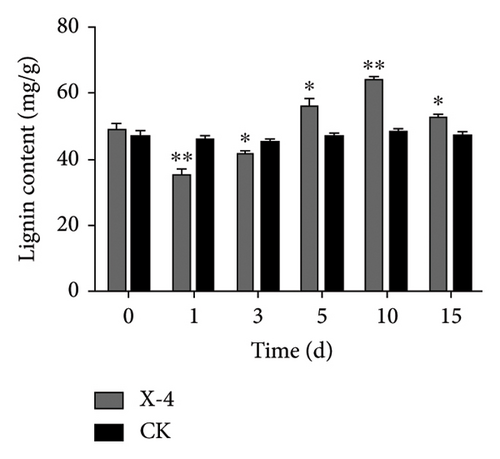
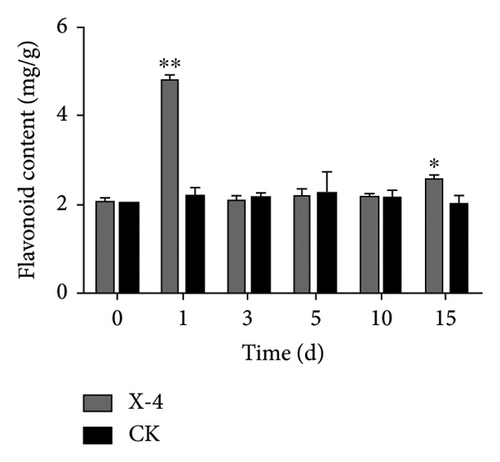
Total phenolics, a diverse group of natural compounds characterized by an aromatic ring bearing one or more hydroxyl groups, are widely recognized for their importance in plant health. Numerous studies have consistently demonstrated their upregulation in response to insect herbivory, pathogen infection, or colonization by beneficial microorganisms [39]. Lignin constitutes an indispensable component of the secondary cell walls in plants, conferring enhanced mechanical strength and serving as a chemical and physical barrier to bolster resistance against pathogen invasion [40]. Flavonoids, specialized metabolites frequently implicated in plant defense against biotic and abiotic stresses, have been extensively reported [41]. The results depicted in Figure 1(a) show that following treatment with amino-oligosaccharide for 1 day, there was a significant increase in the total phenolic content to 5.74 mg/g compared to the CK group. Subsequently, no significant differences were observed in the total phenolic content between the amino-oligosaccharide treatment group and CK group after this time period elapsed, indicating that amino-oligosaccharide induced an initial rise in total phenolics to improve plant antioxidant capacity. Meanwhile, Figure 1(b) illustrates the capacity of amino-oligosaccharide to increase the lignin content in dragon fruit stems at 5, 10, and 15 days post-treatment, while decreasing the lignin content at 1 and 3 days post-treatment. The lignin contents were recorded as 59.21, 65.48, and 54.53 mg/g at intervals of 5, 10, and 15 days post amino-oligosaccharide treatment, respectively, reaching its highest value on day 10, which surpassed those of the CK group. In addition, Figure 1(c) shows that amino-oligosaccharide had the ability to enhance the content of flavonoid in dragon fruit stems. Notably, on day 1, the flavonoid content reached its highest value (4.86 mg/g), surpassing that of the CK group. Our results show that the amino-oligosaccharide significantly augmented the total phenolic, lignin, and flavonoid contents, which may be related to enhance resistance to stem canker disease in dragon fruit.
3.3. Effect on the Antioxidant Enzyme Activity
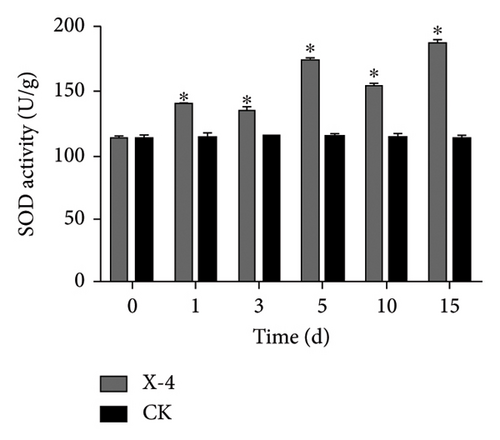
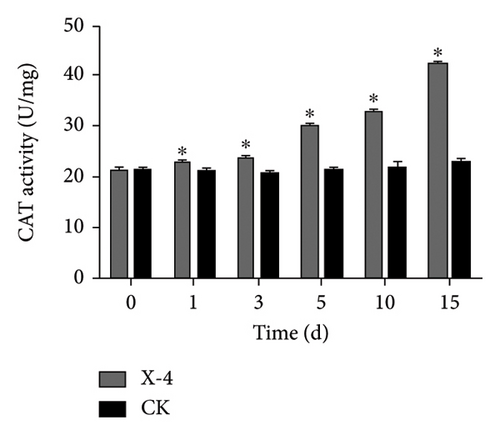
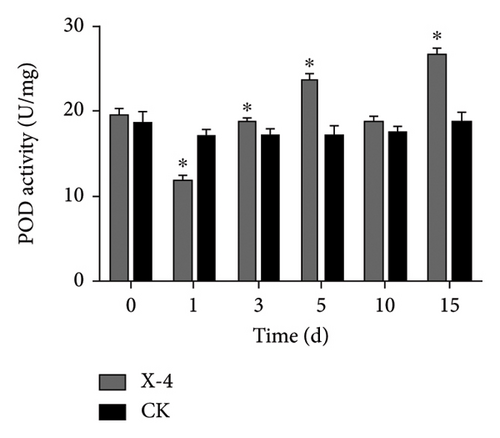
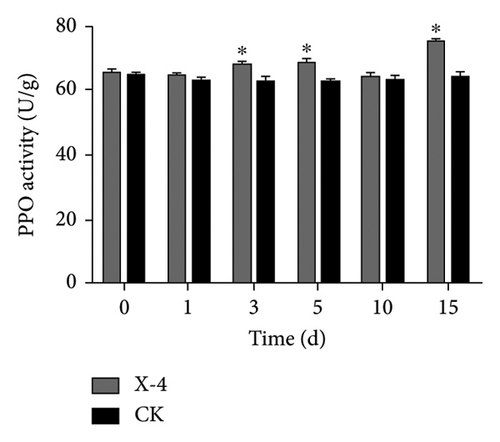
Enzymes are important biological catalysts, which play a crucial role in the life activities of organisms. The resistance of plants to diseases is associated with the activation of a cascade of defense reactions, including the induction of defense-related enzymes such as SOD, CAT, POD, PPO, and others [42–48]. These enzymes facilitate organism metabolism, catalyze various physiological and biochemical reactions, repair cellular damage, and enhance immune capacity [44]. During the entire detection period, as shown in Figure 2(a), the SOD activity of dragon fruit plants in the amino-oligosaccharide treatment group showed an upward trend. Before the treatment, the SOD activity was 113.11 U/g. On the 1st and 3rd days after treatment, the SOD activity values were 138.70 and 134.61 U/g, respectively, significantly higher than that of the CK group. Subsequently, on the 5th, 10th, and 15th days, the SOD enzyme activity of the amino-oligosaccharide treatment group showed a trend of rising, falling, and then rising again, each measurement being significantly higher than that of the CK group during this period. In the entire detection period, the SOD enzyme activity in the amino-oligosaccharide treatment group reached a maximum of 186.56 U/g on the 15th day, while the SOD activity in the CK group hardly changed. After application of amino-oligosaccharide, as shown in Figure 2(b), the CAT activity of dragon fruit plants showed an upward trend during the entire detection period. Before treatment, the CAT activity was 21.30 U/g. On the 1st and 3rd days after treatment, the CAT activity increased slowly, with activity values of 22.95 and 23.83 U/g, respectively, significantly higher than that of the CK group. Subsequently, on the 5th, 10th, and 15th days, the CAT activity values of the amino-oligosaccharide treatment group were 30.07, 32.64, and 41.33 U/g, respectively, all significantly higher than the CAT activity values of the CK group. In the entire detection period, the CAT enzyme activity of the amino-oligosaccharide treatment group reached a maximum of 41.33 U/g on the 15th day, while the CAT activity of the control group hardly changed. During the entire detection period, as shown in Figure 2(c), the change of POD activity in dragon fruit plants treated with amino-oligosaccharide showed a trend of first decreasing, then increasing, decreasing again, and finally increasing. Before the treatment with amino-oligosaccharide, the POD activity value was 19.68 U/g. On the 1st day after the treatment, the POD activity decreased to 11.91 U/g, representing a 40% drop compared to before the treatment, and it was significantly lower than the POD activity of the control group. Subsequently, on the 3rd and 5th days, the POD activity values of amino-oligosaccharide treatment group rose to 18.97 and 23.70 U/g, respectively. On the third day, the POD activity of amino-oligosaccharide treatment group was significantly higher than that of the CK group, and on the 5th day, the POD activity of amino-oligosaccharide treatment group was extremely significantly higher than that of the CK group. On the 10th day, the POD activity of amino-oligosaccharide treatment group decreased to 18.8 U/g, with no significant difference from the CK group. Later, on the 15th day, the POD enzyme activity of amino-oligosaccharide treatment group rose to 26.92 U/g, which was the highest during the entire detection period. The amino-oligosaccharide treatment group was 13.28% higher than the CK group on average during the entire detection period. As shown in Figure 2(d), on the 1st day after treatment, the PPO activity increased to 64.10 U/g, significantly higher than that of the CK group. Then, on the 3rd, 5th, and 15th days, the PPO activity values of amino-oligosaccharide treatment group rose to 67.53, 68.06, and 74.65 U/g, respectively, which were extremely significantly higher than the PPO activity values of the CK group. While, on the 10th day, the PPO activity of the amino-oligosaccharide treatment group decreased to 64.11 U/g, with no significant difference from the CK group. The average PPO activity value of the amino-oligosaccharide treatment group was 7.69% higher than that of the CK group throughout the detection period. The present findings are generally in line with the previous research results reported by Liu et al., which demonstrate that the application of amino-oligosaccharide can augment SOD, CAT, POD, and PPO activity while enhancing the plant’s resistance to pathogen invasion [16, 49, 50].
3.4. Nontargeted Metabolomics Detection
3.4.1. Differential Metabolite Analysis
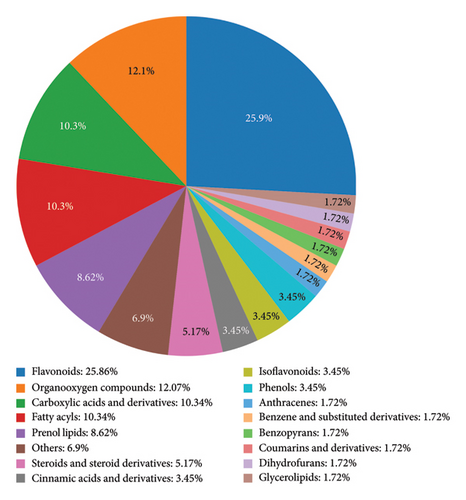
The UHPLC-MS/MS technique was used to detect the metabolites in the amino-oligosaccharide treatment group and CK group samples. As shown in Table S1, a total of 434 metabolites were identified in the amino-oligosaccharide treatment group and CK group samples. After that, the classification of metabolites was statistically analyzed and visually represented using a pie chart, based on the classification information provided by Chemical Taxonomy. The majority of the metabolites observed in Figure 3 can be attributed to prenol lipids, constituting approximately 18.75% of the total content, followed by flavonoids and organo-oxygen compounds, which exhibit a content exceeding 10.00%.

3.4.2. PCA, PLS-DA, and OPLS-DA
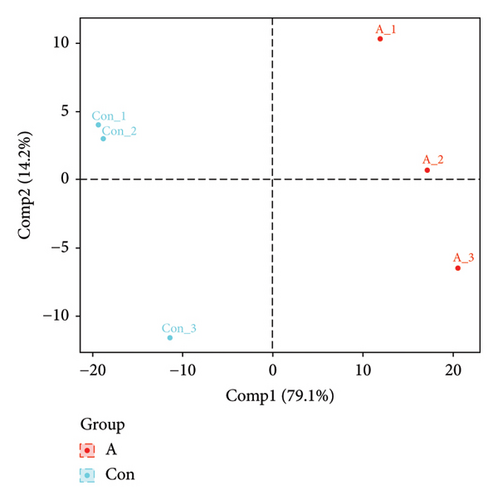
The PCA, PLS-DA, and OPLS-DA models are the unsupervised multivariate statistical analysis methods that effectively capture the overall dissimilarity among samples within each group and the variability between samples across the entire group [51]. In the PCA, PLS-DA, and OPLS-DA score charts, the horizontal coordinate Comp1 represents the first principal component, while the vertical coordinate Comp2 represents the second principal component. The numerical percentage in parentheses indicates the variance explained by each respective principal component. The PCA, PLS-DA, and OPLS-DA score charts delineated the initial state of the sample data, thereby reducing the datasets’ complexity. Based on the outcomes presented in the PCA, PLS-DA, and OPLS-DA score charts (Figures 4(a), 4(b) and 4(d)), it effectively discriminated between the amino-oligosaccharide treatment group (designated as A) and CK group (designated as Con) across all metabolites. The distinct quadrants representing PC1 accounted for 79.1%, 81.4%, and 81.0%, respectively, while PC2 accounted for 14.2%, 14.5%, and 14.9%, respectively. Meanwhile, the primary criterion for evaluating the quality of PLS-DA and OPLS-DA models is R2Y (cum), which represents the proportion of data interpretation achieved through dimensionality reduction compared to the original dataset. A value closer to 1 indicates a more desirable outcome, and an R2Y (cum) exceeding 0.5 implies a favorable model performance [52]. The permutation testing results (Figures 4(c) and 4(e)) demonstrated a high R2Y (cum) value more than 0.6, indicating exceptional repeatability and stability in the analysis system, thereby ensuring reliable data acquisition.
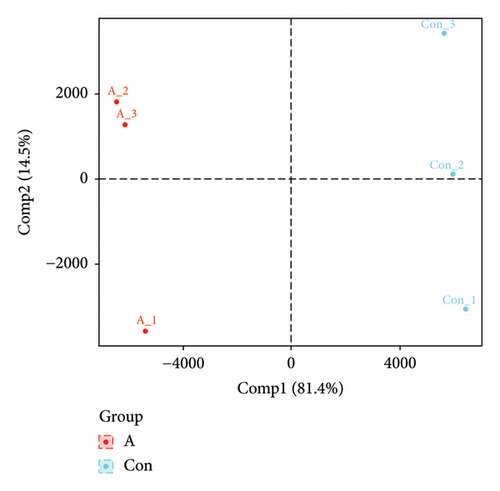
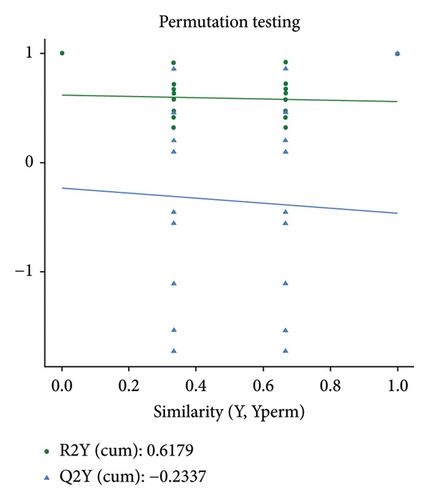
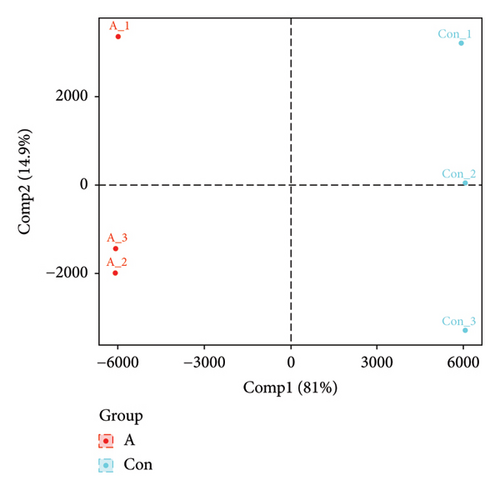
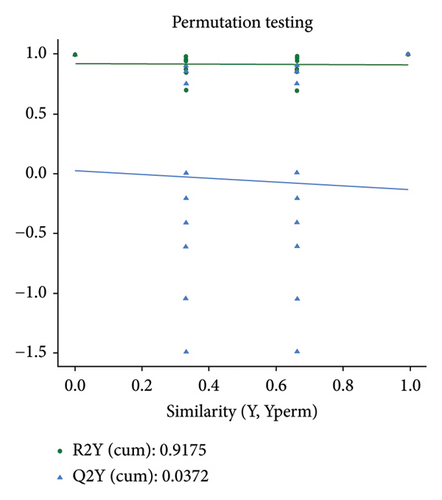
3.4.3. Differential Metabolite Analysis
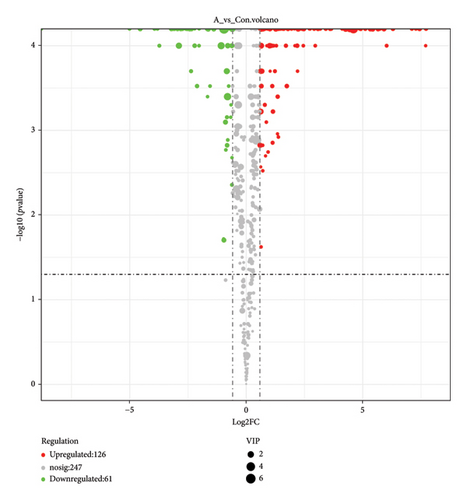
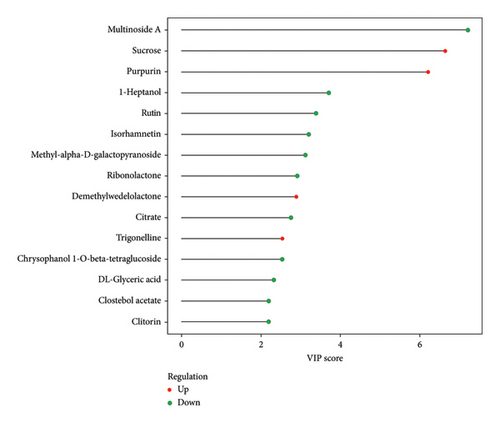
The VIP and p value are two primary parameters used for screening differential metabolites [53]. The VIP value represents the projected importance of variables in the first principal component of the PLS-DA and OPLS-DA models, indicating the contribution degree of each metabolite to the differences observed in these models. Generally, a VIP value greater than 1 is considered significant [54]. On the other hand, p value refers to the statistical test value for evaluating differences in metabolite expression, calculated using Student’s t-test. In combination with multivariate analysis using PLS-DA and OPLS-DA models, metabolites with a p value less than 0.05 and VIP greater than 1 are selected as differential metabolites for subsequent biogenic analysis [55]. The results of differential metabolite screening were subjected to statistical analysis, and the specific findings are presented in Figure 5. The results presented in Figure 5 demonstrate that amino-oligosaccharide treatment led to the identification of a total of 187 metabolites showing differential expression patterns in dragon fruit stems, with 126 being upregulated and 61 being downregulated. Especially, Figure 6 shows that the contents of sucrose, purpurin, demethylwedelolactone, and trigonelline were significantly increased in amino-oligosaccharide treatment group with VIP greater than 1. After that, the majority of the differential metabolites observed in Figure 7 can be grouped to flavonoids, constituting approximately 25.86% of the total content. Previous research has found that the diverse biological activities exhibited by flavonoids have led to their frequent identification as specialized metabolites involved in plant defense against biotic or abiotic stresses [44, 56]. Therefore, our findings demonstrate that amino-oligosaccharides can effectively stimulate the biosynthesis of secondary metabolites, thereby enhancing the defense mechanism against stem canker disease in dragon fruit.
3.4.4. KEGG Pathway of Differential Metabolites
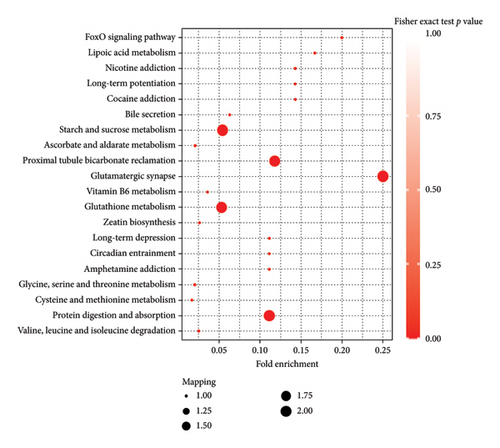
The KEGG database facilitates the annotation of differential metabolites’ pathways and the classification of metabolites based on their participation in specific pathways or functions [57, 58]. Figure 8 shows that the top five metabolic pathways that contain the most differentiated metabolites were starch and sucrose metabolism (ko00500), proximal tubule bicarbonate reclamation (ko04964), glutamatergic synapse (ko04724), glutathione metabolism (ko00480), and protein digestion and absorption (ko04974). Several studies have demonstrated that host–pathogen interaction induces resistance-specific metabolic pathways associated with plant disease resistance, including starch and sucrose metabolism (ko00500), proximal tubule bicarbonate reclamation (ko04964), glutamatergic synapse (ko04724), glutathione metabolism (ko00480), and protein digestion and absorption (ko04974) [59–61].
4. Conclusion
In conclusion, our study demonstrates that amino-oligosaccharide has lower in vitro antifungal activity against N. dimidiatum at 50 μg/mL but shows significant in vivo efficacy in controlling stem canker disease during field trials. Additionally, amino-oligosaccharide significantly increases the total phenolic, lignin, and flavonoid contents and enhances antioxidant enzyme activities in dragon fruit. Furthermore, nontargeted metabolomics analysis reveals that amino-oligosaccharide treatment greatly affects flavonoid content and primarily influences key KEGG pathways associated with plant disease resistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by the Science and Technology Program of Guizhou Province, grant number QKHZC[2023]080.
Supporting Information
Table S1: All metabolites identified in the amino-oligosaccharide treatment group and CK group samples.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




