Ovicidal Activity of 2-Hydroxy-4-Methoxybenzaldehyde, Derivatives and Structural Analogues on Anopheles gambiae Eggs
Abstract
Effective remedies for disrupting An. gambiae metamorphosis at the egg stage are crucial in suppression of the malaria vector populations that result in the reduction of disease burden. 2-Hydroxy-4-methoxybenzaldehyde (the major component of Mondia whytei roots), its derivatives, structural analogues and their blends were evaluated against the eggs of An. gambiae in the search for ovicidal compounds with potential use in mosquito control programs. Mature roots were harvested from Mondia whytei (Hook) Skeels (Asclepiadaceous) plants grown in the Center for African Medicinal and Nutritional Flora and Fauna (CAMNFF) herbal medicinal garden and cleaned with distilled water. 2-Hydroxy-4-methoxybenzaldehyde (1) was isolated by steam distillation of the chopped roots. The selected derivatives and/or analogues were prepared using established chemical procedures and their structures confirmed by NMR spectroscopy and ESI-MS. Ovicidal activity of the pure compounds, derivatives, structural analogues and/or formulated blends was tested at 1, 10, 25 and 50 parts per million (ppm) on An. gambie eggs. Eleven mono-substituted (3–7), di-substituted (8–10), tri-substituted (1-2) aromatic compounds were assayed for ovicidal activity against Anopheles gambiae eggs singly or as blends. Benzaldehyde (4) and 4-methoxybenzaldehyde (9) were further converted into 2-hydroxy-1, 2-diphenylethanone (11), 1, 5-diphenylpenta-1, 4-diene-3-one (12) and 1, 5-bis (4-methoxyphenyl) penta-1, 4-diene-3-one (13) and evaluated for ovicidal activity individually or as blends. Of the 13 compounds evaluated individually, 2-hydroxy-4-methoxybenzaldehyde (1) exhibited the highest ovicidal activity at LC50 0.7075 ppm while anisole had the lowest activity at LC50 40.342 ppm. The derivatives exhibited moderate activity: 2-hydroxy-1, 2-diphenylethanone (LC50 10.599 ppm), 1, 5-diphenylpenta-1, 4-diene-3-one (LC50 9.019 ppm) and 1, 5-bis (4-methoxyphenyl) penta-1, 4-diene-3-one (LC50 15.642 ppm). The blends exhibited intriguingly high ovicidal efficacy with the mixture of benzaldehyde and phenol showing the highest (LC50 0.332 ppm) while phenol and anisole exhibited the lowest activity (LC50 9.9909 ppm). From the activity of the blends, it is evident that anisole is antagonistic to the efficacy of phenol and benzaldehyde. It is also apparent that aldehyde and hydroxyl groups, when directly attached to the phenyl ring, provide the critical structural characteristics that contribute to the ovicidal activity of the aromatic compounds.
1. Introduction
Malaria remains the most important parasitic disease in the world [1]. Africa with an estimated 215 million annual malaria cases accounts for 94% of the global cases leading to 3,84,000 deaths [2]. It is estimated that there were 33 million pregnancies in Africa in 2020 with 35% of the expectant mothers being exposed to malaria infection resulting in about 82,000 children with low birth weight [2].
Mosquitoes are important public health vectors of malaria, filariasis and arboviral diseases that cause millions of infections and death worldwide [3]. Malaria is transmitted by infected female An. gambiae which feed on human blood meal for viability of its eggs [4]. Effective vector control methods at the egg, larval or adult stages are therefore critical in controlling the malaria vector populations and mitigating the harmful effects on human health [5]. Most malaria control strategies: environmental management (breeding/resting sites), sterile insect technique: biological control agents (predators, parasitoids and entomopathogens); chemical repellents and insecticide/pesticides (natural and synthetic), depend heavily on insect vector population control at the larval or adult stages with little effort on the eggs [6].
Natural insecticide/pesticides are generally nonpest specific, biodegradable, nonallergic to humans, safe to nontarget organisms [7] and have wide spectrum of application [8]. They offer good alternatives to synthetic chemical insecticides which are deleterious to the environment, harmful to non-target organisms and are ineffective due to development of resistance [9–13]. Many reports of plant extracts, secondary metabolites, essential oils and lectins which exhibit: general insect toxicity; growth and/or reproductive inhibition; insect repellency; and larvicidal activity against mosquito vectors have been documented; and are important and potentially suitable for use in integrated vector management (IVM) [6, 14]. However, little work has been documented on ovicidal activity and oviposition deterrence of anti-mosquito plants and/or derived compounds.
In that regard, the ovicidal activity and oviposition deterrence of leaf extracts of Ipomoea cairica against dengue vectors [6]; ovicidal and repellent activity of several botanical extracts against Culex quinquefasciatus, Aedes aegypti and Anopheles stephensi [14]; and the ovicidal and larvicidal activity of some plant extracts against Cx. quinquefasciatus and Ae. aegypti [13] have been documented. The larvicidal, ovicidal and oviposition deterrent potential of neem oil water dispersible tablets have also been reported against An. culicifacies [15] while azadirachtin from neem plant exhibited ovicidal activity against Cx. tarsalis and Cx. quinquefasciatus [16]. The larvicidal and ovicidal activity of Artocarpus blancoi extracts were noted against Ae. aegypti [17] while the larvicidal, ovicidal, and repellent activity of Sophora alopecuroides and synergistic activity of its dominant constituents have been documented against Ae. albopictus [18].
Efforts to control malaria transmission in disease endemic areas are heavily reliant on suppression of the vector populations through a combination of chemical toxins, biological agents and management of breeding sites [19]. Consequently, application of adulticides and larvicides has been a common strategy used in vector control. It is of essence that focus be also directed to the egg stage in the mosquito development cycle due to its limited mobility compared to the free flying and swimming adult mosquitoes and larvae, respectively [20]. Consequently, discovery and development of effective and environmental-friendly ovicidal compounds alongside the identification and focus on most productive/viable breeding sites/habitats for mosquito is crucial for malaria vector and disease control [21].
2-Hydroxy-4-methoxybenzaldehyde (1), a structural isomer of vanillin (2), is an aromatic taste-modifying compound commonly found in the root bark of Mondia Whytei (Hook) Skeels (Asclepiadaceous) plant [22]. It has been previously reported as a tyrosine inhibitor [23] and potent larvicide against An. gambiae [24]. However, we could not find any information on its ovicidal activity or any structure-activity relationship studies on it or related compounds against An. gambiae eggs in the literature. Consequently, this project was designed to investigate the ovicidal activity of 2-hydroxy-4-methoxybenzaldehyde, its derivatives, structural analogues and their blends on An. gambiae eggs in order to understand the structure-ovicidal activity relationships therein.
2. Materials and Methods
2.1. Insect Culture
The An. gambiae mosquitoes that produced eggs used in this study were reared under ambient conditions of 27 ± 1°C and 85% relative humidity (RH), in the insectary situated at Centre for Disease Control CDC at the Kenya Medical Research Institute, Kisumu, Kenya. They were fed on 10% sucrose solution and a blood meal, to ensure that they produced viable eggs for the ovicidal experiments.
2.2. Compounds for Ovicidal Assay
2-Hydroxy-4-methoxybenzaldehyde (1) was isolated from Mondia whytei (Hook) Skeels (Asclepiadaceous) while compounds 2–10 were procured from LOBA CHEMIE PVT LTD.
Compounds 11–13 were prepared in the laboratory as described below.
2.3. Isolation of 2-Hydroxy-4-Methoxybenzaldehyde
Fully developed roots were harvested from mature Mondia whytei (Hook) Skeels (Asclepiadaceous) plants cultivated at the Centre for African Medicinal and Nutritional Fauna and Flora (CAMNFF) herbal garden at Masinde Muliro University of Science and Technology, Kakamega, Kenya. They were cleaned with water and stored under shade awaiting extraction. The roots were chopped into small pieces and subjected to isolation using the established procedures [25]. The white crystalline compound [10 g, mp 41–43°C] was obtained from 1000 g (10% yield) of the roots and confirmed to be 2-hydroxy-4-methoxybenzaldehyde (1) from NMR and ESI-MS data [22]. It was stored in sealed amber bottles and refrigerated at 4°C awaiting ovicidal assays.
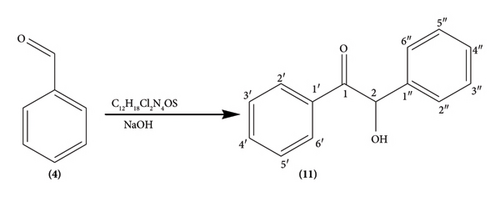
2.3.1. Hydroxy-1, 2-Diphenylethanone (11)
The compound was prepared through established procedures summarized in Scheme 1 [26].
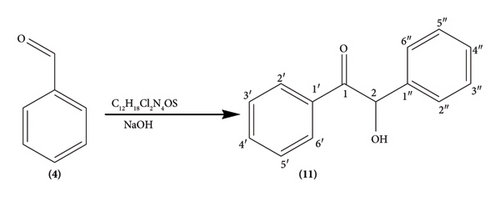
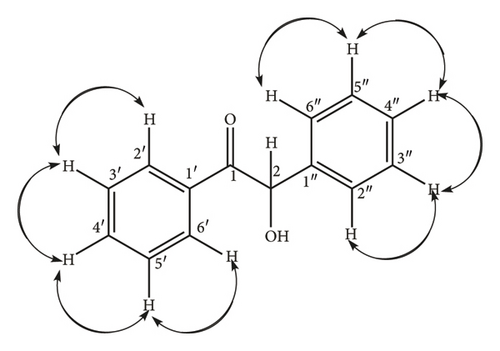
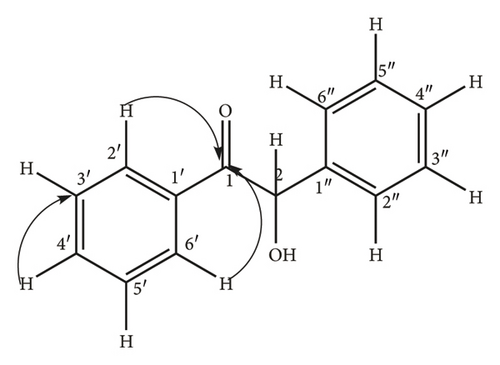
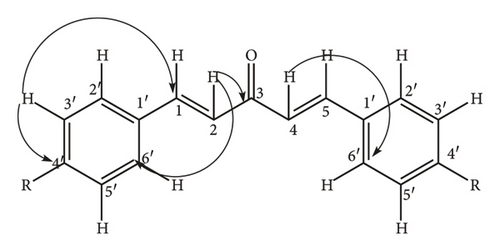
Briefly, thiamine hydrochloride (3 g, 8.04 mMol.) was dissolved in distilled water (4.5 mL) in a 250 mL conical flask, ethanol (30 mL) was added to the solution and the contents of the flask swirled by hand for 15 min until homogeneity was achieved. Addition of NaOH (9 mL, 0.25 Mol.) solution turned the mixture from colorless to bright yellow and the flask put on a mechanical shaker at 300 rpm for 20 min until the bright yellow color changed to pale yellow. Benzaldehyde (9 mL, 9.5 g, 85 mMol.) was slowly added to the mixture and the flask loaded onto a mechanical shaker at 300 rpm for 20 min until the mixture became homogeneous, the flask stoppered and left to stand in the dark for 72 h. The yellow crystals (8.25 g, 38.9 mMol.) were re-crystallized from hot ethanol to give white needle-like crystals of 2-hydroxy-1, 2-diphenylethanone (11) (7.92 g, 37.4 mMol., 95% yield) as confirmed by physical and spectroscopic data: mp 135–137°C (literature 134–136°C) [26]); chemical formulae; C14H12O21H NMR (400 MHz, CD3)2SO) δ 7.82 (2H, d, J = 5.16 Hz, H-2′, H-6′), 7.46 (2H, t, J = 4.28 Hz, H-3′, H-5′), 7.76 (1H, m, H-4′), 7.39 (2H, d, J = 6.8 Hz, H-3″, H-5″), 7.25 (2H, m, J = 7.8 Hz, H-2″, H-6″) 7.06 (1H, d, J = 5.08 Hz, H-4″); 5.92 (1H, d, H-2), 3.17 (1H, s, OH); 1H-1H COSY (CD3)2SO) see Figure 1; 13C NMR (δ, CD3)2SO) 199.65 (C-1) 140.2 (C-1″), 135.22 (C-1′), 133.69 C-4′), 128.93 (C-2′, C-6′), 128.17 (C-2′, C-6′) 129.3 (C-2″, C-6″), 129.06 (C-3″, C-5″), 127.73 (C-4″), 76.14 (C-2); DEPT 135 (CD3)2SO) 133.69 (C-4′H-4′), 128.17 (C-2″ & C -6″H-2″ & H-6″), 129.06 (C-3″ & C-5″H-3″ & 5″), 128.93 (C-2′ &C-6′H-2′ & H-6′), 127.73 (C-4″H-4″), 128.93 (C-2′ & C-6′H-2′ & 6′), and 76.14 (C-2H-2); 1J C-H, HSQC (CD3)2SO) C-2′ H-2′, C6′ H-6′, C-3′ H-3′ C-5′H-5′ C-3″ H-3″ C-5″H-5″, C-4″ H-4″, C-2″ H-2″ C-6″H-6″, C-4″ H-4″, C-2 H-2; 3J, 4J H-C HMBC (CD3)2SO): see Figure 2; EIMS (m/z) 39, 51, 63, 77, 105 (100%) [C7H5O]+, 139, 165, 210 [M+].
Compounds 12 and 13 were prepared through the established procedures summarized in Scheme 2 [27].
2.3.2. 1, 5-Diphenylpenta-1, 4-Diene-3-One (12)
Briefly, 0.25 M NaOH solution (100 mL) was transferred into a 500 mL conical flask, ethanol (80 mL) added and the mixture loaded onto a mechanical shaker at 200 rpm for 15 min to attain homogeneity. Acetone (4 mL, 3.16 g, 54.4 mMol.) and benzaldehyde (12 mL, 12.47 g, 117.5 mMol.) were added and the mixture shaken at 200 rpm for 20 min until the colour changed from pale yellow to deep yellow. On settling down, two layers were observed with the yellow crystals in the organic layer. The layers were separated, the organic layer filtered using a suction pump, the crystals collected, and dried to give 8.90 g. The crystals were carefully cleaned with methanol to obtain shiny disk like yellow crystals of 1.5-diphenylpenta-1,4-diene-3-one (12) (8.74 g, 37.5 mMol., 92% yield) and identified by physical and spectroscopic methods: mp 109–111°C (literature 108–110°C) [27]; chemical formulae C17H12O 1H NMR (δ, CD3OD) 7.61 (4H, d, J = 16.18 Hz, H-2′, H-6′), 7.32 (4H, d, J = 8.04 Hz, H-3′, H-5′), 7.16 (2H, t, J = 8.2 Hz, H-4′), 6.32 (2H, d, J = 16.12 Hz, 12.64 Hz, H-2, H-4), 7.49 (2H, d, J = 16.12 Hz, H-1, H-5); 1H-1H COSY (CD3OD): see Figure 3; 13C NMR (δ, CD3OD) 190.11 (C-3), 143.8 (C-1, C-5), 134.8 (C-1′), 130.38 (C-4′), 128.7 (C-2′, C-6′), 128.3 (C-3′, C-5′), 124.97 (C-2, C-4); DEPT 135 (δ) 143.8(C-1H-1, C-5 H-5) 130.38 (C-4′H-4′), 128.7 (C-2′H-2′, C-6′ H-6′), 128.3 (C-3′H-′), 124.97 (C-2 H-2, C-4 H-4); 3J, 4J H-C HMBC: see Figure 4; and EIMS (m/z) 39, 51, 63, 77, 91, 103, 115, 131, 156, 191, 205, 215., 233 (100%) [M+ − H], 234 [M+].
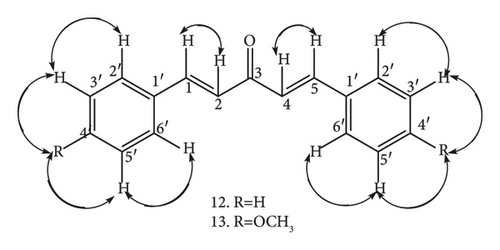
2.3.3. 1, 5-Bis (4-Methoxyphenyl) Penta-1, 4-Diene-3-One (13)
Briefly, 0.25 M NaOH solution (25 mL) was poured into a 100 mL conical flask, 10 mL of ethanol added, and the mixture shaken at 200 rpm for 15 min to attain homogeneity after which acetone (1.4 mL, 1.11 g, 19 mMol.) and 4-methoxybenzaldehyde (5.3 mL, 5.51 g, 40 mMol.) were sequentially added to form a white emulsion. The emulsion was shaken at 200 rpm for 10 min, allowed to settle down and form two layers: pale yellow and deep yellow, with fat-like droplets on top of the deep yellow layer, which changed into yellow crystals after 30 min. The mixture was filtered using a suction filtration pump to afford yellow crystals which were dried and cleaned carefully with methanol to give shiny disk-like yellow crystals of 1.5-bis-(4-methoxyphenyl) penta-1,4-diene-3-one (13) (3.83 g, 13 mMol, 85% yield): mp 105–107oC (literature 105–107°C) [28]; chemical formulae; C19H18O31H NMR (δ CDCl3) 7.89 (4H, d, J = 8.8 Hz, H-2′, H-5′), 7.65 (4H, d, J = 8.8 Hz, H-3′, H-5′), 7.42 (2H, d, J = 15.8 Hz, H-1, H-5), 6.99 (2H, d, J = 15.8 Hz, H-2 H-4). 3.87 (3H, s, OCH3); 1H-1H COSY (CDCl3): see Figure 3; 13C NMR (δ, CDCl3) 188.86 C-3) 161.57 (C-4′), 142.67 (C-1, C-5), 130.07 (C-2′, C-5′), 127.68 (C-1′), 123.54 (C-2, C-4), 114.43 (C-3′, C-5′), 55.41 (OCH3); DEPT 135 (δ, CDCl3) 142.67(C-1H-5), 130.07 (C-2′H-2′), 130.07 (C-5′, H-5′), 123.54 (C-2H-2), 114.43 (C-3′, H-3′), 114.43 (C-5′H-5′), 55.41 (OCH3); 1JC-H, HSQC (δ, CDCl3) C-2 H-2 4C-4 H-4, C-1 H-1 C-5H-5, C-3′ H-3′, C-5′H-5′, C-2′ H-2′; 3J, 4J H-C HMBC: see Figure 4; EIMS (m/z)[31, 39, 41, 53, 56, 57, 59 (100%) [C3H4O]+, 63, 73, 83, 87, 100, 294 [M+].
2.4. Preparation of Stock Solution and Dilutions
The pure compounds (10 mg) were dissolved in 10 mL of ethanol and topped up to 100 mL with distilled water to prepare 100 parts per million (ppm) or milligrams per liter (mg/L) stock solutions. The stock solutions were diluted appropriately with distilled water to obtain 1, 10, 25 and 50 parts per million (ppm) solutions for ovicidal assays.
Blends were formulated from benzaldehyde (4) (B), phenol (5) (P) and anisole (6) (A). Briefly, the individual compounds (3.33 mg each) were mixed to form blend BPA, which was dissolved in ethanol (10 mL) and topped up to 100 mL of distilled water to make a stock solution of 100 ppm. For blends PA, BP and BA, equal amounts of individual compounds (5 mg) were mixed and dissolved in 10 mL of ethanol and topped up to 100 mL with distilled water to make 100 ppm stock solutions. The stock solutions were diluted appropriately with distilled water to make 1, 2, 10, 25 and 50 ppm for ovicidal assays.
2.5. Ovicidal Activity
Ovicidal activity was determined by measuring the inhibition of egg hatchability. Briefly, freshly laid eggs of An. gambiae were counted and divided into groups of 100 using a hand magnifying lens and each group submerged into 25 mL of 1, 2, 10, 25 and 50 ppm solutions of each pure compounds in transparent plastic containers for 48 h or until they hatched into larvae or completely inhibited from hatching. Each treatment was replicated 4 times, with eggs exposed to 1% ethanol in water and plain distilled water serving as controls. The hatchability was assessed after 48 h of post treatment. The emergent larvae were further observed for longevity, fecundity and any physical deformity.
2.6. Statistical Analysis
The ovicidal data were compared using Students Newman Kuel t-tests (SNK t-test) to obtain the means for eggs that hatched and the dose-response-relationships determined using probit analysis. The LD50 and LD90 values obtained from the regression analysis [29]. The level of significance of statistical data was set at p < 0.05 or lower. The software used was SAS version 8.2.
3. Results and Discussion
Thirteen compounds (1–13) grouped into five molecular structures (1-2, 3–7, 8–10, 11, 12-13) were tested individually and as blends for ovicidal activity using the eggs of Anopheles gambiae mosquitoes.
The results in Table 1 indicate the hatchability rate of the An. gambiae eggs in different treatments at various concentrations. Highest egg mortality or un-hatchability was observed at 50 ppm in nearly all the compounds tested. Poor ovicidal activity was noted for all the treatments at 1 ppm since almost all of the An. gambiae eggs hatched into larvae. The emergent larvae had no significant deformities observed.
| Compound/blend | Concentration | |||
|---|---|---|---|---|
| 1 ppm | 10 ppm | 25 ppm | 50 ppm | |
| 1 | 44.33 ± 5.93c | 0.0 ± 0.0h | 0.0 ± 0.0h | 0.0 ± 0.0h |
| 2 | 75.00 ± 8.90a | 83.25 ± 7.05a | 68.75 ± 4.89a | 0.0 ± 0.0h |
| 3 | 67.50 ± 1.85a | 83.25 ± 7.05a | 48.25 ± 5.54c | 0.0 ± 0.0h |
| 4 | 59.00 ± 3.74b | 69.75 ± 3.15a | 0.0 ± 0.0h | 0.0 ± 0.0h |
| 5 | 84.25 ± 3.86a | 45.00 ± 3.35a | 2.00 ± 1.23g | 0.0 ± 0.0h |
| 6 | 92.00 ± 2.38a | 54.00 ± 3.54a | 49.25 ± 1.93c | 0.0 ± 0.0h |
| 7 | 80.25 ± 8.35a | 66.50 ± 5.27a | 76.00 ± 6.34a | 0.0 ± 0.0h |
| 8 | 69.75 ± 4.50a | 0.0 ± 0.0h | 0.0 ± 0.0h | 0.0 ± 0.0h |
| 9 | 62.67 ± 17.84a | 0.0 ± 0.0h | 0.0 ± 0.0h | 0.0 ± 0.0h |
| 10 | 85.75 ± 3.50a | 75.00 ± 2.58a | 19.50 ± 3.88e | 0.0 ± 0.0h |
| 11 | 67.00 ± 7.94a | 57.50 ± 3.71b | 19.00 ± 3.49e | 0.75 ± 0.75g |
| 12 | 76.00 ± 9.44a | 55.75 ± 8.43b | 0.75 ± 0.48g | 0.0 ± 0.0h |
| 13 | 85.00 ± 3.50a | 75.00 ± 2.58a | 19.00 ± 3.88e | 0.0 ± 0.0h |
| BPA | 57.67 ± 16.19a | 25.00 ± 11.00 | 4.67 ± 3.71g | 0.0 ± 0.0 |
| BA | 75.33 ± 6.74a | 69.00 ± 3.605a | 0.0 ± 0.0 | 0.0 ± 0.0 |
| BP | 45.67 ± 4.91c | 16.33 ± 10.398ee | 0.0 ± 0.0 | 0.0 ± 0.0 |
| PA | 56.33 ± 23.82a | 41.67 ± 3.180d | 14.33 ± 2.03f | 0.0 ± 0.0 |
| Ethanol | 71.25 ± 4.96a | 73.00 ± 1.958a | 78.75 ± 3.01a | 57.50 ± 6.86b |
| Water | 83.00 ± 4.55a | |||
- Note: Means with the same letter are not significantly different (SNK test, p ≤ 0.001).
- Abbreviations: BA, benzaldehyde and anisole; BP, benzaldehyde and phenol; BPA, benzaldehyde, phenol and anisole; PA, phenol and anisole.
3.1. Structure Activity Relationship
2-Hydroxy-4-methoxybenzaldehyde (1), a trisubstituted aromatic compound with hydroxyl, methoxyl and aldehydic groups, has been previously isolated from the roots of Mondia whytei, shown to be as a tyrosine inhibitor [23] and a potential larvicide for An. gambiae [24]. The compound is also responsible for the characteristic smell and taste of roots from the plant [22]. The reported insecticidal properties and the diverse functional groups prompted us to probe its ovicidal activity against An. gambiae eggs to establish whether it has any potential to inhibit the eggs from hatching and if so which functional groups confer the activity. The ovicidal activity of 2-hydroxy-4-methoxybenzaldehyde (1), related compounds (2–10), structural analogues (11–13) and formulated blends against An. gambiae are summarized in Table 2. With an LD50 value of 0.7075 ppm, compound 1 prompted a structure activity relationship to probe the functional groups responsible for the observed ovicidal efficacy. The readily available and a closely related congener; 4-hydroxy-3-methoxybenzaldehyde/vanillin (2) was also bioassayed and the activity found to be 34 times lower (LD50 24.177 ppm) than 1. Coincidentally, a much lower tyrosinase inhibition and larvicidal activity of vanillin (2) and other related congeners have also been reported [23, 24]. The significant difference in the biological activity of the two compounds is quite intriguing given that they have the same functional groups arranged differently on the aromatic skeleton. This observation prompted further investigations on the cause of observed varied activity in regard to the ovicidal activity. Consequently, related compounds with similar functional groups but different functional group arrangement on the benzene skeleton were assayed for comparison.
| Compound/blend | LC50 | 95% Cl | LC90 | 95% Cl | Chi-square | p value |
|---|---|---|---|---|---|---|
| 1 | 0.7075 | 0.1537–1.0696 | 4.5928 | 3.7631–6.1956 | 1.5398 | 0.1000 |
| 2 | 24.177 | 5.2523–36.551 | 48.971 | 40.1243–66.0609 | 187.1978 | 0.0001 |
| 3 | 19.494 | 4.235–29.4711 | 48.087 | 38.555–66.9155 | 145.6925 | 0.0001 |
| 4 | 5.584 | 1.213–8.442 | 19.844 | 15.91–27.614 | 43.4529 | 0.0001 |
| 5 | 9.9354 | 7.0038–13.2425 | 20.289 | 16.2672–28.2332 | 6.4655 | 0.0394 |
| 6 | 40.342 | 28.438–53.770 | 100.894 | 80.894–140.399 | 90.6057 | 0.0001 |
| 7 | 25.633 | 23.993–27.461 | 49.830 | 45.5035–56.134 | 188.5348 | 0.0001 |
| 8 | 1.4516 | 1.3587–1.5551 | 2.5707 | 2.3475–2.8959 | 0.000 | 0.1000 |
| 9 | 1.3899 | 0.851–2.123 | 2.9366 | 2.187–5.205 | 0.000 | 0.1000 |
| 10 | 15.642 | 9.574–23.892 | 30.626 | 22.804–54.28 | 15.0007 | 0.0006 |
| 11 | 10.599 | 2.345–17.233 | 32.582 | 24.072–55.387 | 10.1236 | 0.0063 |
| 12 | 9.019 | −5.8937–31.7726 | 20.252 | 12.4073–34.327 | 45.4403 | 0.0001 |
| 13 | 15.642 | 9.574–23.892 | 30.626 | 22.804–54.28 | 15.0007 | 0.0006 |
| BPA | 2.944 | 1.384–4.275 | 19.047 | 17.24–21.339 | 1.5464 | 0.1000 |
| BA | 5.129 | 2.411–7.45 | 27.661 | 24.549–31.726 | 85.1797 | 0.0001 |
| BP | 0.3320 | −1.1544–1.5121 | 11.9848 | 10.6365–13.7459 | 2.5026 | 0.1000 |
| PA | 9.9909 | −3.103–10.237 | 20.8138 | 18.4722–42.28 | 4.6059 | 0.1000 |
- Abbreviations: BA, benzaldehyde and anisole; BP, benzaldehyde and phenol; BPA, benzaldehyde, phenol and anisole; PA, phenol and anisole.
Simple aromatic compounds constituting the same functional groups as those on 1 and 2 were assayed. Anisole or methoxybenzene (6) (LD50 40.342 ppm), benzoic acid (7) (LD50 25.633 ppm) and benzene (3) (LD50 19.494 ppm) exhibited low ovicidal activity while benzaldehyde (4) at LD50 5.584 ppm had slightly higher activity than phenol (5) (LD50 9.9354 ppm) (also toxicological reports reveal that phenol is harmful to humans depending on the route of exposure [30]). The results revealed an interesting trend in the potency of derivatives of benzene (3); due to substituent variation that enhance or lower activity of the resulting aromatic compound confirming that the aldehyde functional group activates the benzene ring more potent than carboxylic acid as demonstrated in compound 4, which exhibited higher ovicidal activity than compound 7. The observation is consistent with earlier reports where 2-hydroxy-4-methoxybenzaldehyde was found to exhibit higher larvicidal activity than 4-methoxysalicylic acid [24]. The enhanced ovicidal activity of compounds 4 and 5 suggest that attachment of an aldehyde or hydroxyl group on 3 enhances its efficacy. On the hand, methoxyl and carboxylic acid groups on benzene ring reduce the ovicidal activity of the resultant compound substantially. While the bioassay data of these simple compounds were supposed to help us understand the contribution of the individual functional groups on the activity of 2-hydroxy-4-methoxybenzaldehyde (1) and vanillin (2), they could not satisfactorily explain the observed activity of compounds 1 and 2, and therefore more assays using di-substituted aromatic compounds were undertaken to investigate the structure-activity relationships in the two compounds. Due to relatively strong ovicidal activity, benzaldehyde (4) was chosen as the starting point for the structure-activity studies. The introduction of an electron donating hydroxyl group at ortho position in compound 4 resulted in 2-hydroxybenzaldehyde (8) with improved ovicidal activity (LD50 1.452 ppm), confirming that ortho-hydroxyl group enhances the ovicidal activity of benzaldehyde. However, when the hydroxyl group was shifted to para position as in 4-hydroxybenzaldehyde (10), the activity was significantly reduced to LD50 15.642 ppm, confirming that para-hydroxyl group is antagonistic to the ovicidal activity of benzaldehyde. The two observations unravel the contribution of the hydroxyl group on the activity of 1 and 2 and confirm that the relative position of the substituents on the benzene skeleton is critical. Interestingly, addition of a stronger electron donating methoxy group at the para position of 4 gives 4-methoxybenzaldehyde (9) with increased activity at LD50 of 1.390 ppm. Consequently, the activity of compound 9 helped us to explain the poor ovicidal activity observed in vanillin (2), a tri-substituted aromatic compound. Considering the observed poor ovicidal activity of 4-hydroxybenzaldehyde (10), the presence of a methoxyl group ortho to the hydroxyl and meta to the carbonyl as vanillin (2) should confer lower activity as already observed. It is interesting to note that shifting the hydroxyl in 2-hydroxybenzaldehyde (8) to para reduces the activity of the resulting 4-hydroxybenzaldehyde (10) which on further addition of methoxyl ortho to the hydroxyl of 4-hydroxybenzaldehyde (10), gives vanillin (2) with much lower activity than 4-hydroxybenzaldehyde (10). The trend in the activity of vanillin (2), 2-hydroxybenzaldehyde (8) and 4-hydroxybenzaldehyde (10) assisted in assessing the effect of substituent position on the aromatic ring. It further demonstrates that the hydroxyl and methoxyl groups are either synergistic or antagonistic to the ovicidal effect of the carbonyl group when on the same ring depending on the position of attachment. In addition, the hydroxyl and methoxyl groups are antagonistic when ortho relative to each other as earlier reported for larvicidal activity [24]. On the contrary, 2-hydroxy-4-methoxybenzaldehyde (1) displays quite an interesting trend in activity. Considering 2-hydroxybenzaldehyde (8) and 4-methoxybenzaldehyde (9), the addition of methoxy at para to the carbonyl and meta to the hydroxyl of 8 as in 1 enhances the ovicidal activity. Similarly, the addition of hydroxyl ortho to the carbonyl and meta to the methoxyl group in 4-methoxybenzaldehyde (9) gives 2-hydroxy-4-methoxybenzaldehyde (1) with increased activity suggesting that methoxyl and hydroxyl groups are potentiating the benzaldehyde group depending on their positions relative to the carbonyl and to each other when all the three functional groups are on the same benzene ring.
The electron donating hydroxyl and methoxyl groups gave interesting results when attached at para to aldehyde carbonyl. It is important to note the fundamental role played by the slightly bulky methoxyl in increasing activity as in 4-methoxybenzaldehyde (9); while on the other hand, the hydroxyl group in 4-hydroxybenzaldehyde (10) substantially reduced activity. The para-hydroxyl group plays more antagonistic ovicidal role to the aldehydic carbonyl position probably due to stronger inter-molecular H-bonding than when it is at ortho position where there is intra-molecular H-bonds as in 8 which enhance activity. These observations can also be rationalized by the stronger electron donating property of para-methoxy than para hydroxyl group and the intra-molecular hydrogen bonding to the carbonyl by the ortho-hydroxyl group. The enhanced activity of compounds 8, 9 and 10 therefore reflects the effectiveness of the position of hydroxyl and methoxyl groups in relation to aldehyde group as demonstrated in compounds 1 and 2 where it was noted that the position of methoxyl or hydroxyl has impact on activity.
Compound 4 was modified to 11 and 12 while compound 9 gave 13. The structural analogs were assayed for ovicidal activity. Compounds 11 (LD50 10.599 ppm) and 12 (LD50 9.019 ppm) exhibited significantly lower ovicidal activity than the parent compound 4. Similarly, compound 13 exhibited significantly lower activity (LD50 15.642 ppm) than the parent compound 9. The observations confirm that the aldehyde and hydroxyl groups are critical for ovicidal activity of An. gambiae eggs as previously reported for larvicidal activity [24].
The blends assayed for ovicidal activity included benzaldehyde (4), phenol (5), and anisole (6) (BPA); benzaldehyde (4) and anisole (6) (BA), benzaldehyde (4) and phenol (5) (BP) and phenol (5) and anisole (6) (PA). BPA exhibited better ovicidal activity at LD50 2.944 ppm than any of the individual components, but was four times lower than 2-hydroxy-4-methoxybenzaldehyde (1) and eight times higher than vanillin (2), suggesting that the compounds exert synergy when in the blend. PA, equivalent to subtraction of compound 4 from BPA, significantly lowered its ovicidal activity by to LD50 5.129 ppm thus indicating that benzaldehyde is a critical component of the blend. BP, equivalent to substituting compound 6 with 4 or subtracting 6 from blend BPA, exhibited the highest ovicidal activity LD50 0.332 ppm which was nine (9) times higher than that of blend BPA and fifteen (15) times higher than that of blend PA confirming that benzaldehyde is a critical component of the blend. BA, equivalent to the subtraction of compound 5 from BPA or substitution of compound 5 from BP, exhibited the lowest ovicidal activity of all the blends at LD50 9.990 ppm confirming that anisole is an antagonistic component of the blend. The observed results revealed that the synergistic interaction of the individual compounds is much stronger when the compounds are blended than when all the functional groups are incorporated in one compound suggesting that intra-molecular interactions have higher positive impact on ovicidal activity than the inter-molecular interactions.
Several structure-larvicidal activity relationships have been documented with all the studies linking functional groups of different compounds to the resulting activity of the compounds. For instance, acetyl derivatives of monoterpenoid compounds were reported to exhibit high larvicidal activity against Ae. aegypti [31]. In another case, presence of hetero-atoms in the basic monoterpene structure for instance neoisopulegol reduced the potency of the compound. It was further noted that conjugated or exo-carbon-carbon double bonds and epoxidation increased larvicidal activity [32]. Eugenol derivatives were found to exhibit lower larvicidal activity than the parent compound [33]. It has been also been reported that conversion of phenol to diphenyl ether substantially enhanced the larvicidal activity against An. gambiae [24]. A preprint of the findings of this work has previously been published [34]”
4. Conclusion
The presence of aldehyde and hydroxyl groups on mono-substituted benzene confers strong ovicidal activity while methoxyl group lowers activity. While simple mono-functional compounds: BPA exhibited relatively lower activity when evaluated individually, the ovicidal activity was significantly enhanced when they were formulated as blends. The blend of BP exhibited the highest activity, while BA had the lowest efficacy. For di-substituted simple aromatic compounds, methoxyl group is an activity-potentiating group at para position to the aldehyde group and hydroxyl when in ortho-position to the aldehyde. Hydroxyl, methoxyl and aldehyde functional groups on an aromatic skeleton confer ovicidal activity when appropriately located in one compound but are strongly synergistic when in different molecules. 2-Hydroxy-4-methoxybenzaldehyde exhibited the highest ovicidal activity against An. gambiae, while anisole exhibited the lowest efficacy.
Disclosure
A preprint has been previously published [34].
Conflicts of Interest
The corresponding author is an employee of Masinde Muliro University of Science and technology, which financially supported the research work. The remaining authors declare no conflicts of interest.
Funding
This research work got financially supported by partial funding from Masinde Muliro University of Science and Technology’s University Research Fund (URF). The University is the employer of the corresponding author.
Acknowledgements
We appreciate Mr. Richard Amito and his team at KEMRI for the supply of An. gambiae eggs and the help accorded to us during the bioassays. University of Western Cape is appreciated for Spectroscopic experiments and data produced, the Center for African Medicinal and Nutritional Flora and Fauna (CAMNFF) was acknowledged for provision of Mondia whitei roots and the laboratory space for synthetic work.
Appendix A: Identity of the Compounds Tested for Hatchability of the Anopheles gambiae Eggs
- 1.
2-Hydroxy-4-methoxybenzaldehyde
- 2.
4-Hydroxy-3-methoxybenzaldehyde/vanillin
- 3.
Benzene
- 4.
Benzaldehyde
- 5.
Phenol
- 6.
Anisole or methoxybenzene
- 7.
Benzoic acid
- 8.
2-Hydroxybenzaldehyde
- 9.
4-Methoxybenzaldehyde
- 10.
4-Hydroxybenzaldehyde
- 11.
2-Hydroxy-1, 2-diphenylethanone
- 12.
1, 5-Diphenylpenta-1, 4-diene-3-one
- 13.
1, 5-Bis (4-methoxyphenyl) penta-1, 4-diene-3-one
Open Research
Data Availability Statement
The OVICIDAL ACTIVITY and SPECTROSCOPIC data used to support the findings of this study are available from the corresponding author upon request.




