Latex of Calotropis gigantea-Based Biogenic Magnesium Hydroxide Nanoparticles: Synthesis, Characterization, Antioxidant and Antibacterial Activity
Abstract
The current investigation determines the fabrication and characterization of magnesium hydroxide nanoparticles (Mg(OH)2 NPs) with the help of C. gigantea latex via biological method. The size, shape, morphology, functional groups and stability of biogenic Mg(OH)2 NPs were analysed by microscopic and spectroscopical analyses. Mg–O vibration, C–O stretch, O–H and C–H bend were detected by FTIR analysis. Crystalline nature of biogenic Mg(OH)2 NPs was confirmed by X-ray diffraction (XRD). FESEM with EDX analysis revealed the morphology and elemental composition of biogenic Mg(OH)2 NPs and proved that formation of nano petal-shaped Mg(OH)2 NPs. Moreover, the synthesized biogenic Mg(OH)2 NPs were studied for antioxidant and antibacterial activities against wound-causing bacteria by standard methods. As-synthesized Mg(OH)2 NPs showed significant antioxidant activity as compared with ascorbic acid and it had potential antibacterial activity. The study concluded that Mg(OH)2 NPs with antioxidant and antibacterial properties can be synthesized using C. gigantea latex by green chemistry approach and may be used for formulation of new drugs and gel for wound healing and any other medical application.
1. Introduction
Nanotechnology is the term used to describe the manufacturing, characterization, manipulation and application of structures at the nanoscale [1]. It is considered the most dynamic field of research in material sciences, with the synthesis of nanoparticles (NPs) gaining significant momentum worldwide. NPs exhibit unique or enhanced properties based on specific characteristics such as size (1–100 nm), shape and structure [2]. They can be broadly categorized as organic or inorganic NPs. Inorganic NPs include metallic NPs (Au, Ag, Cu and CuNi [3]), semiconductor NPs (ZnO, ZnS and CdS) and different composite particles for broad areas of use [4–7]. On the other hand, organic NPs encompass carbon NPs (such as fullerenes, quantum dots and carbon nanotubes) [8]. Researchers have increasingly prioritized the advancement of effective green chemistry techniques in synthesizing metal NPs [9, 10]. Plants, in particular, have emerged as the most promising candidates for large-scale biosynthesis of NPs. Notably, NPs derived from plants exhibit enhanced stability and a faster synthesis rate compared with those produced by microorganisms [11]. Green synthesis methods utilize environmentally friendly chemicals to produce NPs and utilize gentle solvents like water, natural extracts. The goal of green chemistry is to minimize pollution from the beginning. It focuses on minimizing waste rather than managing or cleaning up waste once it is generated [12].
Magnesium hydroxide (Mg(OH)2) NPs are prepared by various methods, such as precipitation, hydrothermal synthesis and thermal decomposition of magnesium salts [13]. Among these methods, the most popular is chemical precipitation, which uses the reaction between magnesium salt solutions and alkalis. However, such traditional approaches often require aggressive chemicals and harsh reaction conditions, making the process less environmentally friendly. In recent years, there has been a significant increase in interest in green methods for the synthesis of NPs, which use plant extracts as reducing agents and stabilizers [14, 15]. This approach is based on the use of bioactive compounds contained in plants, such as polyphenols, flavonoids and organic acids, which can reduce and stabilize NPs under mild conditions effectively. Aqueous extracts of plants such as aloe vera, mint, turmeric and green tea have shown high efficiency in the synthesis of Mg(OH)2 NPs. Green synthesis allows for the production of NPs with improved characteristics, including smaller size and more uniform particle distribution. This approach not only reduces the environmental impact but also simplifies the process of producing nanomaterials for further use in biomedicine and industry. Mg(OH)2 NPs have unique antioxidant and antibacterial properties, making them promising for use in various fields [16, 17]. These NPs’ antioxidant properties manifest in their ability to neutralize reactive oxygen species (ROS), preventing oxidative stress and protecting cells from damage. These properties are especially in demand in biomedicine, where NPs can be used to protect tissues from inflammatory processes and accelerate wound healing. In addition, Mg(OH)2 demonstrates pronounced antibacterial activity against a wide range of pathogenic microorganisms [18]. This is due to the alkaline nature of the NPs and their ability to destroy bacterial cell walls. Such properties open up broad prospects for the use of Mg(OH)2 NPs in the production of antibacterial coatings, medical implants and packaging materials to extend the shelf life of products. In industry, they can be used to prevent corrosion of materials and protect surfaces from microbial fouling. Thus, the antioxidant and antibacterial properties of Mg(OH)2 NPs make them promising for solving urgent problems in medicine, the food industry and environmentally friendly technologies.
Calotropis gigantea is a xerophytic, belongs to Asclepiadaceae family and grown in the tropical and subtropical regions of Asia and Africa. It is popularly known because it produces large amount of latex. The plant has potential pharmacological properties [19]. It is often referred to as the Swallow-Wort or Milkweed. The latex of this plant contains various cardiac glycosides such as calotopin, uscharin, calotoxin, calactin, uscharidin and gigantin [20]. Rajkuberan et al. [21] were produced silver nanoparticles (Ag NPs) by utilizing the aqueous latex extract of C. gigantea L. via green approach for various biomedical applications. Das et al. [22] utilized aqueous extract of C. procera latex for the production of gold NPs. Pradeepkumar et al. [23] assembled multifunctional latex-based hybrid nanocarriers using C. gigantea for sustained doxorubicin releases. Murugesan et al. [24] produced the photoluminescent-reduced graphene oxide quantum dots using latex of C. gigantea for cytotoxicity, radical scavenging, bioimaging and metal sensing in Artemia salina.
The current work was describing on synthesis of Mg(OH)2 NPs using the aqueous latex extract of C. gigantea and studied of its size, shape, crystalline nature and purity via microscopic and spectroscopic analysis. Moreover, the antimicrobial and antioxidant activities of as-synthesized Mg(OH)2 NPs were determined.
2. Materials and Methods
2.1. Collection of C. gigantea Latex
Latex of C. gigantea was collected from in and around PSG College of Arts & Science, Coimbatore, on May 2024. The plant was authenticated by Scientist F, Botanical Survey of India, Coimbatore, India.
2.2. Green Synthesis of Mg(OH)2 NPs Using Latex
One mL of C. gigantea latex was obtained and it was mixed with 100 mL of distilled water. Then, the prepared extract was boiled at 80°C for 15 min. Simultaneously, a 0.1 M MgSO4 solution with a pH of 8 was prepared. The ratio of 1:1 of C. gigantea latex and MgSO4 solution were taken and mixed well. Then, it was allowed to stirrer until precipitation occurred. After precipitation, the white colour powder pellet was obtained and it was dried at 80°C under hot air oven. Finally, the fine powder was collected and stored in sterile bottles for further analysis.
2.3. Characterization of Biogenic Mg(OH)2 NPs
One mg of biogenic Mg(OH)2 NPs was mixed with 10 mL of deionized water. Then, it was kept in a sonicator to prepare the nanosuspension. The optical property of as-prepared Mg(OH)2 NPs was assessed by Shimadzu UV-1601 spectrophotometer at range of 200 to 800 nm. To find the possible biomolecules responsible for the reduction of the Mg(OH)2 and capping of the bio reduced Mg(OH)2 NPs synthesized by the latex extract, FTIR analyses were performed. FTIR spectra of biogenic Mg(OH)2 NPs were observed using the KBr pellets by Perkin–Elmer FT-IR spectrophotometer at a range of 4000–400 cm−1, with 2 cm−1 of resolution. Crystalline nature, structural characteristics and diffraction pattern of as-synthesized biogenic Mg(OH)2 NPs was analysed by X-ray diffraction (XRD). Sample was scanned at a rate of 0.026°/min in the region of 2θ from 10° to 80° via Cu-Kα radiation source (λ = 1.54 Å). The average crystalline size of biogenic Mg(OH)2 NPs was calculated using the Debye–Scherer equation. The morphological structure of biogenic Mg(OH)2 NPs was investigated using FESEM analysis (JEOL Model JSM-7600F) (at 500,00× magnification and at an accelerating voltage of 10 kV). A thin film of sample was prepared on carbon coated copper grid using dropping a small amount of Mg(OH)2 NPs into the grid. Different magnification was employed to take micrograph of Mg(OH)2 NPs. To determine the purity and elements of as-synthesized biogenic Mg(OH)2 NPs, the energy dispersive X-ray spectroscopy (XL30, Philips microscope) analysis was executed.
2.4. Antioxidant Activity
2.5. Antibacterial Activity
The antibacterial activity of biogenic Mg(OH)2 NPs was determined by agar-well diffusion method against pathogens. The test was carried out to observe the Mg(OH)2 NPs inhibitory effect. Gel puncture was used to make wells (6 mm diameter) on Muller–Hinton agar plates. Sterile cotton swabs were used to evenly distribute each strain on each plate. Using a micropipette, 25–100 μg/mL of biogenic Mg(OH)2 NPs solution were added to each well on all plates. The zones of inhibition were measured after 24 h of incubation at 37°C, with tetracycline (0.01 g/mL) as the positive control. Duplicates were maintained for each plate. The zone of inhibition was measured in diameters. The experiment was repeated five times, and the average value was determined and represented in mean ± SD [25].
3. Results and Discussion
3.1. Samples Characterisation
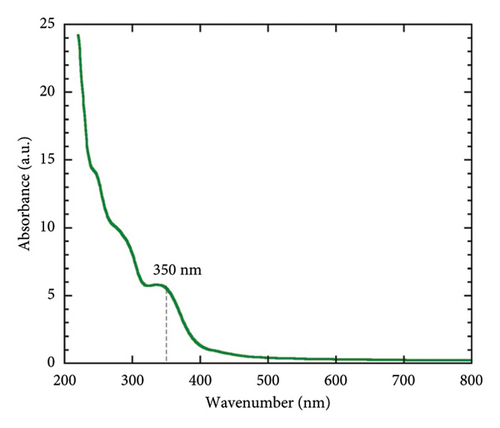
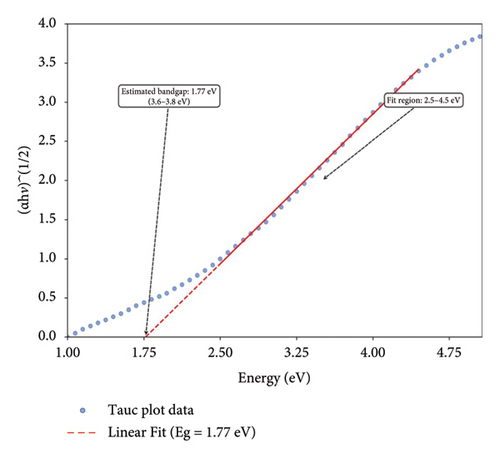
In the current investigation, the absorption spectra of biogenic Mg(OH)2 NPs were observed at the wavelength range of 200–800 nm in UV-visible spectrophotometer. Figure 1(a) shows the UV-Vis absorption spectra of biogenic Mg(OH)2 NPs and show the peak around 350 nm. Similarly, the optical properties of Mg(OH)2 NPs were verified through UV-visible spectrophotometer and the peak at 300 nm was observed [26]. The optical band gap of the biogenic Mg(OH)2 NPs was determined using the Tauc plot (Figure 1(b)), which plots (αhν)n versus photon energy (hν) to identify the transition type [9]. The extrapolation of the linear region of the plot to the x-axis yielded a band gap energy of 1.77 eV, confirming the material’s suitability for potential applications in photocatalysis and biomedical coatings. This analysis further highlights the influence of C. gigantea latex-mediated synthesis on the electronic properties of the NPs.
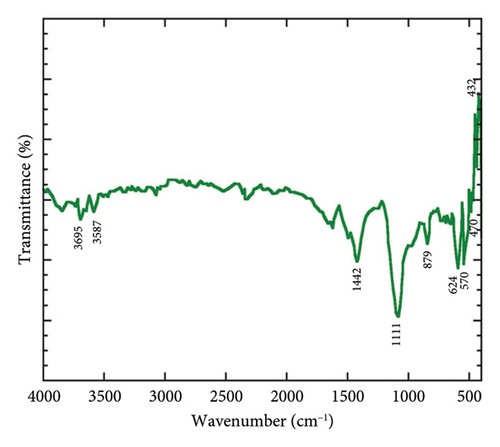
The synthesized Mg(OH)2 NPs was additionally analysed using FTIR spectroscopy to determine its functional groups. Figure 1(b) illustrates the distinctive peaks of different functional groups. The peaks of 432, 470, 570, 624, 879, 1111, 1442, 3587 and 3695 cm−1 were observed in FTIR spectra of biogenic Mg NPs. The peaks of 432, 470, 570, 624 and 879 cm−1 were referred the Mg–O vibration. A peak at 1111 cm−1 was occurred due to existence of C–O stretch. The peak at 1442 cm−1 corresponded to C–H bend. The O–H stretch and H-bonded were found and denoted by the occurrence of peaks 3587 and 3695 cm−1. The authors in [27] reported the different functional groups and Mg–O vibration for Camellia sinensis extract-mediated Mg(OH)2 NPs.
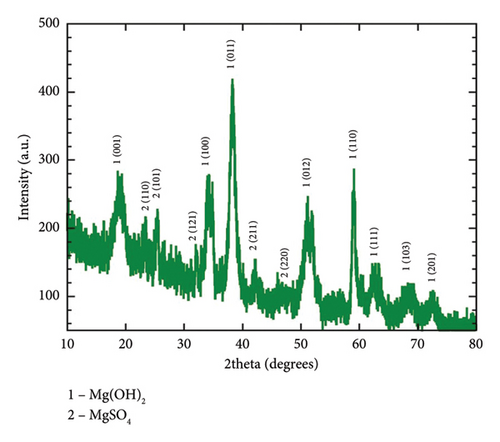
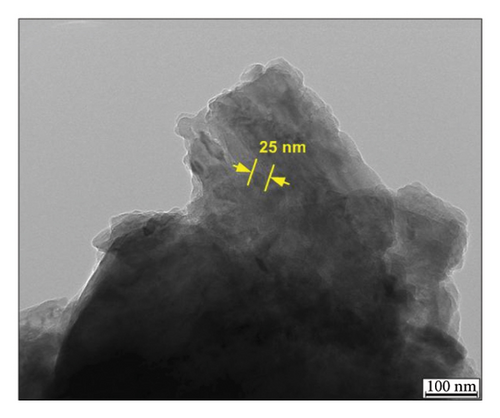
The investigation of the crystalline phase and structure of the fresh-synthesized Mg(OH)2 NPs was conducted using XRD. Figure 1(c) present the XRD pattern of C. gigantea latex-mediated Mg(OH)2 NPs with hexagonal crystal system and P-3m1 space group. The rest of the peaks belongs to the MgSO4 impurities. The average size of biogenic Mg(OH)2 NPs was determined using Scherrer’s formula and found to be 22 nm [28].
Figure 2 illustrate the surface morphology of biogenic Mg(OH)2 NPs, which were acquired by the FESEM analysis. It clearly reveals that biogenic Mg(OH)2 NPs were nano petals with an average size of 20.98 nm.
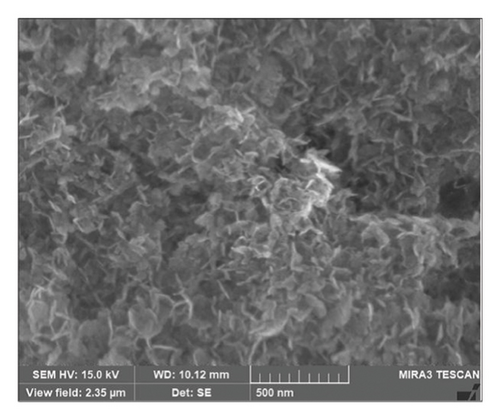
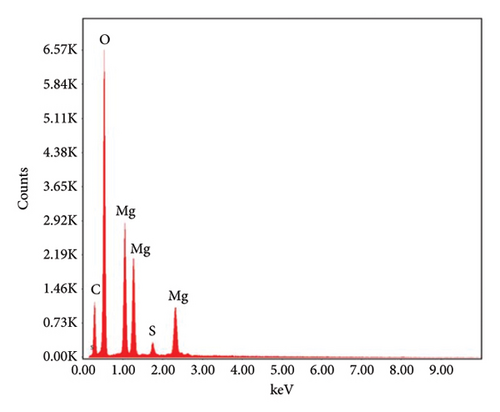
EDX analysis was carried out to find out the elemental composition of biogenic Mg(OH)2 NPs. In addition, other elemental signals such as C, O and S were observed due to capping agents and other phytomolecules. Moreover, they concluded that the occurrence of carbon in EDX spectra was due to organic biomolecules in plant extract as reducing and capping agents for formation of Mg(OH)2 NPs.
3.2. Analysis of Antioxidant Activity
The antioxidant properties of biogenic Mg(OH)2 NPs were assessed by DPPH radical scavenging assay (Table 1). Ascorbic acid was employed as a standard. As the results clearly reveal, the antioxidant properties increase with increasing concentration of biogenic Mg(OH)2 NPs (6–300 μg/mL) in the % of DPPH-free radical scavenging activities. The maximum free radical scavenging activities (65.57 ± 1.85) were found at the concentration of 300 μg/mL of biogenic Mg(OH)2 NPs. The lowest free radical scavenging was observed at 6 μg/mL of biogenic Mg(OH)2 NPs. Kharat and Mendhulkar [29] investigated and found the antioxidant activity of Elephantopus scaber leaf extract-based Ag NPs through DPPH assay.
| Concentrations (μg/mL) | % inhibition |
|---|---|
| 6 | 45.08 ± 2.53 |
| 30 | 46.72 ± 1.24 |
| 90 | 50.00 ± 2.90 |
| 150 | 53.28 ± 1.52 |
| 300 | 65.57 ± 1.85 |
3.3. Antibacterial Activity
C. gigantea latex-mediated Mg(OH)2 NPs have good antibacterial activity against wound-causing bacteria such as K. pneumonia, Micrococcus Spp., Proteases, S. aureus and P. aeruginosa (Figure 3). The maximum zone of inhibition was observed against Micrococcus Spp. at different concentration of C. gigantea latex-mediated Mg(OH)2 NPs. The green-synthesized Mg(OH)2 NPs using various plants extract have a similar antibacterial activity [30–33].
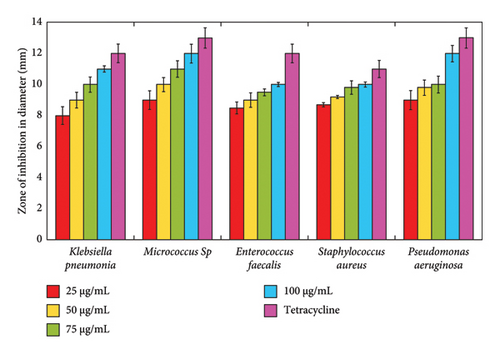
The antioxidant activity of biogenic Mg(OH)2 NPs is primarily attributed to their ability to neutralize ROS, thereby mitigating oxidative stress and cellular damage. The DPPH radical scavenging assay demonstrated a concentration-dependent increase in antioxidant activity, with a maximum inhibition of 65.57 ± 1.85% at 300 μg/mL, comparable with ascorbic acid. This suggests that the bioactive compounds present in C. gigantea latex, such as flavonoids and polyphenols, play a crucial role in stabilizing Mg(OH)2 NPs and enhancing their radical scavenging potential. Furthermore, the antibacterial efficacy of Mg(OH)2 NPs was evaluated against wound-causing bacteria, including Klebsiella pneumoniae, Micrococcus spp., Enterococcus faecalis, Staphylococcus aureus and Pseudomonas aeruginosa. The NPs exhibited significant inhibitory effects, with the highest zone of inhibition observed against Micrococcus spp. at 100 μg/mL. The antibacterial mechanism is likely due to the alkaline nature of Mg(OH)2, which disrupts bacterial cell membranes, leading to increased permeability and cell death. In addition, the presence of hydroxyl ions (OH−) may contribute to oxidative stress in bacterial cells, further enhancing antimicrobial activity. These findings underscore the potential of C. gigantea latex-mediated Mg(OH)2 NPs as multifunctional agents for biomedical applications, particularly in wound-healing formulations and antimicrobial coatings.
4. Conclusion
Biogenic Mg(OH)2 NPs were synthesized by simple and ecofriendly manner. The aqueous-based latex extract was used to fabricate the Mg(OH)2 NPs. The synthesized biogenic Mg(OH)2 NPs were confirmed and characterized by different spectroscopic and microscopic analyses. The synthesised NPs were crystalline and nano petals shaped with an average size of 20.98 nm. Biosynthesized biogenic Mg(OH)2 NPs have good antioxidant properties. The antibacterial activity of biogenic Mg(OH)2 NPs was determined against wound-causing bacteria. The highest zone of inhibition was found against Micrococcus spp. The study concluded that as-synthesized biogenic Mg(OH)2 NPs have excellent biocompatibility and can be suggested to formulate the new cream or gel for upcoming therapeutic applications.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jayalekshmi C.: investigation and writing the original draft; Rajiv Periakaruppan: conceptualization, supervision and administration; A. Bharathi, Karungan Selvaraj Vijai Selvaraj and Noura Al-Dayan: review and data curation; Valentin Romanovski: formal analysis, data curation, validation, writing the original draft and reviewing and editing.
Funding
The authors have nothing to report.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




