Characteristic Improvement of Reduced Graphene Oxide From Battery Waste Synthesized Using Modified Hummer’s Method
Abstract
In this study, the reduced graphene oxide (rGO) using the modified Hummers method from battery waste materials has been successfully carried out. The results obtained were compared with the conventional Hummers method. XRD analysis showed that the characteristic peaks of graphene oxide (GO) were reduced to rGO using both the conventional and modified Hummers methods. The results of the UV-vis analysis also revealed that the absorption peak of the rGO sample showed a shift toward a longer wavelength, located between 200 and 270 nm, compared to GO. The FTIR results showed that the intensity of all oxygen-containing functional groups was reduced in rGO (the presence of several spectral peaks and functional groups after reduction), thus confirming the successful reduction of GO using both the modified and conventional Hummers methods. The Raman spectroscopy results showed that the defects in rGO using the modified Hummers method were smaller than those in rGO using the conventional Hummers method. This can be seen from the results of the D-band and G-band intensity ratios (ID/IG), which were 1.108 and 1.292 in rGO using the modified and conventional Hummers methods, respectively. SEM analysis shows morphological changes indicating changes in graphite to GO and then to rGO. The EDX results obtained are also reinforced by the increase in carbon elements (84.21% = conventional rGO and 82.80% = modified rGO) and a decrease in oxygen elements in all methods for rGO. These findings suggest that the modified Hummers method, which utilizes environmentally friendly chemicals instead of hazardous ones (convention Hummers method), is a viable approach for converting battery waste into rGO applications. Additionally, the oxygen content produced by the modified Hummers method is higher than that of the conventional Hummers method.
1. Introduction
The increasing demand for electronic devices has led to an exponential rise in the consumption of batteries, both rechargeable and disposable. Disposable batteries, including zinc–carbon, lithium, and alkaline varieties, are commonly used in household devices such as flashlights, radios, remote controls, and toys [1–3]. Among these, zinc–carbon batteries are particularly popular due to their affordability and wide availability. However, the extensive use of these batteries results in significant environmental concerns [4]. As these batteries reach the end of their lifespan, improper disposal can lead to the accumulation of hazardous waste that can negatively impact both the environment and human health. This is primarily because the components of the batteries, such as zinc and manganese oxide, pose environmental risks when not properly handled [4–6].
Zinc–carbon batteries consist of carbon rods, which contain graphite, and zinc electrodes. Graphite, a material known for its inert properties, is essential in preventing the electrodes from deteriorating during the electrochemical reactions within the battery [7–9]. However, as these batteries age, the graphite in the carbon rods can be recovered and repurposed. One promising method for recycling graphite from spent batteries is its transformation into graphene oxide (GO) through the oxidation of graphite [10–12]. GO is a derivative of graphene, a remarkable two-dimensional material known for its exceptional conductivity, chemical stability, and mechanical properties [13–16]. GO retains many of the advantageous characteristics of graphene but also includes oxygen-containing functional groups, which enhance its versatility in a wide range of applications [17–22]. However, the presence of these functional groups can also introduce structural imperfections, which may affect the material’s properties. To address this, GO can be reduced to reduced graphene oxide (rGO), a material with properties closer to pure graphene [23–25]. rGO has shown significant promise in diverse fields, including sensor technology [26], energy storage [27], and catalysis [28]. Its potential applications span from ammonia detection sensors to energy storage devices such as supercapacitors and lithium-ion batteries, demonstrating the versatility and value of repurposing graphite from waste batteries into functional materials for modern technologies [29–32]. The Hummers method is a commonly used in GO synthesis. However, this method involves the use of hazardous chemicals such as NaNO3 [33]. Recent modifications, such as the Marcano method, offer a safer and more efficient alternative [34, 35]. This improved process, which utilizes H3PO4, not only avoids the production of toxic gases but also yields a more highly oxidized and structurally uniform GO, making it a valuable material for various applications [36–42]. In addition, the research on the modified Hummers method also aligns with mainstream recycling practices by focusing on resource efficiency, waste reduction, and environmentally friendly processes. Like recycling, it minimizes harmful byproducts and energy consumption, supporting sustainable manufacturing. This approach also enhances the longevity and performance of materials such as rGO, contributing to a circular economy by reducing waste and promoting reuse, which is in line with sustainable production goals. The use of battery waste in this study is also a key factor in the research, coherent with mainstream recycling practices around sustainability.
This research explores the potential of transforming graphite from discarded zinc–carbon batteries into GO and its reduced form, rGO, as a sustainable approach to recycling and reusing valuable materials. By advancing methods to enhance the quality and safety of the process, this study aims to contribute to both environmental protection and the development of cutting-edge technologies.
2. Methods
2.1. Graphite Purification
The battery parts were carefully separated and cleaned to remove impurities and metal particles, such as MnO2, through sanding and repeated washing with water. Then, carbon rod of battery as graphite source was pulverized using a mortar and pestle, and its particle size was homogenized using a 200-mesh sieve. An acid treatment was performed using a 37% HCl and 65% HNO3 solution in a 3:1 ratio to purify the graphite from inorganic materials. The graphite was then subjected to centrifugation and washed multiple times with distilled water until a neutral pH was achieved. Finally, the graphite was dried at 60°C for 24 h.
2.2. Synthesis of GO via the Hummers Method (GO)
The synthesis process of GO commenced by dissolving 1.5 g of NaNO3 and 3 g of purified graphite, obtained from carbon rods of battery waste, into 69 mL of H2SO4, while stirring for 15 min. Subsequently, 9 g of KMnO4 was carefully introduced to the solution, maintaining a temperature below 20°C while stirring for 2 h. The solution was then transferred to an oil bath, where it was heated gently to a temperature range of 35°C–45°C and stirred for 5 h.
Afterward, 138 mL of distilled water was added slowly, followed by 15 min of stirring. An exothermic reaction occurred during this stage. The solution temperature was raised to 90°C–92°C and stirred for an additional 15 min. The mixture was then transferred to plain water and allowed to stand for 10 min. Then, 420 mL of distilled water was added and stirred for 30 min.
To stop the oxidation process, 3 mL of 30% H2O2 was introduced, and the solution was stirred for 30 min, with the formation of bubbles indicating the completion of oxidation. The solution was left at room temperature for 12 h. Following this, the solution was sonicated for 30 min.
The synthesis process continued with the washing of the solution via centrifugation. The solution was washed twice with 100 mL of distilled water and twice with 100 mL of 30% HCl to eliminate impurities formed during the synthesis and enhance purity. The solution was then washed multiple times with 98% absolute ethanol until a neutral pH was achieved. The resulting precipitate was collected and dried in an oven at 60°C for 12 h.
2.3. Synthesis of GO via the Modified Hummers Method (GOI)
The synthesis of GOI was conducted by modifying the Hummers method, refer to the study by Marcano et al. [34, 35]. A mixture of H2SO4 and H3PO4 in a 9:1 ratio (360 mL H2SO4 to 40 mL H3PO4) was prepared, and 3 g of graphite powder obtained from used batteries was added. Subsequently, 18 g of KMnO4 was slowly introduced, generating an exothermic reaction that remained below 35°C–40°C during the addition. The mixture was stirred at 50°C for 12 h, followed by dilution with distilled water. To halt the oxidation process, 400 mL of ice and 3 mL of 30% H2O2 were added. The solution was then washed sequentially with 200 mL of distilled water, 200 mL of 30% HCl, and 200 mL of ethanol, each wash repeated twice, before being centrifuged. Finally, the product was heated at 60°C for 24 h.
2.4. Reduction Synthesis of GO
GO was reduced using hydrazine hydrate as the reducing agent. The reduction process began by preparing a mixture of 1 g of GO and 100 mL of distilled water. Subsequently, 6 mL of hydrazine hydrate was added to the mixture, which was then stirred using a mechanical stirrer in an oil bath at 90°C for 12 h, after which precipitation occurred. The resulting black mixture was washed several times with distilled water and filtered until the pH reached neutral. Finally, the product was dried in an oven at 60°C for 24 h, yielding rGO powder (rGO = Hummers method and rGOI = modified Hummers method).
2.5. Characterization Techniques
X-ray diffraction (XRD, PanAnalytical X’pert Pro) using X-rays, the average wavelengths of Cu-Kα1 = 1.5406 Å and Cu-Kα2 = 1.5444 Å in the range of 5°–90° with a step size of 0.02°, was used to characterize the crystalline structure. The electrical structure was analyzed using a UV-vis spectrophotometer (Shimadzu UV-1601, lambda 200–400 nm, and double beam). Additionally, Raman spectroscopy (Horiba Scientific—InVia) with a 532-nm excitation laser was utilized to evaluate the structural quality of the materials. Fourier transform infrared spectroscopy (FTIR, Shimadzu IF Prestige 21) was used to identify functional groups in the wavenumber range of 350–4000 cm−1. Desktop scanning electron microscopy (SEM, FEI Inspect-S50) with a magnification of 20,000x was used to investigate the morphology. The grain-size distribution was determined by selecting the SEM picture for 100 randomly selected granules. Finally, energy dispersive X-ray (EDS, FEI Inspect-S50) was used to estimate the element concentration.
3. Results and Discussion
3.1. Analysis Results of XRD Spectra
Figure 1 displays the XRD patterns of graphite, GO, modified graphene oxide (GOI), rGO, and modified reduced graphene oxide (rGOI) derived from battery waste. The diffraction peaks of graphite align with those reported by Popova [43] (ICDD: PDF 00-056-0159), confirming its rhombohedral structure. The most intense peak corresponds to the (002) plane at 26.52°, with an interplanar spacing (d) of 3.36 Å (calculated using Bragg’s equation: d = λ/2sinθ [44]). Additional minor peaks at 54.59° (assigned to the (004) plane, d = 3.34 Å) further validate the characteristic graphite structure, confirming the successful purification of graphite from battery waste. A weak impurity peak observed at ∼21° suggests residual contaminants due to imperfect synthesis processes.
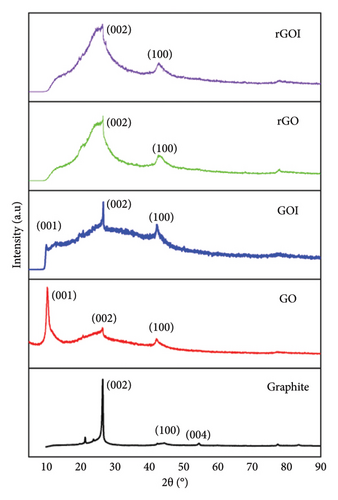
For GO samples (GO and GOI), a prominent diffraction peak at 10.38° (assigned to the (001) plane, d = 8.34 Å) confirms the intercalation of oxygen-containing functional groups and water molecules between graphite layers during oxidation via both conventional and modified Hummers’ methods [45, 46]. Notably, the (002) graphite peak at 26.52° (d = 3.36 Å) disappears entirely in dried GO samples, as reported in prior studies [47]. For GOI, the reduced intensity of the (001) peak suggests partial reduction (phase transition) during the modified Hummers’ process.
Upon reduction to rGO and rGOI, the dominant (001) peak at 10.38° shifts to 26.6° and 26.51°, corresponding to the (002) plane with reduced interlayer spacings of 3.34 and 3.36 Å, respectively. This shift, accompanied by peak broadening, reflects decreased crystallite size (D) and increased structural disorder due to oxygen group removal, indicative of partial restoration of graphene-like layers [48]. The broad, low-intensity (002) peaks in rGO/rGOI contrast sharply with the sharp graphite peaks, confirming successful reduction despite residual defects.
All five samples (graphite, GO, GOI, rGO, and rGOI) exhibit minor diffraction peaks at 2θ ≈ 42°–44.8°, assigned to the (100) plane. These peaks signify the microcrystalline nature of the system, reaffirming the structural reformation of natural graphite during purification and modification processes.
3.2. UV-Visible Spectrophotometer Analysis
Sample preparation for UV-vis analysis involved dissolving graphite in dimethyl sulfoxide (DMSO) at a concentration of 0.5 mg/mL, while GO and GOI and rGO and rGOI were dispersed in distilled water at concentrations of 0.5 and 0.2 mg/mL, respectively. As depicted in Figure 2, the UV-vis spectrum of graphite exhibits a distinct absorption peak at 233 nm, characteristic of its pristine sp2-hybridized carbon network. Following oxidation, the absorption profiles of GO and GOI reveal prominent peaks at 264 nm and 247 nm, respectively, attributed to the π⟶π∗ transitions of conjugated aromatic C-C bonds. These shifts signify the introduction of oxygenated functional groups and the partial conversion of sp2 to sp3 hybridization during oxidation, consistent with the formation of GO. Notably, additional absorption bands observed at 331 nm (GO) and 391 nm (GOI) correspond to n⟶π∗ transitions of C=O bonds, further corroborating the successful oxidation of graphite [34, 49, 50].
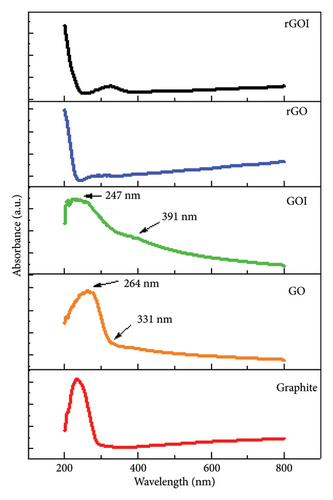
The enhanced water solubility of GO and GOI compared to pristine graphite underscores the efficacy of oxidative functionalization in modifying hydrophilicity, a critical factor for applications in aqueous systems. Subsequent chemical reduction of GO to rGO aimed to restore the sp2 carbon network by eliminating oxygen-containing moieties, thereby improving electrical conductivity and mechanical robustness. Postreduction, the UV-vis spectra of rGO and rGOI exhibit a redshifted absorption profile within the 200–270-nm range compared to GO. This redshift reflects the recovery of sp2 domains, reduction of defect states, and increased π-electron conjugation density—a hallmark of restored graphitic character. The observed spectral evolution aligns with the structural transition from oxidized, disordered layers (GO/GOI) to partially reduced, conductive frameworks (rGO/rGOI), highlighting the tunability of electronic properties through controlled reduction [51–53].
These findings underscore the critical interplay between oxidation/reduction processes and the optoelectronic behavior of graphene-based materials. The retention of residual absorption features in rGO/rGOI, however, suggests incomplete restoration of the sp2 network, likely due to persistent structural defects or residual oxygen groups. Such insights emphasize the need for optimization of reduction protocols to achieve desired performance metrics in advanced applications [54, 55].
3.3. FTIR Spectrum Analysis
The oxidation of graphite to GO involves a structural transition from sp2 to sp3 hybridization, driven by the covalent bonding of oxygen atoms to the carbon lattice, which expands the interlayer spacing due to the introduction of hydroxyl and epoxide groups on the basal plane [50, 55]. Conversely, reduction processes (e.g., chemical reduction) reverse this trend by partially restoring sp2 hybridization through the removal of oxygen functional groups, as evidenced by spectroscopic analyses [52]. The FTIR spectra (Figure 3) of graphite, GO, GOI, rGO, and rGOI corroborate these structural changes. A broad absorption band near 3400 cm−1 (observed at 3416.6, 3409.20, 3402.57, 3424.12, and 3387.54 cm−1 for graphite, GO, GOI, rGO, and rGOI, respectively) corresponds to O–H stretching vibrations from adsorbed water and hydroxyl groups, with heightened intensity in GO/GOI (3409.20 and 3402.57 cm−1) confirming extensive oxygen functionalization postoxidation [34].
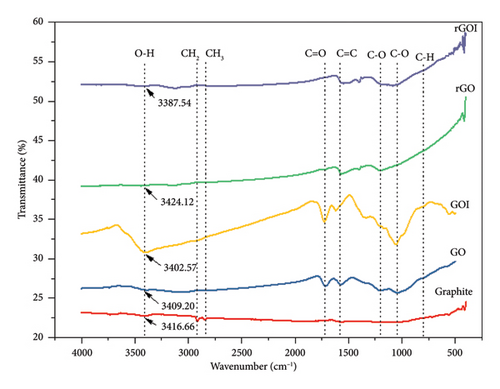
Key functional groups include symmetric/asymmetric CH2 stretching at 2851 and 2904.22 cm−1, C=O carbonyl stretching at 1721.80 cm−1 (GO) and 1729.27 cm−1 (GOI), and C=C aromatic stretching at 1580.84 cm−1 (GO) and 1617.33 cm−1 (GOI) [51]. Postreduction, these oxygen-related peaks diminish significantly in rGO/rGOI (e.g., C=C at 1565.92 and 1573.38 cm−1), alongside weakened signals for C–O epoxy (1248 cm−1) and alkoxy (1045.19 cm−1 for GO and 1067.57 cm−1 for GOI) groups, indicative of successful deoxygenation [53]. Residual C–H bending vibrations at 807.21 cm−1 (GO) and 799.75 cm−1 (GOI) further highlight incomplete structural restoration, consistent with residual defects in reduced materials [54]. These spectral trends align with prior studies demonstrating that reduction efficacy is reflected in the attenuation of oxygenated moieties and partial recovery of sp2 domains [49].
3.4. Raman Spectroscopy Results
Raman spectroscopy, performed using a 532-nm laser excitation wavelength (λlaser), provides critical insights into the structural evolution of graphene-based materials. The spectra are characterized by two primary bands: the D-band (disorder-induced mode, ∼1350 cm−1), reflecting defects and edge effects in the carbon lattice, and the G band (E2g phonon mode, ∼1580 cm−1), associated with in-plane sp2-bonded carbon domains [56]. Upon oxidation of graphite to GO, both the D and G bands exhibit broadening (Figure 4), attributable to the introduction of oxygen functional groups (e.g., epoxide and hydroxyl) that disrupt the planar sp2 network and induce sp3 hybridization. Concurrently, the G band undergoes a blue shift from 1580.68 cm−1 (graphite) to 1602.86 cm−1 (GO) and 1592.63 cm−1 (GOI), consistent with the amorphization of carbon structures and increased electron-phonon coupling due to oxidative strain [57].
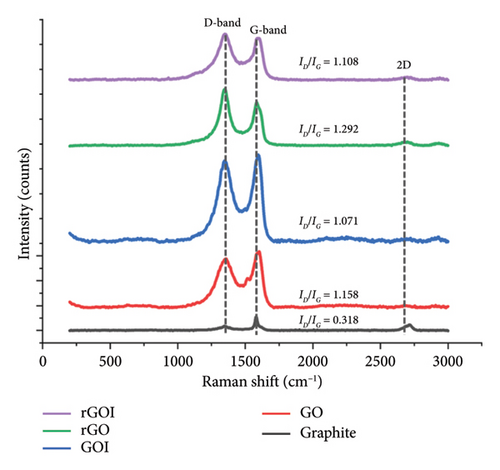
| Samples | D-band position (cm−1) | G-band position (cm−1) | 2D-band position (cm−1) | ID/IG ratio | Average defect density ηD (cm−2) (× 1011) |
|---|---|---|---|---|---|
| Graphite | 1342.62 | 1580.68 | 2720.43 | 0.318 | 0.54–0.93 |
| GO | 1363.79 | 1602.86 | 2693.67 | 1.158 | 1.95–3.40 |
| GOI | 1347.92 | 1592.63 | 2715.78 | 1.071 | 1.81–3.14 |
| rGO | 1351.45 | 1582.39 | 2687.76 | 1.292 | 2.18–3.79 |
| rGOI | 1347.92 | 1589.22 | 2700.03 | 1.108 | 1.87–3.25 |
3.5. SEM-EDX Analysis
Figure 5 illustrates the morphological evolution of (a) graphite, (b) GO, (c) GOI, (d) rGO, and (e) rGOI at 20,000x magnification. Pristine graphite exhibits a characteristic curved and folded two-dimensional layered structure (Figure 5(a)). Upon chemical oxidation, the morphological integrity of graphite is disrupted, leading to exfoliation into GO and GOI (Figures 5(b) and 5(c)). These oxidized derivatives display pronounced interlayer spacing, along with folds and wrinkles attributed to the introduction of oxygen-containing functional groups (e.g., epoxy, hydroxyl, and carboxyl) and structural defects during the oxidation process [64]. Successful exfoliation confirms the efficacy of the chemical treatment in disrupting van der Waals interactions between graphite layers.
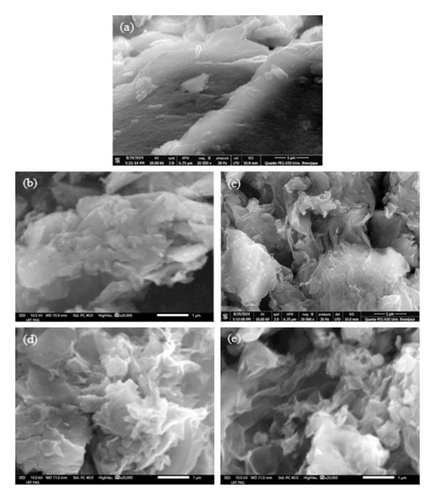
Postreduction, rGO and rGOI (Figures 5(d) and 5(e)) exhibit partial restoration of the graphitic structure, evidenced by reduced interlayer spacing and fewer surface wrinkles. This is attributed to the removal of oxygen moieties via chemical reduction, which mitigates structural defects and enhances π-conjugation [52]. The smoother morphology correlates with improved electrical conductivity, as oxygen functional groups in GO/GOI disrupt the sp2-hybridized carbon network, impeding charge transport [50]. These observations align with established mechanisms of oxidation-reduction-driven structural evolution in graphene-based materials [50, 52, 55].
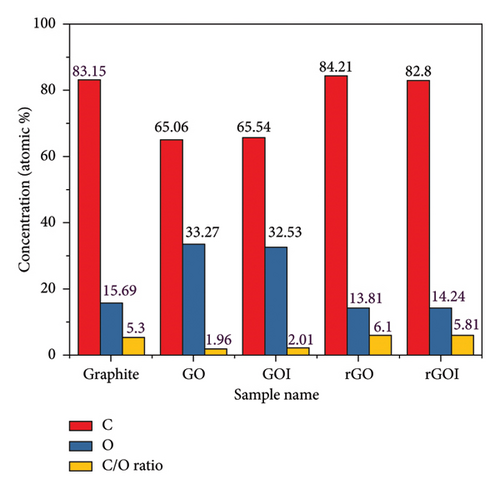
Table 2 summarizes the percentages of carbon, oxygen, aluminum, silicon, sulfur, and chlorine present in each sample (GO, GOI, rGO, and rGOI). The presence of aluminum (Al) and silicon (Si) is attributed to electrochemical reactions between the cathode and anode during battery operation. A small amount of chlorine (Cl) is derived from the HCl used in the graphite purification process. The EDX spectra of GO and GOI show a significant increase in oxygen content due to the insertion of oxygen-containing groups during the oxidation process. The synthesis of GO and GOI was confirmed by the increase in oxygen percentage from 15.69% in graphite to 33.27% in GO and 32.53% in GOI. The oxygen content in GOI is similar to that in GO, indicating that oxidation was successfully carried out using the modified Hummers method, which is analogous to the original Hummers method. During graphite oxidation, the carbon content decreases due to the incorporation of oxygen functional groups along the basal plane and edges of the graphene layers. Consequently, the C/O ratio of GO decreases from 5.3 (in graphite) to 1.96 (in GO), as shown in Figure 6. In rGO and rGOI, the oxygen content decreases to 13.81% and 14.24%, respectively, indicating the successful removal of oxygen groups and the restoration of the sp2 carbon network, a desirable feature of graphene [65]. This reduction of oxygen functional groups in GO and GOI using hydrazine hydrate as a reducing agent confirms the successful reduction process, which is critical for improving the material’s electrical conductivity. The residual oxygen content in rGO synthesized by the modified method plays a key role in its applications. Lower oxygen content improves electrical conductivity and mechanical strength, making rGO ideal for high-performance applications such as energy storage and flexible electronics. However, moderate oxygen content provides functional groups for further chemical modifications, useful in catalysis or drug delivery. Additionally, residual oxygen can enhance chemical stability, ensuring durability in various harsh environments. This balance makes rGO versatile across a range of technological applications.
| Samples | Weight (%) | C/O ratio | |||||
|---|---|---|---|---|---|---|---|
| Carbon | Oxygen | Silicon | Aluminum | Sulfur | Chlorine | ||
| Graphite | 83.15 | 15.69 | 0.42 | 0.30 | 0.22 | 0.22 | 5.30 |
| GO | 65.06 | 33.27 | 0.33 | 0.22 | 0.64 | 0.49 | 1.96 |
| GOI | 65.54 | 32.53 | 0.64 | 0.36 | 0.46 | 0.47 | 2.01 |
| rGO | 84.21 | 13.81 | 1.00 | 0.67 | 0.31 | — | 6.10 |
| rGOI | 82.80 | 14.24 | 1.59 | 0.96 | 0.26 | 0.15 | 5.81 |
Overall, it has been found that the modified Hummers method, which replaces hazardous reagents with environmentally friendly alternatives, is equally effective in producing rGO while being in line with sustainable practices. The slightly higher residual oxygen content in rGO with the modified method suggests its tunable properties for targeted applications. The contrast between the modified and conventional methods of rGO production has important implications for waste management and environmental sustainability. The modified method generally produces fewer hazardous by products and requires less energy, making it more environmentally friendly compared to the conventional method, which often generates more toxic waste and consumes more resources. By reducing the use of harmful chemicals and improving efficiency, the modified method supports sustainable manufacturing practices. Additionally, as rGO is used in applications such as energy storage and water filtration, the environmentally responsible production of rGO can contribute to addressing global sustainability challenges. Ultimately, adopting the modified method can help minimize waste, reduce environmental impact, and promote a more sustainable approach to manufacturing and resource use. Thus, these findings underscore the potential of utilizing battery waste for environmentally friendly rGO synthesis, which offers a scalable pathway for advanced materials’ development.
4. Conclusion
In this study, rGO was successfully synthesized from battery waste via a modified Hummers’ method and compared with rGO produced through the conventional Hummers’ approach. XRD analysis confirmed the reduction of GO to rGO, as evidenced by the disappearance of characteristic GO peaks in both methods. UV-vis spectroscopy revealed a redshift in the rGO absorption peak (200–270 nm), consistent with restored π-conjugation. FTIR spectra demonstrated a significant reduction in oxygen-containing functional groups (e.g., C=O, O-H) in rGO, validating successful deoxygenation. Raman spectroscopy further indicated lower structural defects in rGO synthesized via the modified method, as reflected by the smaller D-band to G-band intensity ratio (ID/IG = 1.108) compared to the conventional method (ID/IG = 1.292). SEM images corroborated morphological evolution from layered graphite to exfoliated GO and restacked rGO, while EDX analysis highlighted increased carbon content (84.21% conventional; 82.80% modified) and reduced oxygen levels in rGO. Notably, the modified Hummers’ method, which substitutes hazardous reagents with environmentally friendly alternatives, proved equally effective in producing rGO while aligning with sustainable practices. The slightly higher residual oxygen content in the modified rGO method suggests tunable properties for targeted applications. These findings underscore the potential of utilizing battery waste for eco-friendly rGO synthesis, offering a scalable pathway for advanced material development.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors extend their appreciation to Universitas Sebelas Maret for funding this research work through the Indonesian Collaborative Research Grant (RKI-21 PTNBH) with contract no. 285/UN27.22/PT.01.03/2024.
Acknowledgments
The authors extend their appreciation to Universitas Sebelas Maret for funding this research work through the Indonesian Collaborative Research Grant (RKI-21 PTNBH) with contract no. 285/UN27.22/PT.01.03/2024.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




