Efficacy of Dorzagliatin and Related Factors in a Real-World Cohort of Type 2 Diabetes
Abstract
Background: This study is aimed at evaluating the efficacy of dorzagliatin and identify factors associated with better glucose control in a cohort of patients with T2D in a real-world setting.
Methods: This is a single-center cohort study conducted in a real-world setting. Patients with T2D who had newly initiated dorzagliatin were enrolled in the study. The follow-up period ranged from at least 3 to up to 9 months, during which FBG, PBG, HbA1c, lipid profile, renal function, and MA/UCREA were measured and recorded every 3 months.
Results: The study cohort of patients with T2D had a mean age of 64.0 years, a diabetes duration of 17.9 years, an average of 4 antidiabetic drugs, and an HbA1c of 7.55%. FBG decreased by 0.60 mmol/L (p = 0.026), 2hPBG reduced by 2.15 mmol/L (p < 0.001), and HbA1c declined by 0.30% (p < 0.001) after treatment with dorzagliatin. Renal function significantly improved following dorzagliatin intervention (eGFR, estimated treatment difference 4.20 mL/kg/1.73m2, 74.5 vs. 78.7 mL/kg/1.73m2, p < 0.001). A regression model (B = 0.556, Exp = 1.744, p = 0.006) suggested that BMI, baseline HbA1c, and duration of diabetes were independently associated with the treatment effectiveness of dorzagliatin (BMI, B = −0.191, p = 0.009; baseline HbA1c, B = 1.060, p < 0.001; and duration of diabetes, B = −0.058, p = 0.034).
Conclusions: Dorzagliatin is effective in lowering glucose levels in patients with T2D in a real-world setting. Patients with lower BMI, higher baseline HbA1c, and shorter duration of disease may be robust responders to dorzagliatin treatment.
1. Introduction
The overall prevalence of diabetes in Chinese adults has gradually increased to 12.8%, and the number of patients with diabetes ranks first globally [1]. Type 2 diabetes (T2D) is characterized by persistent insulin resistance and progressively declining insulin secretion function [2]. A cross-sectional study revealed that in Chinese patients with T2D, beta cell function deteriorates progressively by 2% annually, with the decline being even faster in patients with poor glycemic control [3]. Despite the emergence of new therapeutics in the past decade, including glucagon-like peptide-1 receptor agonists (GLP-1RAs), sodium-glucose cotransporter 2 inhibitors (SGLT2i), and dipeptidyl peptidase 4 inhibitors (DPP4i), the glycemic control rate among patients with T2D in China was only 49.2%, failing to achieve the target glycated hemoglobin (HbA1c) level of <7.0% [4].
The physiological effect of glucokinase was first reported by Matschinsky in 1968, who identified the expression of glucokinase in hepatic cells, pancreatic alpha cells, and beta cells [5]. Glucokinase is recognized as a glucose sensor that regulates the set points in glucose homeostasis and subsequently plays a critical role in triggering early-phase insulin secretion in glucose-stimulated insulin secretion (GSIS), intrinsic regulation of glucagon secretion in alpha cells, and control of hepatic glucose uptake for glycogen synthesis in hepatic cells [6–8]. In addition to impaired beta cell function, evidence has shown that hepatic glucokinase activity is defective in patients with T2D [9].
Dorzagliatin is an orally bioavailable, dual-acting glucokinase activator (GKA) that binds to glucokinase and restores glucokinase function and glucose homeostasis. Multiple Phase 1 trials and one Phase 2 trial have been completed, demonstrating the glucose-lowering effect and safety of dorzagliatin in T2D [10–12]. Two Phase 3 trials demonstrated that dorzagliatin decreased HbA1c by 0.57% in drug-naïve patients with T2D and by 0.66% in patients treated with metformin [13, 14].
In recent years, dorzagliatin has been widely used in patients with T2D in China. Accumulating real-world evidence has indicated the robust effect of dorzagliatin on glucose control, but with heterogeneity. A small proportion of patients failed to show any reduction in fasting or postprandial blood glucose levels [15]. The efficacy of dorzagliatin in real-world practice and the characteristics of patients who experience treatment failure with dorzagliatin remain unknown. The objective of this study is aimed at evaluating the efficacy of dorzagliatin in a cohort of patients with T2D in a real-world setting and exploring the possible factors associated with successful glucose control using dorzagliatin.
2. Methods
2.1. Settings
The study design is a single-center, cohort study conducted in a real-world setting. From January 1st, 2024 to August 31st, 2024, patients diagnosed with T2D, at the age of 18–80 years, who newly initiated the treatment of dorzagliatin based on the judgement of the physicians at the research site, and were regularly followed up every 3 months in the Integrated Care Outpatient Clinic for Diabetes in Peking University First Hospital, were enrolled in the study. All participants met the diagnostic criteria for T2D [16]: (1) typical diabetes symptoms, (2) fasting blood glucose (FBG) ≥ 7.0 mmol/L, (3) random plasma glucose ≥ 11.1 mmol/L, (5) 2-hour postprandial blood glucose (2hPPG) ≥ 11.1 mmol/L in oral glucose tolerance test (OGTT), and (5) HbA1c ≥ 6.5%. Patients met the criteria (1) and any of (2)/(3)/(4)/(5) were confirmed to have the diagnosis of diabetes. The exclusion criteria for the study are as follows: (1) failure to attend on-site follow-up after treatment, (2) severe end-stage organ diseases, (3) pregnant or lactating women, and (4) Type 1 diabetes and other special types of diabetes. The enrolled patients were confirmed diagnosed with T2D who met the inclusion criteria at the diagnosis, and their blood glucose were partially decreased after treatment of antidiabetic drugs. All study participants provided informed written consent prior to study enrollment.
Baseline data, including sex, age, duration of diabetes, current treatment of glucose-lowering drugs, FBG, 2hPBG, HbA1c, lipid profile, renal function, albumin–creatinine ratio (MA/UCREA), body height, and body weight, were collected from the medical records prior to the initiation of dorzagliatin treatment. FBG and 2hPBG were measured and recorded using an online timely self-glucose monitor, with 2hPBG representing self-monitored blood glucose 2 h after one of the three meals. Other antidiabetic agents were adjusted based on the judgment of the physicians at the research site, in accordance with diabetes management guidelines. The follow-up period lasted at least 3 and up to 9 months, during which FBG, PBG, HbA1c, lipid profile, renal function, and MA/UCREA were remeasured and recorded every 3 months. Patients who failed to re-evaluate HbA1c, FBG or PBG, after at least 3 months of dorzagliatin treatment were excluded. Missing data for lipid profile and renal function were replaced with sequence average value. A decrease exceeding 0.5% in HbA1c after at least 3 months of dorzagliatin treatment was considered indicative of effective glycemic control, and potential covariates of effective glycemic control with dorzagliatin were further explored.
2.2. Statistical Analysis
Statistical analysis was conducted using SPSS 22.0 (IBM, United States). Continuous variables are expressed as mean (standard deviation, SD) or median (interquartile range, IQR) and analyzed using the paired t-test or Wilcoxon rank-sum test, depending on whether the data conform to a normal distribution. Categorical variables are presented as percentages and assessed using chi-square analysis. Binary logistic regression analysis was conducted to explore potential confounding factors associated with effective glycemic control. A p value of <0.05 was considered statistically significant.
3. Results
3.1. Baseline Clinical Characteristics
From January 1st, 2024 to August 31st, 2024, 145 eligible patients with T2D, who had recently initiated dorzagliatin, were enrolled in the study cohort. Among them, 55.2% were female, with a mean age of 64.0 years, a diabetes duration of 17.9 years, an average of 4 antidiabetic drugs, a body height of 1.66 cm, a body weight of 66.2 kg, a body mass index (BMI) of 24.0 kg/m2, a FBG level of 7.83 mmol/L, a 2hPBG level of 10.52 mmol/L, an HbA1c of 7.55%, a fasting C-peptide of 2.48 ng/mL, a high-density lipoprotein cholesterol (HDL-c) of 1.26 mmol/L, a low-density lipoprotein cholesterol (LDL-c) of 2.29 mmol/L, a median MA/UCREA of 10.81, and an estimated glomerular filtration rate (eGFR) of 74.4 mL/min/1.73m2 (Table 1). At the first visit, 54.5% had comorbid hypertension, and 71.0% had hyperlipidemia. At baseline, 71.7% of the patients were receiving metformin, 35.2% were treated with SGLT2i, 20.0% with GLP-1RAs, 41.4% with thiazolidinediones (TZDs), 29.0% with sulfonylureas or glinides, 29.7% with α-glucosidase inhibitors (AGIs), 44.8% with DPP4i, and 42.8% with insulin therapy (Table 1).
| Items | n | Value |
|---|---|---|
| Sex (male/female, female %) | 145 | 65/80, 55.2% |
| Age (year) | 145 | 64.0 ± 10.5 |
| Hypertension (n, %) | 145 | 79, 54.5% |
| Hyperlipidemia (n, %) | 145 | 103, 71.0% |
| Duration of diabetes (year) ∗ | 145 | 17.0 (9.0, 23.0) |
| Number of current glucose-lowering drugs (n) ∗ | 145 | 4 (2, 5) |
| Currently using metformin (n, %) | 145 | 104, 71.7% |
| Currently using SGLT2i (n, %) | 145 | 51, 35.2% |
| Currently using GLP-1RAs (n, %) | 145 | 29, 20.0% |
| Currently using TZDs (n, %) | 145 | 60, 41.4% |
| Currently using sulfonylureas or glinides (n, %) | 145 | 42, 29.0% |
| Currently using AGI (n, %) | 145 | 43, 29.7% |
| Currently using DPP4i (n, %) | 145 | 65, 44.8% |
| Currently using insulin (n, %) | 145 | 62, 42.8% |
| Body height (m) | 144 | 1.66 ± 0.08 |
| Body weight (kg) | 144 | 66.2 ± 10.9 |
| BMI (kg/m2) | 144 | 24.0 ± 3.3 |
| FBG (mmol/L) | 111 | 7.83 ± 1.89 |
| 2hPBG (mmol/L) | 111 | 10.52 ± 2.49 |
| HbA1c (%) | 144 | 7.55 ± 1.19 |
| HDL-c (mmol/L) | 145 | 1.26 ± 0.34 |
| LDL-c (mmol/L) | 145 | 2.29 ± 0.76 |
| MA/UCREA (mg/g) ∗ | 129 | 10.81 (5.98, 29.00) |
| eGFR (mL/kg/1.73m2) | 145 | 74.4 ± 18.2 |
- Note: N = 145, which indicated the total number of all participants, and n for subset.
- ∗Continuous variables inconsistent with normal distribution were represented as median (IQR).
- Abbreviations: 2hPBG, 2-hour postprandial blood glucose; AGI, α-glycosidase inhibitor; BMI, body mass index; DPP4i, dipeptidyl peptidase 4 inhibitor; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; MA/UCREA, albumin creatinine ratio.
3.2. Efficacy Outcomes
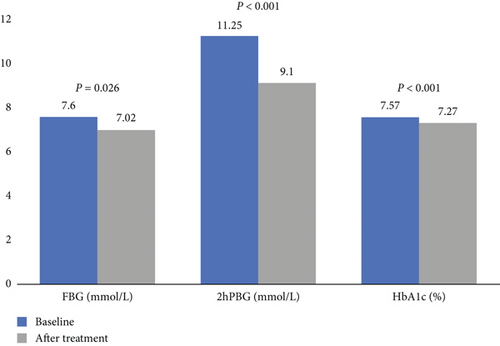
A significant glucose-lowering effect of dorzagliatin was demonstrated in the real-world study. After up to 9 months of dorzagliatin treatment, FBG decreased by 0.60 mmol/L (7.60 vs. 7.02 mmol/L, p = 0.026), 2hPBG decreased by 2.15 mmol/L (11.25 vs. Nine.10 mmol/L, p < 0.001), and HbA1c declined by 0.30% (7.57% vs. 7.27%, p < 0.001) (Figure 1). Regarding the lipid profile, a significant decrease of 0.04 mmol/L in HDL-c was observed after dorzagliatin treatment (1.26 vs. 1.21 mmol/L, p = 0.018), while no change in LDL-c levels was observed (2.27 vs. 2.23 mmol/L, p = 0.369). Renal function significantly improved after dorzagliatin intervention (eGFR, estimated treatment difference of 4.20 mL/min/1.73 m2, 74.5 vs. 78.7 mL/min/1.73 m2, p < 0.001), whereas MA/UCREA did not change significantly (92.9 vs. 84.6 mg/g, p = 0.704) (Table 2).
| Baseline | n/N | After dorzagliatin intervention | Variation (95% CI) | p values | |
|---|---|---|---|---|---|
| FBG (mmol/L) | 7.60 ± 1.50 | 38/145 | 7.02 ± 1.52 | 0.60 (0.07 to 1.09) | 0.026 |
| 2hPBG (mmol/L) | 11.25 ± 2.92 | 44/145 | 9.10 ± 1.83 | 2.15 (1.46 to 2.85) | <0.001 |
| HbA1c (%) | 7.57 ± 1.19 | 141/145 | 7.27 ± 1.07 | 0.30 (0.14 to 0.47) | <0.001 |
| HDL-c (mmol/L) | 1.26 ± 0.34 | 141/145 | 1.21 ± 0.32 | 0.04 (0.01 to 0.08) | 0.018 |
| LDL-c (mmol/L) | 2.27 ± 0.75 | 142/145 | 2.23 ± 0.71 | 0.05 (−0.06 to 0.15) | 0.369 |
| MA/UCREA (mg/g) | 92.9 ± 47.6 | 97/145 | 84.6 ± 28.1 | 8.26 (−34.71 to 51.23) | 0.704 |
| eGFR (mL/kg/1.73m2) | 74.5 ± 18.1 | 142/145 | 78.7 ± 18.8 | −4.20 (−6.01 to −2.38) | <0.001 |
- Note: N indicated the total number of all participants, and n for subset. p value < 0.05 was considered clinically significant and emphasized in bold.
- Abbreviations: 2hPBG, 2-hour postprandial blood glucose; CI, confidence interval; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; MA/UCREA, albumin creatinine ratio.
3.3. Related Factors of Treatment Effectiveness
Among the 145 eligible patients, 59 (40.7%) failed to achieve a reduction of more than 0.5% in HbA1c and were considered to have treatment failure. The remaining patients with an HbA1c reduction exceeding 0.5% were classified into the effective treatment group (n = 86, 59.3%). The baseline characteristics between responders to dorzgliatin and nonresponders were compared in Table 3, and baseline HbA1c was significantly increased in responders than nonresponders (7.85 ± 1.26 vs. 7.11 ± 0.91 mmol/L, p < 0.001). Other baseline characters including age, FBG, 2hPBG, BMI, renal function, and duration of diabetes were comparable between responders and nonresponders (Table 3).
| Items | Nonresponders (n = 59) |
Responders (n = 86) |
p value |
|---|---|---|---|
| Age (year) | 64.9 ± 10.6 | 63.4 ± 10.5 | 0.401 |
| Duration of diabetes (year) | 17.5 ± 8.7 | 16.0 ± 8.4 | 0.285 |
| Number of current glucose-lowering drugs (n) | 3.1 ± 1.3 | 3.2 ± 1.3 | 0.744 |
| BMI (kg/m2) | 28.1 ± 3.5 | 23.6 ± 3.1 | 0.120 |
| FBG (mmol/L) | 7.71 ± 1.57 | 7.89 ± 2.06 | 0.617 |
| 2hPBG (mmol/L) | 9.99 ± 2.14 | 10.81 ± 2.64 | 0.096 |
| HbA1c (%) | 7.11 ± 0.91 | 7.85 ± 1.26 | <0.001 |
| eGFR (mL/kg/1.73m2) | 73.1 ± 18.0 | 75.3 ± 18.4 | 0.465 |
- Note: p value < 0.05 was considered clinically significant and emphasized in bold.
- Abbreviations: 2hPBG, 2-hour postprandial blood glucose; BMI, body mass index; eGFR, estimated glomerular filtration rate.; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c.
Confounding factors, including age, sex, comorbidities such as hypertension and hyperlipidemia, duration of diabetes, current use of metformin, SGLT2i, GLP-1RAs, TZDs, sulfonylureas or glinides, AGI, DPP4i, insulin, BMI, baseline FBG, 2hPBG, HbA1c, and eGFR, were evaluated using binary logistic regression analysis to identify independent factors associated with treatment effectiveness. The regression model (B = 0.556, Exp = 1.744, p = 0.006) indicated that BMI, baseline HbA1c, and duration of diabetes were independently associated with the treatment effectiveness of dorzagliatin (BMI, B = −0.191, p = 0.009; baseline HbA1c, B = 1.060, p < 0.001; duration of diabetes, B = −0.058, p = 0.034) (Table 4). Therefore, a longer duration of diabetes, lower baseline HbA1c, and higher BMI were independently associated with a poor treatment response to dorzagliatin.
| Items | B | Exp(B) | 95% CI | p value |
|---|---|---|---|---|
| Sex | — | — | — | 0.470 |
| Age | — | — | — | 0.684 |
| Hypertension | — | — | — | 0.911 |
| Hyperlipidemia | — | — | — | 0.701 |
| Duration of diabetes | -0.058 | 0.943 | 0.894–0.996 | 0.034 |
| Currently using metformin | — | — | — | 0.759 |
| Currently using SGLT2i | — | — | — | 0.338 |
| Currently using GLP-1RAs | — | — | — | 0.749 |
| Currently using TZDs | — | — | — | 0.934 |
| Currently using sulfonylureas or glinides | — | — | — | 0.972 |
| Currently using AGI | — | — | — | 0.595 |
| Currently using DPP4i | — | — | — | 0.907 |
| Currently using insulin | — | — | — | 0.470 |
| BMI | -0.191 | 0.826 | 0.716–0.954 | 0.009 |
| FBG | — | — | — | 0.089 |
| 2hPBG | — | — | — | 0.964 |
| HbA1c | 1.060 | 2.885 | 1.581–5.266 | <0.001 |
| eGFR (mL/kg/1.73m2) | — | — | — | 0.946 |
- Note: p value < 0.05 was considered clinically significant and emphasized in bold.
- Abbreviations: 2hPBG, 2-hour postprandial blood glucose; AGI, α-glycosidase inhibitor; BMI, body mass index. CI, confidence interval; DPP4i, dipeptidyl peptidase 4 inhibitor; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; GLP-1RAs, glucagon-like peptide 1 receptor agonists; HbA1c, glycosylated hemoglobin A1c; SGLT2i, sodium glucose cotransporter 2 inhibitor; TZDs, thiazolidinediones.
Subgroup analysis was further performed in patients currently in use of different antidiabetic drugs including metformin, SGLT2i, GLP-1RAs, TZDs, sulfonylureas or glinides, AGI, DPP4i, and insulin, and its association with treatment effectiveness of dorzagliatin, and no significant difference was found using chi-square analysis between the groups (Table S1). In terms of the characteristics between patients with the age 65 ≥ years (n = 84) and <65 years (n = 61), eGFR in the elder group were higher compared with those aged less than 65 years (90.2 vs. 69.6 mL/min/1.73 m2, p < 0.001), and HbA1c after dorzagliatin treatment was significantly decreased in patients <65 years compared with elder patients (7.04 vs. 7.42%, p = 0.042) (Table S2). The HDL-c level was lower in the male subgroup compared with the female subgroup (1.12 vs. One.29 mmol/L, p < 0.001) (Table S3). In the subgroup analysis between patients with eGFR < 60 mL/kg/1.73 m2 (n = 33) and with eGFR ≥ 60 mL/kg/1.73m2 (n = 110), the glucose level and lipid profile after dorzagliatin treatment were of no significant difference (Table S4).
4. Discussion
This single-center, real-world study evaluated the efficacy of dorzagliatin, a dual-acting GKA, and the factors related to treatment effectiveness in a cohort of T2D patients. Patients with a mean duration of diabetes of 17 years and a baseline HbA1c of 7.55% exhibited a significant glucose-lowering effect after dorzagliatin treatment, with a reduction in HbA1c of 0.30% when dorzagliatin was added to an average of four other antidiabetic drugs. Due to its unique mechanism as a GKA, dorzagliatin demonstrated good tolerability and efficacy as an add-on therapy to various other antidiabetic drugs in the real-world setting.
Patients enrolled in the study was at the mean age of 64 years, with a mean duration of diabetes of 17 years. The previous Phase 2 trial enrolled patients aged 40–75 years [12], and the two Phase 3 trials involved patients aged 18–75 years [13, 14]. The 3 trials included 1333 patients, and a proportion of them were elders aged 65–75 years, in which the safety of dorzagliatin for the elders were proved. Our study provided real-world evidence for the safety and effectiveness of dorzagliatin in the elders, with relatively long disease duration.
Two Phase 3 trials suggested the effectiveness and safety of dorzagliatin in drug-naïve patients or as an add-on therapy to metformin [13, 14]. However, real-world evidence regarding its antidiabetic function when added to multiple other types of medications remains limited. One Phase 1 trial demonstrated the safety of the combination therapy of sitagliptin and dorzagliatin [17]. Another clinical trial assessed the combination therapy and drug interactions between dorzagliatin and empagliflozin (NCT03790787), which was completed with no solid results. Due to the unique mechanism of dorzagliatin as an antidiabetic drug, it is rational to be an add-on therapy with other kinds of glucose-lowering drugs, with accumulating real-world evidence. Safety concerns on GKA treatment involving recurrent hyperlipidemia and hyperuricemia in monotherapy or combination therapy with other antidiabetic drugs, which was reviewed in a recent meta-analysis [18]. Our study demonstrated that dorzagliatin maintained significant efficacy and safety when combined with one to seven other antidiabetic drugs in the real-world setting. Since the main mechanism of GKA focuses on the restoration of GSIS, dorzagliatin decreases PBG to a greater extent than FBG, as shown in our study and previous trials [13, 14].
Our study revealed the potential renal protective function of dorzagliatin, as evidenced by the improvement in eGFR, rather than in MA/UCREA. Since the glucokinase was not directly expressed in the renal system, the improvement in eGFR may be due to GKA in an indirect manner. A pharmacokinetics study demonstrated that the plasma exposure of dorzagliatin was similar between patients with end-stage renal disease (ESRD) and healthy volunteers, indicating the safety of dorzagliatin in patients with any level of renal function [19]. However, a cohort study in Chinese patients with diabetes revealed that mutations in GKRP genes such as rs1260326 T allele and rs1799884 T alleles, which caused glucokinase activation, was correlated with macroalbuminuria and hampered glomerular filtration [20]. The above study concluded in contrast to our study, and the clinical significance of the findings needs to be confirmed through real-world studies. Additionally, experiments using rodent models should be conducted to explore the underlying mechanism of the impact of dorzagliatin on renal system. Therefore, our study innovatively indicated an improvement in renal function after dorzagliatin treatment. A randomized controlled trial with a longer intervention period, focusing on renal endpoints, may further confirm the potential renal protective effects of dorzagliatin.
Continuous activation of glucokinase was considered to be linked with an elevated risk of hypertriglyceridemia and hepatic steatosis [21]. A previous meta-analysis suggested that triglyceride (TG) levels increased by 0.43 mmol/L after dorzagliatin treatment, rather than HDL-c or LDL-c [22]. Since an increase in TG is commonly accompanied by a decrease in HDL-c, our study revealed a similar deterioration in the lipid profile, characterized by the decrease in HDL-c. Evidence from the real-world or other clinical trials are needed to validate the impact of dorzagliatin on lipid profile, and clinicians should re-examine TG and HDL-c for patients after the add-on therapy of GKA.
Our study found that a shorter disease duration, higher baseline HbA1c, and lower BMI were associated with greater effectiveness of dorzagliatin treatment. Unfortunately, only a small proportion of patients had their fasting C-peptide measured before dorzagliatin initiation, and the direct assessment for β-cell function was in lack. In compensate, a longer duration of T2D is strongly associated with impaired β-cell function [23]. In Chinese adults, insulin resistance is more strongly associated with the incidence of diabetes than β-cell dysfunction, although this pattern is less prominent among lean adults [24]. Consequently, lean patients with T2D may exhibit relatively poor beta cell function and lower insulin resistance, potentially resulting in a better response to dorzagliatin treatment, which inversely affects GSIS. Previous study concerning the treatment effectiveness of other antidiabetic drugs revealed that sitagliptin provided better glycemic control in patients with better insulin sensitivity and higher baseline HbA1c [25]. A treatment effective study suggested that the combination therapy of metformin and sitagliptin exert profound reduction in HbA1c in patients with high baseline HbA1c, low beta-cell function, and short duration of diabetes, after adjusting for age, sex, BMI, blood pressure, TGs, creatinine, high-sensitivity CRP, glucagon, C-peptide, HOMA-B, and HOMA-IR [26], which indicated that the confounding factors included in the regression model is of clinical significance. The DAWN trial enrolled T2D patients with a mean disease duration of 6 years and a baseline HbA1c of 8.3%, showing a placebo-adjusted decrease in HbA1c of 0.66% with add-on therapy of 1500 mg of daily metformin [14]. Similarly, a reduction in HbA1c of 0.51% was observed in T2D patients with a mean disease duration of 1 year and a baseline HbA1c of 8.4% [13]. Our study cohort had an average disease duration of 17 years and a baseline HbA1c of 7.55%, which likely diminished the effectiveness of dorzagliatin. In clinical practice, patients with lower insulin resistance, higher baseline glucose levels, and without severe beta cell dysfunction may be more likely to respond effectively to dorzagliatin.
The study had several limitations. Due to the real-world study design, which lacked randomization or a control group, the main limitation arises from inevitable confounding factors, such as the difficulty in ruling out the impact of adjustments to other hypoglycemic medications on the efficacy endpoints during the intervention period. Future randomized clinical trials with a multicenter design, larger sample sizes, and targeted subgroup analyses focusing on BMI, baseline glucose levels, and beta cell function could further confirm the conclusions of our study. Secondly, genetic testing was not performed in the study cohort of T2D, and it is possible that several cases of glucokinase maturity-onset diabetes of the young (GCK-MODY), which have been shown to have a robust response to dorzagliatin treatment [27], may have been misdiagnosed as T2D in the cohort.
5. Conclusion
Dorzagliatin has been shown to be effective in lowering glucose levels in patients with T2D in real-world settings, and it also demonstrates potential renal protective effects. Patients with a lower BMI, higher baseline HbA1c, and a shorter duration of disease may exhibit a stronger response to dorzagliatin treatment.
Ethics Statement
The study was reviewed and approved by the Institutional Review Board of Peking University First Hospital.
Consent
Informed written consent was obtained from all study participants prior to enrollment.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The study is granted by the 2024 Thyroid Youth Research Fund.
Acknowledgments
We would like to express our appreciation to all patients who participated in the follow-up assessments.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




