Proton Pump Inhibitor Use and Its Association With Asthma: A Systematic Review and Meta-Analysis
Abstract
Background: Asthma is a prevalent chronic respiratory condition marked by airway inflammation and hyperresponsiveness, significantly impacting quality of life. Emerging evidence suggests a potential association between proton pump inhibitor (PPI) use and an increased risk of asthma. This systematic review and meta-analysis assessed the relationship between PPI use and the development or exacerbation of asthma.
Methods: A systematic search of PubMed, Web of Science, and Embase databases was conducted, covering studies published from the inception of the database to July 12, 2024. Observational studies examining the association between PPI use and asthma risk were included. Two reviewers independently extracted data using Nested Knowledge software, with study quality assessed via the Newcastle–Ottawa Scale. A random-effects meta-analysis was performed, pooling odds ratios (ORs) and hazard ratios (HRs) to assess the association, with heterogeneity evaluated via the I2 statistic.
Results: Fourteen studies, conducted between 2009 and 2024 and involving over 1.7 million participants, met the inclusion criteria. The pooled HR showed a 38% increased risk of asthma among PPI users compared to nonusers (HR, 1.38; 95% CI, 1.14–1.62). OR analysis indicated a 29% higher risk (OR, 1.29; 95% CI, 1.23–1.35). PPI users had an 81% higher risk compared to histamine H2 receptor antagonist (H2RA) users (HR, 1.81; 95% CI, 1.09–2.53), and asthma patients using PPIs were 61% more likely to experience exacerbations (OR, 1.61; 95% CI, 1.42–1.80).
Conclusion: PPI use is associated with an increased risk of asthma. These findings underscore the need for cautious prescribing and further investigation into underlying mechanisms.
1. Introduction
Asthma is a common noncommunicable disease (NCD) that affects individuals of all ages, particularly children, and is one of the leading chronic conditions worldwide. It is a chronic inflammatory disorder of the airways involving various immune cells, including mast cells, eosinophils, and T lymphocytes [1–3]. Asthma significantly burdens global health, with the World Health Organization (WHO) reporting that 3%–5% of adults and 7%–10% of children are affected [1, 4]. The WHO predicts that the number of people with asthma will rise from 100 to 150 million to over 200 million by 2025, with 180,000 new cases annually [4, 5]. Asthma is characterized by airway inflammation, heightened sensitivity, and obstructed airflow, which challenges healthcare systems and significantly impacts patients’ quality of life [6]. Understanding its risk factors is crucial for effective prevention and management, particularly the potential role of proton pump inhibitors (PPIs) [7].
PPIs are commonly prescribed for acid-related gastrointestinal disorders such as gastroesophageal reflux disease (GERD), peptic ulcer disease, and Zollinger–Ellison syndrome [8]. These medications work by irreversibly inhibiting the hydrogen–potassium ATPase enzyme system in gastric parietal cells, leading to a significant reduction in gastric acid production [9, 10]. While generally considered safe and effective for managing acid-related conditions, emerging evidence suggests a potential association between PPI use and the onset or exacerbation of asthma symptoms [9].
Several hypotheses have been proposed to explain this possible connection. One theory suggests that PPIs disrupt the gut microbiome by altering gastric pH, which impairs natural defenses against pathogenic bacteria and leads to dysbiosis [11–14]. This microbial imbalance may trigger systemic inflammation, including airway inflammation, by hyperactivating Type 2 helper T cells and increasing the production of inflammatory cytokines, such as interleukin-4 (IL-4) and IL-5, which are involved in asthma pathogenesis [14]. In addition, dysbiosis has been linked to an increased risk of enteric infections, such as Clostridium difficile, which may exacerbate asthma symptoms through disruption of the gut–lung axis [15]. Another hypothesis proposes that PPI-induced hypochlorhydria allows pathogenic bacteria to proliferate in the upper gastrointestinal tract, potentially migrating to the respiratory system and promoting inflammation [16]. Studies have also shown that PPI use is associated with small intestinal bacterial overgrowth, which could further contribute to respiratory health issues [17–19]. Furthermore, PPIs may directly affect lung tissue by impairing endothelial function, accelerating endothelial aging, and increasing vascular permeability—all of which could worsen asthma symptoms [20]. In addition, rebound acid hypersecretion upon PPI discontinuation may exacerbate asthma, particularly in susceptible individuals [21].
The association between PPI use and asthma remains uncertain, with observational studies yielding inconsistent findings [11–14, 22, 23]. This systematic review and meta-analysis synthesizes evidence to evaluate the risk of asthma associated with PPI use, including comparisons with nonusers and H2RA users. By addressing gaps in the literature, the study highlights potential respiratory risks, informs clinical practice, and identifies directions for future research on underlying mechanisms.
2. Methods
This systematic review and meta-analysis was carried out according to a preregistered protocol (CRD42024563844) in PROSPERO and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Table S1).
2.1. Eligibility Criteria
The review focused on observational studies that investigated the association between PPI use and the risk of developing asthma. Eligible studies were required to involve participants of any age and gender who had been exposed to PPIs for the treatment of gastrointestinal conditions, with clear documentation of PPI usage, including dosage and duration. Studies needed to report the incidence or prevalence of asthma as a primary outcome, defined by clinical diagnosis, patient history, and physical examination. Only studies published in English were considered. Exclusion criteria included case reports, case series, editorials, review articles without original data, studies involving only animal subjects, studies not specifying asthma diagnostic criteria, participants with preexisting asthma prior to PPI exposure, and studies lacking a clearly defined comparison group or incomplete data. This comprehensive inclusion and exclusion framework ensured the selection of high-quality studies that provided robust evidence on the relationship between PPI use and asthma risk.
2.2. Search Strategy
The search strategy encompassed three primary electronic databases PubMed, Web of Science, and Embase up to July 12, 2024. The search targeted articles published from the inception of each database until the search date, with a restriction to studies published in English. Keywords and Medical Subject Headings (MeSH) terms related to PPIs and asthma were utilized, including terms such as “proton pump inhibitors,” “PPIs,” “asthma,” “respiratory conditions,” and “acid-suppressive medications.” In addition, reference lists of pertinent articles were manually reviewed to identify further studies that met the inclusion criteria. This comprehensive search strategy was meticulously designed to ensure the capture of all relevant studies examining the potential link between PPI use and the risk of developing asthma (Table S2).
2.3. Screening and Data Extraction
Screening and data extraction were meticulously performed using Nested Knowledge software to ensure both accuracy and thoroughness. Two reviewers screened the titles and abstracts of all identified studies separately based on predefined inclusion and exclusion criteria. Studies that met the initial screening criteria proceeded to a full-text review for a more detailed assessment of eligibility. The data extraction process utilized the tagging function within Nested Knowledge, enabling the systematic organization of data by factors such as author, year of publication, country, study design, participant demographics (e.g., age, gender, and population), details of PPI exposure, and asthma-related outcomes. Data extraction was conducted separately by the two reviewers, with any disagreements resolved by consensus.
2.4. Quality Assessment
The quality of the included studies was evaluated using the Newcastle–Ottawa Scale (NOS) for observational studies [24]. The NOS assesses methodological rigor by considering aspects such as participant selection, comparability of study groups, and the accuracy of outcome assessment. Each study was independently reviewed by two reviewers, with any discrepancies resolved through discussion or consultation with a third reviewer.
2.5. Data Analysis
Meta-analysis was performed using R Version 4.4, specifically employing the metagen function from the (metafor) and (dmetar) packages, which are well suited for precalculated effect size data [25]. Odds ratios (ORs) and hazard ratios (HRs) were pooled using a random-effects model, with the results visualized through forest plots showing individual study estimates and the overall pooled estimate [26]. Subgroup analyses were conducted to investigate potential differences based on comparison groups, such as PPI-exposed versus unexposed individuals, PPI users versus histamine H2 receptor antagonist (H2RA) users, and PPI users with asthma versus without asthma. Considering the anticipated between-study heterogeneity, a random-effects model, complemented by Knapp–Hartung adjustments to minimize the risk of false-positive results. Heterogeneity was assessed using the I2 statistic [27, 28].
3. Results
3.1. Literature Search and Summary Characteristics of Studies
The literature search process, as outlined in Figure 1, began with the identification of 2344 records across three databases. After the removal of 904 duplicates, 1440 unique records were screened. Of these, 1357 records were excluded based on title and abstract review, resulting in 83 reports being retrieved for full-text screening. Following detailed evaluation, 69 full-text articles were excluded for reasons including irrelevant population (n = 36), unsuitable study design (n = 24), and irrelevant outcomes (i.e., outcomes not directly associating PPI use with asthma risk or exacerbation) (n = 9). Ultimately, 14 studies met the inclusion criteria.
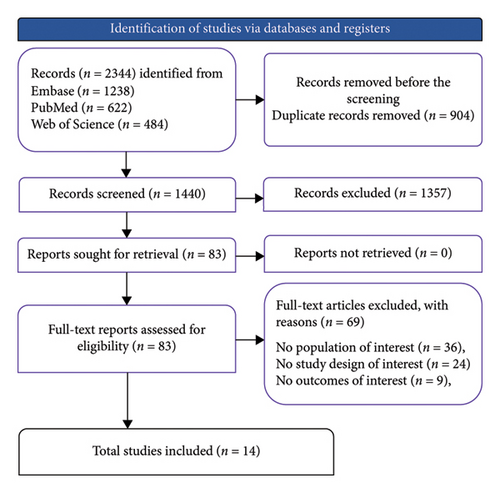
Table 1 provides a summary of the 14 studies reviewed. Sample sizes ranged from 2886 to 625,057 participants, collectively encompassing over 1.7 million individuals. These studies explored the association between PPI use and asthma risk across diverse populations from the United Kingdom [11, 12], South Korea [14, 31, 33], Germany [13], Taiwan [22, 34], the United States [23, 29, 30], the Netherlands [32], Spain [35], and Sweden [36]. Participants ranged in age from less than 1 year to over 60 years. The included studies employed a variety of research designs, including prospective and retrospective cohort studies, as well as case–control studies, reflecting a broad spectrum of methodologies. The quality of the studies, as assessed by the NOS, ranged from moderate to high (Table S3).
| Study | Study design | Country | Age | Male (%) | Sample size | Exposure group | Comparison group | Asthma definition | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Shin and Sachs [9] | Prospective cohort study | UK | < 1 year | NA | 10,116 | Children with prenatal exposure to PPI | Unexposed to PPI | NA | NA |
| Gandhi, Shamim, and Padhi [27] | Nested case–control study | South Korea | ≥ 40 | 45.8 | 129,618 | PPI users | Non-PPI users | ICD-10 | NA |
| Shin et al. [10] | Case–control study | United Kingdom | 3.6 years | 52 | 3748 | Children with drug-treated (PPI) asthma | Nonasthmatic (PPI users) | NA | 3 years |
| Cea Soriano et al. [11] | Prospective multicentre cohort study | Germany | NA | NA | 2886 | Adult outpatients with GERD (PPI users) | Non-PPI users | NA | 5 years |
| Hak et al. [12] | Prospective cohort study | South Korea | 42.6 | 51.3 | 500,082 | PPI users | H2RAs users | Modified from register-based validation study in Sweden | 6.7 years |
| Green and Turner [20] | Retrospective cohort study | Taiwan | 20–60 years | 51.54 | 21,183 | Patients with peptic ulcer and CAD using PPI | H2RAs users | ICD-9: 493 | 10 years |
| Mitre E 2018 [21] | Retrospective cohort study | United States | NA | 50.1 | 625,057 | TRICARE children with birth records in Military Health System database (exposed to PPI) | Unexposed to PPI | NA | NA |
| Freedberg, Lebwohl, and Abrams [29] | Prospective cohort study | Netherlands | 1.9 | 52.8 | 33,366 | Children born 1995–2011 (exposed to PPI) | Unexposed to PPI | Dutch General Practitioner Guidelines | 8 years |
| Langan et al. [28] | Retrospective cohort study | South Korea | 31.8 | NA | 168,526 | Infants with exposure to acid-suppressive drugs (exposed to PPI) | Unexposed to PPI | ICD-10 | 13 years |
| Wang et al. [30] | Observational cohort study | Spain | THIN 12.3, PHARMO-11 | 40.8 (THIN), 45.5 (PHARMO) | THIN = 16,141, PHARMO = 21,530 | Pediatric patients prescribed PPI | H2RAs users | NA | NA |
| Choi et al. [31] | Nested case–control study | Taiwan | NA | NA | 40,688 | PPI users with asthma | PPI users without asthma | NA | NA |
| Mulder et al. [32] | Propensity score–matched cohort study | Sweden | 12.9 | 37 | 161,740 | Children with PPI use | Unexposed to PPI | NA | 3 years |
| Noh et al. [33] | Retrospective cohort study | Taiwan | 54.95 | 45.4 | 48,154 | Adult PPI users | Non-PPI users | ICD-9 | NA |
| Wang et al. [34] | Case–control study | USA | 46.8 | 48.0 | 4481 | Adults 20+ with data on PPI use | Non-PPI users | CDC | NA |
- Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; CAD, coronary artery disease; CDC, Centers for Disease Control and Prevention; GERD, gastroesophageal reflux disease; H2RA, histamine H2 receptor antagonist; HR, hazard ratio; ICD, International Classification of Diseases; NA, not applicable; OR, odds ratio; PHARMO, the PHARMO Institute for Drug Outcomes Research; PPI, proton pump inhibitor; RR, relative risk; THIN, The Health Improvement Network.
3.2. Meta-Analysis
3.2.1. Unexposed vs Exposed to PPIs
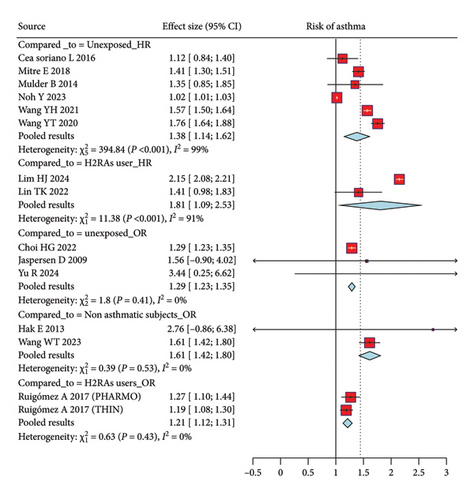
The meta-analysis evaluated the risk of asthma among individuals exposed to PPIs compared to those who were unexposed, with the effect sizes measured using the HR and OR (Figure 2). Six studies contributed to the pooled analysis using the HR, revealing a statistically significant association between PPI use and an increased risk of asthma. The pooled HR was 1.38 (95% CI, 1.14; 1.62), indicating a 38% higher risk of developing asthma among PPI users compared to unexposed individuals. However, this group exhibited substantial heterogeneity (I2 = 99%, p < 0.001). Similarly, the analysis using the OR from three studies also demonstrated a significant association, with a pooled OR of 1.29 (95% CI, 1.23; 1.35) and minimal heterogeneity (I2 = 0%, p = 0.41).
3.2.2. PPI Users vs H2RA Users
Figure 2 depicts the comparison between PPI users and those using H2RAs, and the meta-analysis included studies reporting both the HR and OR. The pooled HR from two studies was 1.81 (95% CI, 1.09; 2.53), suggesting that PPI users have an 81% higher risk of asthma compared to those using H2RAs. This comparison also showed moderate heterogeneity (I2 = 91%, p < 0.001), indicating some variability between the studies included in this analysis. The pooled OR from two additional studies was 1.21 (95% CI, 1.12; 1.31), further supporting the finding that PPI users are at a modestly higher risk of developing asthma compared to H2RA users. The heterogeneity in the OR analysis was minimal (I2 = 0%, p = 0.43), suggesting more consistent results across studies using this measure.
3.2.3. PPI Users With Asthma vs PPI Users Without Asthma
The analysis comparing PPI users with asthma to PPI users without asthma subjects utilized the OR to measure the association between PPI use and the risk of developing asthma (Figure 2). Two studies contributed to this comparison, yielding a pooled OR of 1.61 (95% CI, 1.42; 1.80). This result indicates that PPI users have a 61% higher likelihood of exacerbating asthma compared to nonasthmatic subjects. The heterogeneity among these studies was low (I2 = 0%, p = 0.53), suggesting that the findings were consistent across the included studies.
4. Discussion
This systematic review and meta-analysis provide evidence supporting a significant association between PPI use and an increased risk of developing asthma. The consistency of the findings across various comparison groups such as PPI users versus nonusers, PPI users versus H2RA users, and asthmatic versus nonasthmatic subjects suggests that PPI use may contribute to the development or exacerbation of asthma. These results have critical implications for clinical practice, particularly in the management of patients prescribed PPIs for gastrointestinal disorders.
Our meta-analysis revealed that individuals exposed to PPIs have a notably higher risk of developing asthma compared to those unexposed. The pooled HR indicated a 38% increased risk, while the pooled OR showed a 29% higher risk. Despite the high heterogeneity observed in the HR analysis (I2 = 99%), the consistent association across multiple studies supports the hypothesis that PPI use is linked to an increased asthma risk. This association remained significant even when comparing PPI users to H2RA users, with pooled HR and OR estimates indicating an 81% and 21% increased risk, respectively. Moreover, the comparison between PPI users with asthma and PPI users without asthma showed a 61% higher likelihood of exacerbating the asthma, with low heterogeneity observed in this analysis (I2 = 0%).
Recent studies have demonstrated a significant association between long-term PPI use and the development of asthma. For instance, one study reported that PPI users had a 1.58 times higher incidence of asthma compared with nonusers, with an adjusted HR of 1.76, particularly notable among patients without prior peptic ulcer disease (HR, 1.95) [37]. In pediatric populations, a cohort study found that new PPI users had a 57% increased risk of developing asthma compared with nonusers [36]. Despite these findings, the efficacy of PPIs in asthma management remains controversial. While some studies observed a modest improvement in morning peak expiratory flow rates among adults with asthma, others failed to demonstrate significant benefits in asthma control, even in patients with concurrent GERD [38]. These inconsistencies suggest that although PPIs may address GERD-related symptoms, their role in asthma management is unclear and warrants further investigation [39]. Recent studies utilizing larger cohorts and extended follow-up periods have strengthened evidence of the link between PPI use and asthma, surpassing earlier research limited by small sample sizes and shorter durations [14, 31].
Several plausible biological mechanisms may explain the observed association between PPI use and asthma risk [22]. One leading hypothesis is that PPIs alter the gut microbiome, leading to an overgrowth of pathogenic bacteria and a reduction in beneficial species, a phenomenon known as dysbiosis [14]. This microbial imbalance may promote airway inflammation and increase susceptibility to asthma through the hyperactivation of Type 2 helper T cells and the overproduction of inflammatory cytokines [40]. In addition, PPI-induced hypochlorhydria could allow pathogenic bacteria to colonize the upper gastrointestinal tract, which may subsequently translocate to the respiratory system and trigger inflammatory responses that contribute to asthma [21]. Furthermore, PPIs may directly damage the lung tissue by impairing endothelial function and accelerating endothelial senescence, potentially exacerbating asthma symptoms [20].
The rapid increase in asthma risk observed shortly after the initiation of PPI treatment suggests that these effects may be mediated by swift alterations in the gut microbiome [41]. Moreover, rebound acid hypersecretion upon discontinuation of PPIs may exacerbate asthma symptoms in susceptible individuals, adding further complexity to the relationship between PPI use and asthma [42]. These mechanisms are supported by studies that demonstrate a connection between altered gut flora and the onset of asthma, though the exact pathways remain to be fully elucidated [40, 43].
The clinical implications of these findings are considerable, especially given the widespread use of PPIs for the management of acid-related gastrointestinal disorders [44]. Although PPIs are generally considered safe and effective for conditions such as GERD and peptic ulcers, the potential respiratory risks associated with their use should not be overlooked [45, 46]. Our study highlights a significant association between PPI use and an increased risk of developing asthma, underscoring the need for clinical awareness. Clinicians should exercise caution when prescribing PPIs, particularly for patients with a history of asthma, other respiratory conditions, or factors that may predispose them to adverse pulmonary outcomes. In clinical practice, considering alternative therapies, such as H2RAs, may be prudent for patients at higher risk of asthma, particularly when long-term PPI use is indicated [45, 47]. These findings also emphasize the importance of regular monitoring of respiratory symptoms in patients using PPIs, especially those on prolonged therapy. Early detection of asthma-related symptoms, followed by prompt intervention, could help mitigate the respiratory risks associated with PPI use, potentially improving patient outcomes and quality of life [30]. Furthermore, patient education is essential and clinicians should inform patients of the possible respiratory side effects of PPIs and emphasize adherence to prescribed dosages and treatment durations to minimize the risk of asthma exacerbations or the development of new-onset asthma [48, 49]. A personalized treatment approaches, tailored to individual risk profiles, may optimize the selection of the most appropriate therapy for managing acid-related disorders while minimizing the potential impact on respiratory health [50].
Despite the strengths of this systematic review and meta-analysis, several limitations should be acknowledged. The substantial heterogeneity observed in some analyses, particularly in the HR analysis, suggests variability in study designs, populations, and methodologies, which may affect the generalizability of our findings. Although random-effects models and Knapp–Hartung adjustments were used to account for this heterogeneity, the underlying sources of variability remain unclear and warrant further investigation. Furthermore, the limited number of studies included in the analysis prevented a comprehensive assessment of publication bias, which could potentially affect the robustness of our conclusions. The reliance on observational studies introduces the possibility of confounding factors that may have influenced the observed associations. Although the included studies adjusted for various confounders, residual confounding cannot be entirely ruled out. Randomized controlled trials (RCTs) specifically designed to evaluate the respiratory effects of PPIs would provide more robust evidence of causality, but such trials are currently lacking. In addition, the inclusion of only English language studies may introduce language bias, limiting the comprehensiveness of the analysis and potentially omitting relevant studies published in other languages.
Future research is essential to clarify the biological mechanisms underlying the association between PPI use and asthma. Studies exploring the impact of PPI-induced changes in the gut microbiome on respiratory health would be particularly valuable in advancing our understanding of this relationship. Furthermore, RCTs focusing on the respiratory outcomes of PPI use are needed to establish causality and assess the clinical relevance of these findings. Future studies should also investigate potential subpopulations at greater risk of developing asthma in response to PPI use, including individuals with specific genetic or environmental risk factors. Such insights could help guide more personalized approaches to PPI prescribing and asthma management. As evidence linking PPI use to asthma risk grows, ongoing vigilance in the prescription and monitoring of these medications will be crucial to safeguarding patient health.
5. Conclusion
This systematic review and meta-analysis highlight a significant association between PPI use and an increased risk of asthma. While the underlying mechanisms remain to be fully elucidated, the potential respiratory risks associated with PPI use warrant careful consideration in clinical practice. Future research, including well-designed RCTs and mechanistic studies, is essential to clarify the nature of this association and guide clinical decision-making in the management of patients requiring acid-suppressive therapy.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization: H.A.S., M.N.K., S.B., and P.B. Data curation: B.S.T., A.A., M.R.K., and A.S. Formal analysis: A.M.G., S.S., G.B., and M.S. Investigation: H.A.S., M.N.K., and S.B. Methodology: P.B., B.S.T., A.A., and M.R.K. Project administration: P.R., A.M.G., S.S., and G.B. Resources: B.S.T., A.A., M.R.K., and A.S. Software: A.A., M.N.K., and S.B. Supervision: H.A.S., M.N.K., S.B., P.B., and M.S. Visualization: A.A., M.N.K., and S.B. Writing—original draft: M.R.K., A.S., P.R., A.M.G., S.S., and G.B. Writing—review and editing: M.N.K., S.B., P.B., B.S.T., A.A., M.R.K., A.S., P.R., A.M.G., S.S., M.S., and G.B. G.B. and M.N.K. contributed equally as first authors.
Funding
Qatar National Library funded the publication of this article.
Supporting Information
Table S1: PRISMA checklist.
Table S2: the adjusted search terms as per searched electronic databases.
Table S3: quality assessment of studies.
Open Research
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supporting Information files).




