Efficacy and Safety of Intranasal Dexmedetomidine Combined With Oral Chloral Hydrate for Sedation in Neonatal MRI Procedures: A Single-Center Retrospective Study
Abstract
Background: Pharmacological sedation during neonatal magnetic resonance imaging (MRI) is crucial for procedure success and minimizing artifacts. Hence, it is vital to evaluate the effectiveness and safety of conventional sedatives in this population. In this study, we aim to evaluate the effectiveness of oral chloral hydrate combined with intranasal dexmedetomidine in neonatal MRI.
Methods: Neonates aged 0 to 28 days undergoing MRI were enrolled and received intranasal dexmedetomidine followed by oral chloral hydrate. Subsequently, sedation scores, onset time, and overall time of sedation, as well as any potential adverse reactions, were recorded.
Results: All neonates completed the MRI without notable adverse reactions. 128 neonates (90.1%) completed the MRI study with a single dose, while 14 neonates (9.9%) required additional medications. In the neonates with a single dose, no statistically significant differences in onset time were observed across postnatal days, gender, and weight. And no statistically significant differences in total time were observed across postnatal days and gender. However, the total time was significantly extended in the neonates with a weight under 3 kg. Furthermore, compared to the neonates with a single dose, the total time was significantly extended in the neonates with additional medications.
Conclusion: Oral chloral hydrate combined with intranasal dexmedetomidine is effective and safe for neonatal MRI, but extra attention is needed for neonates under 3 kg.
1. Introduction
Magnetic resonance imaging (MRI) is increasingly used in infants and children, with a common focus on evaluating the brain, particularly in neonates presenting with encephalopathy [1, 2]. MRI provides valuable information while eliminating the risks of radiation exposure and posing minimal harm [3]. However, the time-consuming process and noisy environment present significant challenges in achieving optimal image quality and accurate diagnosis due to the limited ability of neonates to remain still [4].
Procedural sedation is widely employed to alleviate pain and anxiety during diagnostic and therapeutic procedures in individuals of all age groups. Among children, chloral hydrate is among the most commonly administered sedatives, known for its high success rates [5]. However, the unpleasant taste of chloral hydrate may cause nausea, vomiting, and inadequate sedation in patients who are uncooperative [6]. Research has demonstrated that intranasal dexmedetomidine serves as an adequate substitute for oral chloral hydrate, offering a high rate of test completion with a single dose and achieving the desired sedation level in a shorter time [6]. Safety and effectiveness are the primary objectives during procedural sedation. Neonates, a unique subset of the pediatric population, require special attention due to their susceptibility to procedural apnea and the importance of maintaining cardiovascular stability, reducing neurotoxic effects [7]. Therefore, special attention and meticulous consideration are necessary when neonates undergo advanced cross-sectional MRI imaging [8]. However, there is a limited amount of literature on procedural sedation in term and preterm infants undergoing MRI. In this study, our objective was to assess the effectiveness and safety of combining intranasal dexmedetomidine with oral chloral hydrate for sedation during neonatal MRI.
2. Method
2.1. Design and Patients
A retrospective observational study focused on the combined use of dexmedetomidine combined with chloral hydrate was conducted at the Affiliated Children’s Hospital of Jiangnan University (Wuxi Children’s Hospital). Neonates aged 0 to 28 days who performed MRI between January 2022 and December 2023 were enrolled in the present study. The physical condition of neonates was meticulously assessed prior to sedation. The study excluded the neonates presenting with allergy or hypersensitive reaction to dexmedetomidine or chloral hydrate, recent pneumonia, bronchitis, upper respiratory tract infections, bradycardia or conduction blocks, recent vomiting, apnea, and severe organ dysfunction. Neonates with congenital heart conditions but normal cardiac function were included. This study was approved by the ethics committee of the Affiliated Children’s Hospital of Jiangnan University (Wuxi Children’s Hospital).
2.2. Sedation Procedure
On the day of sedation, the neonates were instructed to refrain from breastfeeding for 2 h before sedation. Subsequently, neonates were evaluated by the anesthesiologist, covering demographic details, medical history, diagnosis, allergies, and the American Society of Anesthesiologists (ASA) physical status classification. Once informed consent was acquired from the parents or guardians, the nurse in the sedation room administered the medications and recorded the observational parameters. Neonates were first administered with intranasal dexmedetomidine injection solution (0.1 mg/mL, Batch No. H20213533, Sichuan Meida Kang Huakang Pharmaceutical Co. LTD, Deyang, China). The undiluted drug was drawn into a 1 mL syringe, and an equal volume was dripped on both sides of the nasal mucosa, followed by oral chloral hydrate solution (100 mg/mL, Batch No. H20210013, Tefeng Pharmaceutical Co. LTD, Nanjing, China). The neonates were positioned flat and kept in this position for 1-2 min to optimize drug absorption. After drug administration, sedation scores were recorded, the neonates were connected to a monitor following induction of sleep, and continuous monitoring was performed to ensure that the level of sedation did not exceed the intended depth. Simultaneously, the onset time, overall sedation time, and any potential adverse reactions were documented. Sedation levels were evaluated using the Ramsay score at ten-minute intervals. Once the Ramsay score was > 4, the neonates were swaddled, and the MRI examination was initiated. To minimize the noise from the MRI machine, ear plugs were provided to the neonates during the procedure. If the sedation onset time exceeds 30 min, Ramsay score is ≤ 4, or the neonate awakens during the examination, the pediatric anesthesiologist will assess the situation and determine the appropriate supplemental medication. Options include intranasal dexmedetomidine combined with oral chloral hydrate or sevoflurane inhalation (ensuring the neonate has abstained from breast milk for over 4 h or formula milk for over 6 h). Following the examination, the neonates will be transferred to the recovery room for monitoring of consciousness, SpO2, and HR. If SpO2 < 92% on room air, neonates received additional oxygen of 3 L/min via the nasal cannula. If HR < 100 beats per minute, an intramuscular injection of atropine (0.02 mg/kg) was administered to neonates. When the Steward score was ≥ 4 and after feeding, the neonates were allowed to leave the hospital.
2.3. Outcome Measurement
The primary outcome measured was the initial sedation success rate, along with the success rate following the administration of additional rescue sedative medication. The secondary outcomes encompassed the occurrence of adverse events, the initial and supplementary doses of chloral hydrate or dexmedetomidine, the time taken for sedation induction, and the overall duration of sedation. Specifically, we monitored for events such as bradycardia (heart rate below age-specific thresholds), oxygen desaturation (SpO2 < 90%), vomiting, prolonged recovery time, or agitation requiring intervention.
2.4. Statistical Analysis
Clinical characteristics and data were presented as mean ± standard deviation (SD). Statistical significance was established at p < 0.05. For comparison between two independent samples and among multiple groups, Student’s t-test was employed to determine differences. All statistical analyses were performed using GraphPad Prism Version 8.0.
3. Results
3.1. General Characteristics of Patients
In this study, we conducted a retrospective analysis of 142 neonatal cases in which sedation was administered during MRI procedures. Among these cases, dexmedetomidine and chloral hydrate were utilized. The median postnatal days of all the cases at time of dosing were 19 days (16–23 days). The ratio of boy to girl was 1.45, and the average weight of all the cases was 3.71 ± 0.47 kg. The detailed characteristics of patients are presented in Table 1.
| Study population | |
|---|---|
| Gender, n (%) | |
| Boy | 84 (59.3) |
| Girl | 58 (40.7) |
| Median days of birth, days [IQR] | 19 [16–23] |
| Postnatal day distribution, n (%) | |
| 0–10 | 6 (4.2) |
| 11–15 | 27 (19.0) |
| 16–20 | 49 (34.5) |
| 21–25 | 42 (29.6) |
| 26–30 | 18 (12.7) |
| Mean weight, kg ± SD | 3.71 ± 0.48 |
| Weight distribution, n (%) | |
| 2-3 | 14 (9.9) |
| 3.1–4 | 97 (68.3) |
| 4.1–5 | 31 (21.8) |
3.2. Efficacy and Safety of Combining Intranasal Dexmedetomidine With Oral Chloral Hydrate for Neonatal MRI
In this study, the dosing regimen involved administering dexmedetomidine at a dosage of 1.62 ± 0.36 μg/kg along with chloral hydrate at a dosage of 33.31 ± 7.22 mg/kg. All the neonates successfully completed the MRI studies, and no notable adverse reactions were detected after the study drugs were administered. Among these cases, 128 neonates (90.1%) completed the MRI study with a single administration, and 14 neonates (9.9%) received additional medications. In the neonates with single administration, the average onset time after administration was 20.11 ± 5.23 min, and no statistically significant differences in onset time were found based on postnatal age, gender, or weight. The average total sedation time in neonates with single administration was 80.85 ± 24.10 min. No statistically significant differences in total time were observed across postnatal days and gender. However, the total time was significantly extended in the neonates with a weight under 3 kg (103.62 ± 43.41 min) compared to other neonates (Table 2 and Figure 1(a)).
| Statistic | |
|---|---|
| Dosage regimen, n (%) | |
| Present plan (dexmedetomidine + chloral hydrate) | 128 (90.1%) |
| Present plan + sevoflurane | 7 (4.93%) |
| Present plan + dexmedetomidine (0.8 μg/kg) | 4 (2.82%) |
| Present plan + dexmedetomidine (0.4 μg/kg) + chloral hydrate (4.3 mg/kg) | 3 (2.11%) |
| Onset time (present plan), mean ± SD (min) | 20.11 ± 5.23 min |
| Total time (present plan), mean ± SD (min) | 80.85 ± 24.10 min |
| Total time (additional doses), mean ± SD (min) | 101.43 ± 23.39 min |
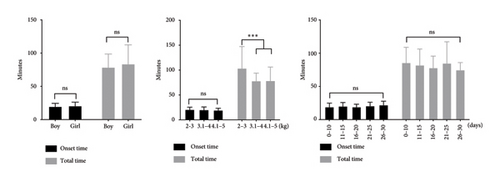
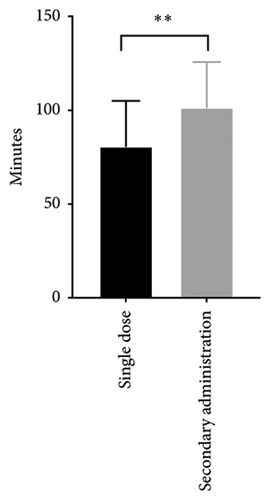
Among the 14 neonates who received additional medications, 7 of them received inhalation anesthetic with sevoflurane (1%; the neonate received sevoflurane via mask for 5 minutes and was transferred back to the MRI examination room once the Ramsay sedation score reached > 4 and vital signs had stabilized), 4 received one dose of dexmedetomidine 0.8 μg/kg, and 3 received one dose of dexmedetomidine 0.4 μg/kg combined chloral hydrate 4.3 mg/kg. Compared to the neonates with single administration, the total time was significantly extended in the neonates with additional medications (Table 2 and Figure 1(b)).
4. Discussion
MRI of the brain is a noninvasive imaging technique that provides detailed insights into brain morphology, structural connectivity, microstructural properties of gray and white matter, and functional organization [9]. However, MRI requires patients to remain still for extended periods, which is challenging for neonates due to the long duration and noisy environment. To minimize motion artifacts and ensure image quality, deep sedation is often necessary in neonatal MRI.
Sedation is typically administered through oral, buccal absorption, nasal drops, enemas, or inhalation routes. Recent studies have investigated the use of chloral hydrate [10], midazolam [11], and dexmedetomidine [12] for procedural sedation in children. Chloral hydrate, a well-established sedative-hypnotic agent, offers safe and effective sedation in clinical settings when administered with proper monitoring and intervention [13]. Compared to other sedative drugs, chloral hydrate has the advantages of convenient administration, relative safety, and minimal side effects [14]. However, chloral hydrate is associated with significant limitations, including vomiting as a common adverse effect and inconsistent sedative responses, which often result in treatment failure after the initial dose [10]. In addition, when used as a standalone sedative, higher doses of chloral hydrate are required, which lowers the sedation success rate and raises the risk of adverse events [15]. To improve efficacy, chloral hydrate is frequently used in combination with other sedative agents.
Dexmedetomidine, a highly selective alpha-2 adrenergic agonist, is commonly used for sedation in older children and is effective in reducing the total amount of opioids required for pain management [16]. Research has indicated that intranasal dexmedetomidine exhibits a more potent sedative effect compared to midazolam and ketamine in outpatient pediatric surgeries and MRI sedation [17, 18], and it was also found to be more effective than melatonin in inducing sleep in electroencephalogram [19]. The intranasal route is a minimally invasive method that obviates the need for intravenous access, making it relatively easy and convenient. Additionally, it mitigates first-pass metabolism and has proven successful in administering dexmedetomidine [7, 20]. In neonates and infants, intranasal dexmedetomidine has been shown to simulate natural sleep while preserving spontaneous breathing and upper airway tone [21, 22]. A review encompassing six studies involving 252 neonates revealed that dexmedetomidine reduces the requirement for adjunctive sedation or analgesia, shortens extubation time, decreases mechanical ventilation duration, and minimizes adverse effects [23]. Moreover, intranasal dexmedetomidine has shown higher sedation success rates in children undergoing electroencephalogram recording, particularly in patients with severe behavioral issues [24]. Collectively, intranasal dexmedetomidine seems to be a potential alternative for procedural pain management and sedation in neonates [25]. It can rapidly deliver dexmedetomidine for short-term sedation in neonates without the need for intravenous access.
The use of intranasal dexmedetomidine in both term and preterm infants has demonstrated safety and good tolerability. Vital signs before and after dosing did not show significant differences [26]. However, dexmedetomidine use alone may not be the ideal design. Traditional pediatric anesthetic dosing using pharmacokinetic estimates based on age and weight is often imprecise, frequently leading to oversedation. Research indicated that high-dose intranasal dexmedetomidine was more likely to induce bradycardia and had a shorter recovery time than intranasal low-dose dexmedetomidine and oral chloral hydrate sedation [27]. Besides, research demonstrated that intranasal dexmedetomidine combined with midazolam resulted in higher sedation success than sole dexmedetomidine [12]. Given the uncertainty regarding the efficacy and safety of chloral hydrate and dexmedetomidine in neonates, further research is crucial to establish optimal dosing strategies and to better understand the potential risks and benefits in this vulnerable population. In this study, we retrospectively summarize the safety and efficacy of oral chloral hydrate and intranasal dexmedetomidine for neonatal MRI examinations. We evaluated the sedative efficacy of dexmedetomidine from the perspectives of gender, birthdays, and weight. The results showed that intranasal dexmedetomidine combined with oral chloral hydrate demonstrated effective sedation with minimal side effects and high safety during neonatal MRI examinations. What is noteworthy is that neonates weighing less than 3 kg should be given adequate attention due to the longer recovery time. In summary, we conclude that neonatal sedation with intranasal dexmedetomidine and oral chloral hydrate for MRI is safe and successful.
There are several limitations to our study. First, the dose–response relationship of the chloral hydrate–dexmedetomidine combination as a premedication treatment is not the main focus of this study. Hence, larger studies are needed to evaluate the optimal dose of combined sedatives. Second, gestational age and postnatal age are both key factors determining the development of neonatal organs, influencing both sedation efficacy and safety, but we did not evaluate the clinical significance of gestational age in this study. Finally, the study was performed in a single clinical center with a small sample size, which not only limits the ability to detect potentially significant associations but also restricts the generalizability of our findings to other clinical settings.
5. Conclusions
For neonatal MRI examinations, oral chloral hydrate and intranasal dexmedetomidine form a readily acceptable protocol, characterized by high efficacy and safety, but neonates weighing less than 3 kg should be given adequate attention.
Ethics Statement
This study was approved by the Institutional Ethics Committee of the Affiliated Children’s Hospital of Jiangnan University (Wuxi Children’s Hospital) (WXCH2024-02-060).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission. Wenyan Dong and Shuoxiong Wu designed the study; Wenyan Dong and Zhenkun Yang prepared the manuscript; Lingdi Zhu and Linlin Xu performed the experiments and acquired the data; Zhenkun Yang analyzed the data.
Funding
This work was supported by funding from Bethune Charitable Foundation: EZMR2022-029.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




