Exploring the Therapeutic Effect of Quercetin in Asthma and Pulmonary Fibrosis Overlap Syndrome Post-COVID-19
Abstract
Backgrounds: Quercetin has potentially beneficial therapeutic effects for several chronic, inflammatory disorders of the airways. Thus, we explore the therapeutic value and mechanism of quercetin in the treatment of asthma and pulmonary fibrosis overlap syndrome after COVID-19.
Methods: The potential targets and molecular mechanisms of quercetin for the treatment of overlap syndrome were predicted using a network pharmacology method. The binding mechanism between the core potential compounds and their targets was predicted using molecular docking with AutoDock Vina. An asthma model induced by ovalbumin was built to evaluate the effect of quercetin on asthma.
Results: 55 common targets and eight key genes between the overlap syndrome and quercetin were obtained for further study. Most of the interested target genes were mainly focused on inflammation-related signaling pathways. Via molecular docking, quercetin showed a high degree of affinity for TNF, IL-6, and MMP9. In addition, quercetin improved asthma symptoms, inflammatory response, and pulmonary fibrosis.
Conclusion: This study, which examined the use of quercetin for the treatment of the overlap syndrome of asthma and pulmonary fibrosis following COVID-19 from the aspect of network pharmacology, might serve as a useful guidepost for future studies and clinical applications.
1. Introduction
Asthma is a chronic inflammatory disease, though its precise underlying mechanisms remain incompletely understood. It is hallmarked by airway hyperresponsiveness as well as reversible airflow limitation and recurrent wheezing, dyspnea, chest tightness, and coughing. Airway remodeling, which ultimately results in permanent structural alterations, is mediated by mucous gland hyperplasia, subbasement membrane thickening, higher mass of smooth muscle in the airways, angiogenesis, and subepithelial fibrosis. Previous studies have shown that glucocorticoids and certain anti-inflammatory medications help lower asthmatic patients’ inflammatory responses and morbidity in mild to moderate cases [1, 2]. Pulmonary fibrosis (PF) is a pathological condition characterized by chronic inflammation and excessive deposition of extracellular matrix (ECM), leading to alveolar destruction, lung tissue stiffening, and impaired elasticity. It results in progressive lung dysfunction, manifesting as dyspnea and dry cough, and may progress to respiratory failure in severe cases [3]. PF is distinct from asthma in its pathological mechanisms, as it primarily affects the lung parenchyma rather than the airways. It is associated with complex fibrotic changes, while it shares some similarities with asthma, including genetic predisposition and environmental exposures, as well as key factors, such as chronic, persistent inflammatory, and structural alterations including fibrosis and tissue remodeling. Given these shared factors, the effects of COVID-19 on preexisting conditions such as asthma and PF may involve overlapping pathological mechanisms. This is particularly relevant in the context of post-COVID-19 pulmonary complications, where both asthma and PF patients exhibit exacerbated symptoms due to the inflammatory and fibrotic changes that are characteristic of both diseases. COVID-19, a β-type coronavirus [4], can affect the pulmonary system in various ways, with disease severity influenced by factors such as the immune system, age, and coexisting conditions. In individuals with preexisting respiratory diseases such as asthma or PF, COVID-19 infection can lead to a significant worsening of symptoms, including increased airway inflammation, fibrosis, and compromised lung function [5–7]. As a result, it is essential to examine both common and specific pathological processes to detect similarities and differences between asthma and PF post-COVID-19 and to search for suitable complementary and alternative medical interventions and methods. Post-COVID-19, respiratory physicians are looking through the library of traditional Chinese medicine (TCM) for any prospective innovative medications that might treat the overlap syndrome, including asthma and PF.
Quercetin is the major representative of the flavonoid subclass of flavonols. It is found in many fruits and vegetables. Animal and human studies have reported different pharmacological effects of quercetin as attenuation of cardiovascular protection [8], antioxidation [9], anti-inflammation [10, 11], neuroprotection [12], blood pressure [13, 14], and anticancer [15]. The literature reveals that quercetin exhibits anti-COVID-19 activity [16] and also ameliorates PF [17]. We hypothesized that quercetin is a potential choice for treating the overlap syndrome asthma and PF post-COVID-19. Herein, we examined the overlap between asthma and PF syndrome post-COVID-19 and the possible therapeutic mechanisms of quercetin by examining the causal relationship and molecular links between the two diseases. Asthma models induced by ovalbumin were built to evaluate the effect of quercetin on asthma. Then, using network pharmacology, we explored the contemporary scientific connotation of TCM theory’s “identical therapy for various diseases” concept.
2. Materials and Methods
2.1. Quercetin Targets
The TCM Systems Pharmacology Database and Analysis Platform (TCMSP) (https://tcmsp-e.com/), a public repository of Chinese herbal medicines that shows associations between medications, targets, and disorders, was searched for the related targets of quercetin. The retrieved targets were aligned with the UniProt database (https://www.uniprot.org/) for standardization. Finally, valid gene symbols were obtained after removing mismatches and duplicates.
2.2. Asthma, PF, and COVID-19–Related Target Gene Prediction
The following five datasets were searched for targets related to the three diseases: the Therapeutic Target Database (http://db.idrblab.net/ttd/) (TTD), Pharmacogenomics Knowledgebase (PharmGKB) (https://www.pharmgkb.org/), DrugBank Online (DrugBank) (https://go.drugbank.com/), Online Mendelian Inheritance in Man (OMIM) (https://omim.org/), and Human Gene Database (GeneCards) (https://www.genecards.org/) (Supporting Table 1). Disease-related gene sets were established by intersecting all disease-associated search results, and their intersection was then visualized in R V4.2.3.
2.3. Network Development
2.3.1. Construction of Protein–Protein Interaction (PPI) Network
The related target of quercetin and the disease-related target were taken as an intersection (Supporting Table 2). The relationship between medications and disease was further clarified by identifying quercetin-related targets that were also associated with the disease. To construct the PPI network, these intersecting relationships were imported into the STRING database (https://string-db.org/). Parameters were set to “Homo sapiens” in the organism and the minimum required interaction score cutoff was set at 0.400. The nodes in the network that were disconnected were hidden. After that, we constructed the PPI network and used Cytoscape V3.7.0 to visualize it.
2.3.2. Identification of Core Genes
Core genes were screened for their topological features in the PPI network utilizing the CytoNCA plugin in Cytoscape. The gene scores were calculated using a formula based on the values of the three factors: degree centralities (DC), closeness centrality (CC), and betweenness centrality (BC). Genes that had scores greater than the median were obtained. The filter method was performed twice to identify crucial core genes.
2.4. GO and KEGG Enrichment Analysis
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were conducted to examine the molecular biological functions of drug–disease common targets. Three primary categories were included in the GO enrichment analysis: molecular function (MF), cellular component (CC), and biological process (BP). Complex biological pathways were revealed by means of KEGG enrichment analysis. Both GO and KEGG analyses were performed in R software V4.2.3 and the corresponding data packages (org.Hs.e.g. db, pathview, enrichplot, BiocManager, ggplot2, clusterProfiler, DOSE, stringi, and colorspace), with q value < 0.05 serving as the screening criterion. Eventually, we used Cytoscape V3.7.0 to develop a signaling pathway–active ingredient–target network.
2.5. Molecular Docking
Quercetin-related molecular targets for the treatment of asthma, PF, and COVID-19 were predicted with the aid of molecular docking simulation technology. Small molecule ligands’ 2D structures were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Conversion of 2D structures into 3D formats with minimum energy was performed by ChemBio 3D software. The forms of protein receptors with the screen criteria of “Homo sapiens,” “X-ray diffraction,” and “Protein” were downloaded from the RCSB Protein Data Bank database (PDB) (https://www1.rcsb.org/). The ligands and the receptors were prepared in PyMOL V2.4.0 and AutoDock V1.5.6 by removing water molecules, which added hydrogen atoms and charges. When conducting molecular docking, we turned to AutoDock Vina. The 2D interacting structure diagrams were thereafter produced utilizing a web-based tool (Protein–Ligand Interaction Profiler). PyMOL was then used to provide a visual representation of the output.
2.6. Animal Experiments
2.6.1. Preparation of the Asthma Model
Female BALB/c mice (weight: 18–20 g, age: 8 weeks, Shanghai BK/KY Biotechnology Co., Ltd) were randomly divided into the normal control (NC) group (n = 5), asthma (asthma) group (n = 5), asthma mice treated with dexamethasone (DXM) (2.0 mg/kg) [18] group (n = 5), and asthma mice treated with quercetin (quercetin) (50 mg/kg) [19, 20] (prepare the mixture of quercetin and β-cyclodextrin in a 1:2 ratio, and then dissolve it in physiological saline) group (n = 5) after adaptive feeding for 1 week. The mice in asthma, DXM, and quercetin groups were intraperitoneally injected with 200 μL sensitizer (sensitizer: dissolve 0.018 g ovalbumin in 6 mL PBS to make a 3 mg/mL solution, dilute 70 μL with 2.03 mL PBS to 0.1 mg/mL, and then mix with 2.1 mL aluminum hydroxide to 0.05 mg/mL) on 0, 7, 14, and 21 days. From Day 28 to Day 32, each mouse was given a nasal drip of activator (50 μL/day) (activator: dissolve 0.204 mg ovalbumin in 4.08 mL saline to prepare a 5% ovalbumin solution), and the treatment group was given the corresponding medication by gavage. The mice were then euthanized 24 h after the completion of the last stimulation on Day 33.
2.6.2. Behavioral Characteristics
Behavioral assessment records body mass and asthma incubation period and observes and records the behavioral status of each group of mice. The severity of asthma is evaluated by observing the mouse behavior. Comprehensive scoring criteria for the mouse asthma model are shown in Supporting Table 3.
2.6.3. Serum
The mouse’s eyeballs were excised, blood was collected, transferred into an Eppendorf tube, and allowed to stand at room temperature for 2 h. The tube was then centrifuged at 3000 rpm for 15 min to separate the serum, which was then stored at −20°C. The levels of IgE and hydroxyproline in serum were measured according to the instructions of the ELISA kit.
2.6.4. Lung Tissue
After blood collection, the mouse was euthanized by cervical dislocation. The lung tissue was taken and washed clean with physiological saline. The left lung was placed in a 5 mL Eppendorf tube (containing 4% paraformaldehyde solution) for morphological analysis of lung tissue. After rapid freezing of the right lung in liquid nitrogen for 30 min, it was transferred to a −80°C freezer for lipid metabolomics analysis.
2.6.5. Morphological Analysis of the Lung Tissue
The lung tissue was fixed with 4% paraformaldehyde for 48 h, during which it was gently shaken, dehydrated, embedded, sliced, and stained with hematoxylin and eosin (HE), Masson, and PAS. HE staining revealed airway inflammation, including cellular infiltration and structural changes. Masson’s staining detected collagen deposition, allowing for the evaluation of airway remodeling and fibrosis. PAS staining identified mucus secretion by Goblet cells, reflecting excessive mucus production in the airway epithelium. The above results were observed under an optical microscope. Moreover, the results of the staining were quantified as follows: Inflammation score: A score (0–4) was assigned based on inflammatory cell infiltration, airway narrowing, and epithelial damage, with the final score being the average of all airways. PAS + area percentage: The proportion of PAS–positive areas in the total airway was calculated by manual counting or image analysis software. Masson-positive area percentage: Collagen deposition was quantified by measuring the percentage of Masson-positive areas in the total airway area.
2.7. Statistical Analysis
All statistical analyses were conducted using GraphPad Prism 8 (GraphPad Software Inc.) and R Version 4.1.3. Comparisons between the two groups were performed using Student’s t-test, while comparisons among multiple groups were analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for multiple comparisons. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Common Targets Between Quercetin and Diseases (Asthma, PF, and COVID-19)
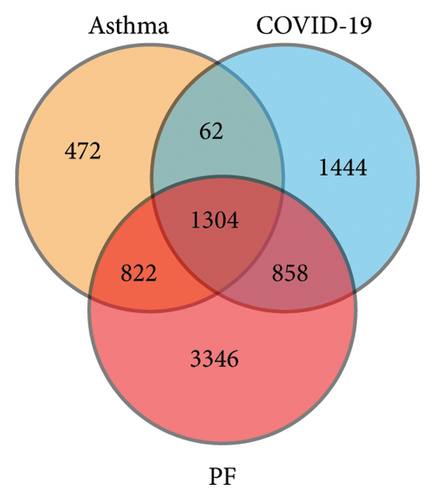
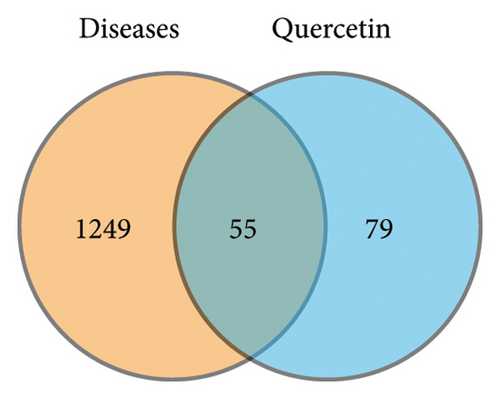
Figure 1 depicts a flowchart of the entire research procedure. In total, 2660 targets associated with asthma, 6330 targets associated with PF, and 3668 targets associated with COVID-19 were identified by searching GeneCards, TTD, DrugBank, OMIM, and the PharmGkb databases (Supporting Table 1). Targets for the aforementioned diseases were compared, and 1304 common targets were identified (Figure 2(a)). Targets of quercetin were predicted by the TCMSP database (Supporting Table 1). The next step was contrasting the targets of quercetin and diseases (asthma, PF, and COVID-19), which revealed 55 common targets between the aforementioned diseases and quercetin (Figure 2(b) and Supporting Table 2).
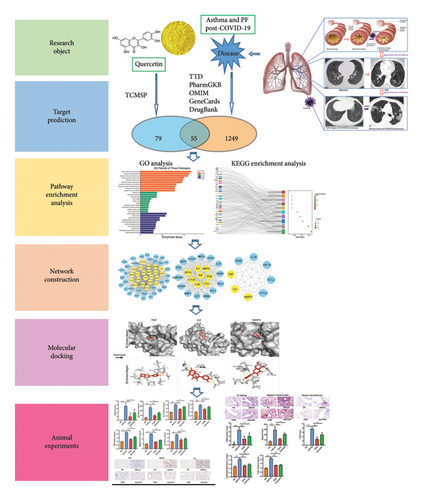
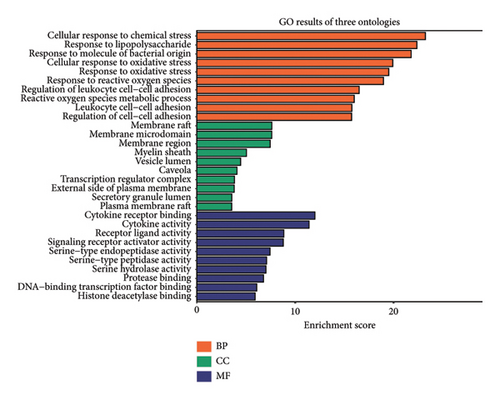
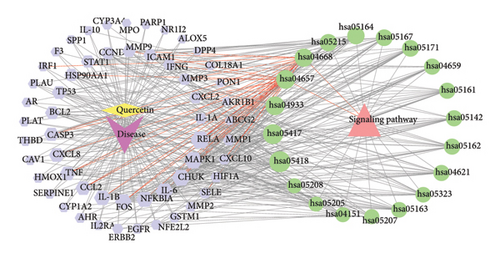

To delve deeper into the effector mechanisms via which quercetin treats asthma and PF post-COVID-19, the R package “cluster profiler” was employed to perform GO and KEGG analyses on the quercetin-disease common targets. In Figure 2(c), the five most popular BP terms and the corresponding genes are response to cellular response to chemical stress (GO:0062197), response to lipopolysaccharide (GO:0032496), response to molecule of bacterial origin (GO:0002237), cellular response to oxidative stress (GO:0034599), and response to oxidative stress (GO:0006979).
The KEGG pathway enrichment study showed the top 20 pathways in Figures 2(d) and the top 10 pathways in Figure 2(e). Most of the interested target genes were mainly focused on the fluid shear stress and atherosclerosis, IL-17 signaling pathway, TNF signaling pathway, influenza A, and coronavirus diseas (COVID-19).
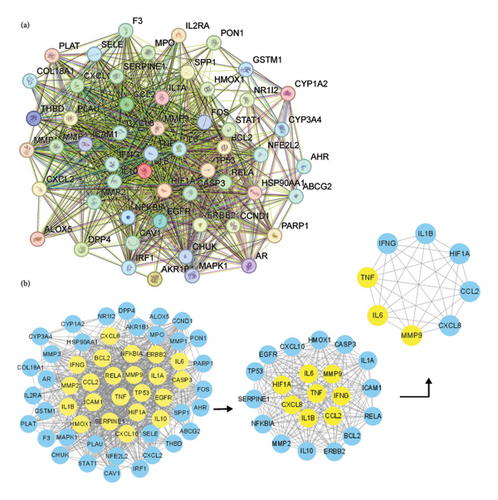
3.2. PPI Network Construction and Identification of Core Genes
Figure 3(a) displays the results of a PPI network analysis performed using the STRING database on shared targets. The PPI network presents the BPs of quercetin in its fight against diseases, with 55 nodes, 828 edges, and an average node degree of 30. The CytoNCA plugin in Cytoscape was used for topological analysis of the targets in the PPI network (Figure 3(b)). Three topological characteristics were used to conduct the analysis process: betweenness, closeness, and degree. The initial filtering step yielded 22 genes by using the criteria BC > 13.432, CC > 0.701, and DC > 32. After a second round of filtering (BC > 36.315, CC > 0.818, and DC > 42), we identified 8 highly connected nodes. These 8 key genes were as follows: IL-6, TNF, MMP9, IL-1B, CCL2, IFNG, HIF1A, and CXCL8. Among them, the degree values of TNF, IL-6, and MMP9 are the highest, all exceeding 70.
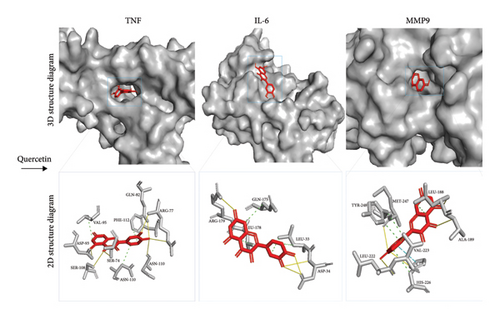
3.3. Validation by Molecular Docking
With the aid of molecular docking simulation technology, quercetin was docked with the top three core targets including TNF, IL-6, and MMP9. Having a binding energy of zero indicates that the ligand and receptor may interact spontaneously, while a lesser value indicates stronger binding. Via molecular docking, quercetin showed a high degree of affinity for TNF (−8.4), IL-6 (−7.1), and MMP9 (−10.9) (Figure 4). The numbers represent the best affinity (kcal/mol).
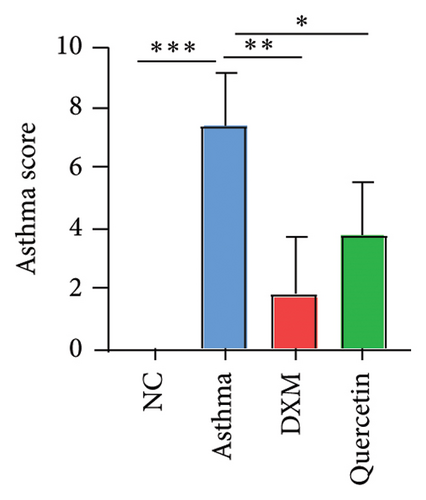
3.4. Effect of Quercetin on Biochemical Indexes of Asthma Model Serum
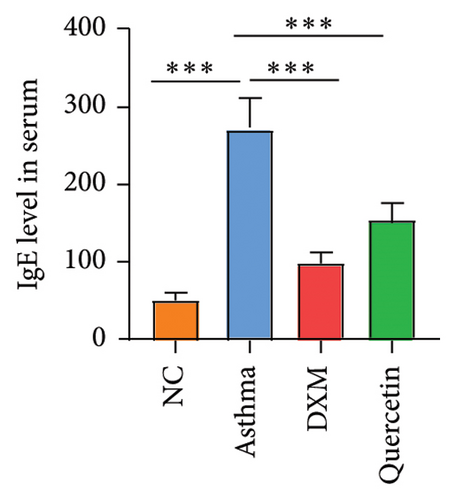
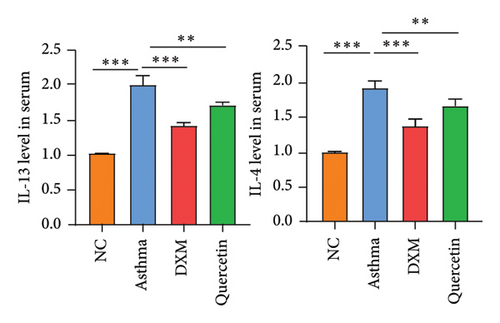
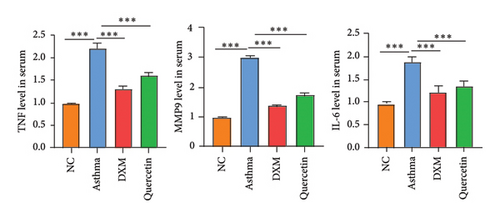
We established the asthma model (Figure 5(a)), and they were divided into the NC group, asthma group (n = 5), DXM (2.0 mg/kg) group (n = 5), and quercetin (50 mg/kg) group (n = 5). We first assessed the severity of asthma by observing the mouse behavior according to the comprehensive scoring criteria shown in Supporting Table 3. A higher total score indicates more severe asthma symptoms. In Figure 5(b), the asthma group has the most severe asthma symptoms, and in the DXM and quercetin groups, asthma scores were reduced. As asthma is a disease with a complicated network of inflammatory responses of cytokines and IgE [21, 22], IL-4 and IL-13 are key cytokines that induce the pathogenic Th2 responses in AR and asthma [23], and we detected levels of IgE, IL-4, and IL-13 in serum. As shown in Figures 5(c) and 5(d), the levels of IgE, IL-3, and IL-4 in the asthma group were significantly higher than those in the NC group. Both dexamethasone and quercetin treatment decreased the levels of IgE, IL-13, and IL-4. We further assessed the levels of the three core targets, TNF, IL-6, and MMP9, using qPCR and immunohistochemistry. They were found to be statistically significantly higher in the asthma group than in the NC group (p < 0.001) and were observed to decrease after treatment with dexamethasone or quercetin (Figures 5(e) and 5(f)).


3.5. Effect of Quercetin on Pathological Changes of Lung Tissue
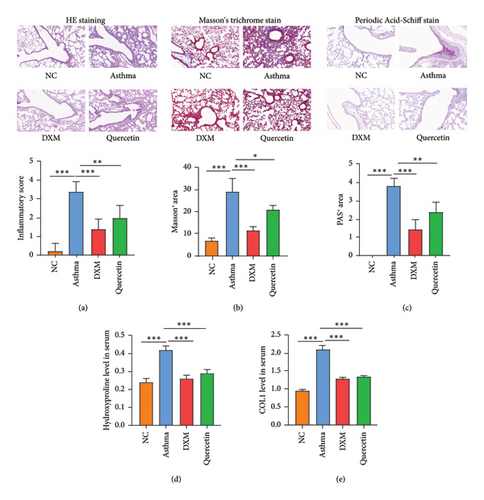
Later, lung tissues were collected and HE staining, Masson’s trichrome staining, and Periodic acid–Schiff staining were performed. Results in Figures 6(a), 6(b), and 6(c) showed that compared to the NC group, the asthma group showed significant infiltration of inflammatory cells, thickening of alveolar walls, widening of septa, and expansion of alveoli, with significant paratracheal and interstitial fibrosis. Both dexamethasone and quercetin treatment significantly ameliorated the above pathological features. Hydroxyproline and COL1 mRNA levels were significantly upregulated in the asthma group compared to the NC group, and both dexamethasone and quercetin treatment decreased the mRNA level of hydroxyproline and COL1 (Figures 6(d) and 6(e)).
4. Discussion
Asthma is among the most prevalent chronic respiratory conditions worldwide. It is marked by variable airflow obstruction, which results in breathing difficulties and wheezing [24]. Existing asthma medications (inhaled bronchodilators and corticosteroids) have been demonstrated in longitudinal trials to decrease the frequency of exacerbations but have little lasting effect on disease outcomes. Furthermore, no treatment or documented preventive measures exist [25, 26]. Evidence suggests that the onset and progression of this disease are influenced by environmental and genetic factors, including occupational irritants, viruses, and allergens. Individuals who are exposed to these variables develop airway inflammation, which is the pathogenic mechanism causing asthma [27, 28]. Epithelial cells, local dendritic cells, vascular cells, and smooth muscle cells may experience a decline in functionality due to the synthesis and secretion of chemokines and cytokines by immunocytes, including platelet-activating factors, kinins, prostanoids, leukotrienes, and histamine. In conjunction with these alterations, the development of extensive pathogenic remodeling stimulates the dynamic growth of fibroblasts, ultimately culminating in airway remodeling [25].
PF is a chronic and progressive lung disorder. It is distinguished by the presence of a scar tissue in the lungs, which contributes to a significant disease burden and a poor prognosis for survival [29]. PF is a chronic progressive fibrotic interstitial lung disease that involves both innate and adaptive inflammatory processes. Moreover, the conventional approach to treating fibrosis is anti-inflammatory combined with immunoregulatory treatment [30]. Also, PF is correlated with an elevated oxidant load and reduced activity of antioxidant enzymes. The family of NADPH oxidases, which is shown to be overexpressed in epithelial cells and (myo) fibroblasts of patients with PF, is a key source of ROS in the lungs. It is believed that this upregulation promotes collagen deposition, (myo) fibroblast differentiation, and alveolar epithelial cell death [31, 32]. Pirfenidone and nintedanib, two clinically approved antifibrotic drugs, have recently demonstrated the ability to impede the disease progression, thereby ameliorating its course. It is hypothesized that these two drugs possess antiangiogenic, antifibrotic, anti-inflammatory, antineoplastic, and antioxidative properties [33]. The antifibrosis effect is reflected in that it inhibits the actions of fibroblasts. However, their efficacy is not universally observed among patients, since disease development persists despite their therapeutic use [34].
COVID-19 has been associated with a broad range of possible clinical manifestations, including fatality, severe myocardial infarction, and asymptomatic conditions [35, 36]. Post-COVID-19 syndrome, which persists for weeks or months beyond the primary COVID-19 infection, is defined as a constellation of signs and symptoms affecting several organ systems. Noticeably, respiratory-related diseases exhibited the most substantial increase in risk. Previous studies have reported that respiratory failure caused by fibrosis, interstitial thickening, and vascular abnormalities may persist in COVID-19 survivors 12 months following their acute infections [37]. PF and asthma are both inflammatory lung disorders distinguished by an inflammatory response, airway damage, and remodeling of the bronchi and parenchyma [38]. Research has demonstrated that the presence of chronic pathogenic diseases, including asthma and PF, may significantly amplify the severity and morbidity associated with COVID-19 [5]. These conditions enhance the susceptibility of respiratory conduit to COVID-19 infections, Even after recovery from COVID-19 infection, specific pathologies such as fibrosis and massive tissue remodeling are mighty [39].
TCM is an all-encompassing therapeutic framework that has been implemented in clinical settings for millennia and is crucial for the global preservation of health. The naturally occurring chemical quercetin has promise as a medicinal agent via certain pharmacological effects (antoxidative and anti-inflammatory effects) [40]. A growing body of data from in vitro and animal research in recent years suggests that quercetin may have a role in mitigating a variety of respiratory disorders, including smoking-induced lung damage, asthma, fibrosis, acute lung injury, and lung cancer [41]. The literature also reveals that quercetin exhibits anti-COVID-19 activity [16]. Therefore, quercetin could have a therapeutic impact on the overlap syndrome of asthma and PF post-COVID-19.
In this study, 1304 proteins were discovered to have overlapped in the progression of asthma, PF, and COVID-19, as determined by constructing asthma-, PF-, and COVID-19–specific PPI networks and comparing the three protein profiles. Subsequently, using the TCMSP databases, a total of 134 predicted targets were ultimately obtained. There were 55 common targets between the aforementioned diseases and quercetin. The outcomes of the GO analysis identified a variety of intricate synergistic actions of quercetin, as well as a few BP categories that are critical for diseases (asthma, PF, and COVID-19). The top five BP terms were cellular response to chemical stress, response to lipopolysaccharide, response to molecule of bacterial origin, cellular response to oxidative stress, and response to oxidative stress. KEGG enrichment analysis found that the top five signaling pathways were the fluid shear stress and atherosclerosis, IL-17 signaling pathway, TNF signaling pathway, influenza A, and COVID-19.
Research has shown that the IL-17 and TNF signaling pathways are significant contributors to the inflammatory process [42]. TNF, the most important cytokine in the TNF pathway, is elevated in asthma, PF, and COVID-19 and can induce the release of other proinflammatory factors [43]. Th17 cells were the prevailing inflammatory cells that managed to infiltrate the lung tissue. Moreover, they infiltrated the tissue extensively throughout the fibrotic process, where they stimulated lung fibroblasts through the secretion of IL-17A, both in vivo and in vitro [44]. IL-17, a signature cytokine secreted by Th17 cells, is a significant proinflammatory player in the IL-17 pathway [45]. The aforementioned findings demonstrated that the signaling pathways obtained from KEGG enrichment analysis were associated with fibrotic processes, inflammation, and oxidative stress.
Eight key genes were found by creating a PPI network of intersecting targets between quercetin and diseases (asthma, PF, and COVID-19). These genes included IL-6, TNF, MMP9, IL-1B, CCL2, IFNG, HIF1A, and CXCL8. Research showed that oxidative stress performs a fundamental function in the induction of proinflammatory responses in response to air pollution exposure [46], hinting that there is a close relationship between oxidative stress and inflammation. In addition, high exposure to O3 could lead to increased expression levels of inflammatory mediators (IL-1β, IL-6, IL-10, TNF-α, HSP70, etc.) [47]. Mark G et al. revealed that TNF-α treatment increased inflammatory marker (IL-6) secretion, reduced the metabolic activity of viable cells, increased endothelial oxidative stress, and induced apoptosis [48]. Through chemotactic neutrophils, CXCL8 is an attractive target for the therapeutic management of neoplastic and inflammatory disorders [49]. Moreover, c-Fos regulates the expression of matrix metalloproteinases (MMPs) and interferes with the prognosis of liver fibrosis by regulating ECM deposition in cirrhosis scar tissue [50]. These researchers suggested that eight key genes had a close relationship with anti-inflammatory, antioxidant, and antifibrosis.
Further molecular docking outcomes indicated that the ligands consisting of docked small molecules demonstrated an excellent affinity and the lowest binding energy toward macromolecular protein receptors. Each of the molecular binding energies was below 5 kcal/mol. It was determined that quercetin had a high degree of affinity with TNF, IL-6, and MMP9.
These results suggest that quercetin intervenes in the treatment of asthma and PF post-COVID-19 through the abovementioned pathways. Previous studies suggested that quercetin can up/downregulate transcriptional factors in inflammtory and antioxidant pathways [51]. Experiments confirmed that both quercetin at 25 μm effectively decreased TNF, IL-6, IFN, and IL-1 production in human whole blood [52] and mitigated TNF-induced cytotoxicity in L929 cells by targeting ROS [53]. These experimental findings align with our network pharmacology analysis, which predicted quercetin’s interactions with key inflammatory mediators and antioxidant pathways. This consistency highlights the reliability of the network pharmacology approach in supporting and explaining the observed therapeutic effects of quercetin. Currently, the top priority in the management of patients with complications of asthma and PF post-COVID-19 is anti-inflammatory and antioxidant stress. The aforementioned findings exhibited a strong correlation with the notion that the active components of quercetin might alleviate inflammation, fibrosis, and remodeling of the airways via binding to IL-6, TNF, and MMP9.
The results of the asthma model showed that quercetin treatment significantly ameliorated the asthma symptoms and lung fibrosis and decreased the IgE, IL-4, IL-13, IL-6, TNF, MMP9, hydroxyproline, and COL1.
Despite the study’s deep relevance, it is important to acknowledge some limitations. First, the network analysis, focusing on separate biological entities, may not fully capture the complex regulatory mechanisms and dynamic changes of disease. In addition, the dose–effect relationship between pharmaceuticals and disorders poses challenges in quantifying results through network pharmacology. The use of β-cyclodextrin to enhance quercetin solubility could influence the results, as it may affect the pharmacokinetics and biological activity of quercetin. Finally, the interaction score cutoff of 0.400 is relatively low and may impact result specificity; a higher threshold could yield more accurate interactions in future studies.
5. Conclusion
Although the comorbidity mechanisms between asthma and PF post-COVID-19 are complex, quercetin’s effectiveness in treating asthma and PF has been partially proven in clinical and experimental research. In the overlap syndrome of asthma and PF post-COVID-19, our findings identified the active components and potential molecular mechanisms via which quercetin treatment is effective against airway inflammation, oxidative stress, and remodeling. This may be achieved via regulation of the IL-17 and TNF signaling pathways. Subsequent molecular docking analysis revealed that bioactive chemicals derived from quercetin bind well to the following proteins: IL-6, TNF, and MMP9. Our results will serve as a comprehensive reference for the investigation of the mechanism via which quercetin intervenes in the overlap syndrome of asthma and PF post-COVID-19. To further validate the network pharmacology predictions and experimental findings, future clinical studies and in vivo experiments are essential. These studies will be critical for confirming quercetin’s therapeutic potential and for better understanding its role in managing this complex comorbidity.
Nomenclature
-
- PF
-
- Pulmonary fibrosis
-
- COVID-19
-
- Coronavirus disease 2019
-
- TCMSP
-
- Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform
-
- PPI
-
- Protein–protein interaction
-
- GO
-
- Gene Ontology
-
- KEGG
-
- Kyoto Encyclopedia of Genes and Genomes
Ethics Statement
The study protocol was approved by the Ethics Committee of Animal Experiments of the Huai’an First People’s Hospital (DW-P-2023–001-22) and is in conformity with the Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Baolan Wang acquired, analyzed the data, and wrote the manuscript. Zhe Zhang, Yi Wang, and Rong Zhu revised the manuscript. Xiuqin Zhang designed the research and analyzed the data. All the authors have read and approved the final submitted manuscript. Baolan Wang, Zhe Zhang, and Yi Wang contributed equally to the work and should be considered co-first authors.
Funding
This work was partly supported by the Jiangsu Provincial Medical Key Discipline (ZDXK202201) and the Jiangsu Science and Technology Association research topic (JSKXKT2023029).
Supporting Information
Supporting tables include the following supporting tables:
Supporting Table 1: Targets of quercetin and diseases (asthma, PF, and COVID-19).
Supporting Table 2: The 55 common targets between the diseases and quercetin.
Supporting Table 3: Asthma scoring criteria.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. All relevant data are included within the manuscript and its Supporting Information.




