Analysis of Predictors of Adverse Events and Mortality Risk Associated With IL-6 Inhibitors: A Pharmacovigilance Study Using the FDA Adverse Event Reporting System Database
Abstract
Aim: This study aimed to investigate post-marketing adverse events (AEs) of interleukin-6 (IL-6) inhibitors, and to explore risk factors for death.
Method: Disproportionality analyses were conducted on adverse event cases of IL-6 inhibitors reported to the US Food and Drug Administration Adverse Event Reporting System (FAERS) from the time of drug launch until the fourth quarter of 2023. Univariate and multivariate logistic regression analyses were carried out utilizing patient-related clinical information, and prediction models for IL-6 inhibitor-related mortality risk were developed by incorporating patient age and weight factors.
Results: A total of 63,445 reports were retrieved, with the majority of known age groups falling between 18 and 64 years. Most reports were submitted by consumers and physicians, predominantly from the United States. Tocilizumab was associated with AEs such as drug intolerance and infection, while sarilumab showed symptoms of pain and condition aggravated. Siltuximab was linked to disease progression and thrombocytopenia. The median time to AEs with IL-6 inhibitors was 74 days (interquartile range [IQR] 10-311), mostly occurring within 1 month. Factors such as age, propionic acid derivative, infections and infestations, nervous system disorders, and immune system disorders were independent risk factors for deaths related to IL-6 inhibitor use (p < 0.05). The mortality risk prediction model demonstrated good discriminatory power and clinical applicability in both the training set (AUC 0.6968) and the validation set (AUC 0.7502).
Conclusion: Our postmarketing pharmacovigilance analysis revealed the types and incidence of AEs related to IL-6 inhibitors. Column line diagrams may be useful for clinical assessment of the occurrence of death and have high clinical utility.
1. Introduction
Interleukin-6 (IL-6) is a versatile cytokine commonly linked to inflammatory conditions like cytokine release syndrome (CRS) and COVID-19 [1–5]. Moreover, IL-6 plays a significant role in the tumor microenvironment, being notably upregulated in various cancers including colorectal and lung cancer. This has sparked considerable interest in exploring IL-6 blockade [6]. A study has shown that the use of IL-6 inhibitors is associated with a lower 28 days all-cause mortality rate compared with conventional treatment or placebo [7]. The IL-6 inhibitor tocilizumab has shown potential in preventing the deterioration of severe COVID-19 patients and effectively improving clinical symptoms [8]. Nevertheless, adverse events (AEs) associated with IL-6 blockade frequently manifest in settings where IL-6 plays a crucial role in maintaining tissue homeostasis or barrier immunity [9]. The use of IL-6 inhibitors is associated with an increased risk of severe infections, along with common AEs such as elevated transaminases and decreased blood cells [10]. Drug instructions from a 6-month clinical trial state that 2.1% of the patients receiving 8 mg/kg of tocilizumab experienced a 3-fold increase in ALT/AST levels. These AEs limit the use of IL-6 inhibitors in patients with underlying complications, such as active infections or liver function impairment.
Clinical trials in the literature often lack comprehensive data on AEs and long-term outcomes. Moreover, only a limited number of perspectives are based on low-level evidence or expert opinion. A meta-analysis was specifically carried out in the ICU setting, aiming to systematically analyze the findings of previous studies. It was worth mentioning that observational studies are vulnerable to different biases [11]. Gao et al. utilized the FAERS database to examine and contrast the variances and correlations in drug-induced liver injury (DILI) among COVID-19 patients who were treated with tocilizumab or sarilumab. Nevertheless, this research did not thoroughly investigate the risk factors for severe AEs induced by IL-6 inhibitors, leading to an insufficient clinical reference for their utilization [12]. Kamath et al. pointed out that compared with other drugs, patients receiving tocilizumab had disproportionately higher reports of pancreatitis [13]. However, such slightly higher reports are unlikely to attract direct clinical attention. Early assessment of patient prognosis is essential for managing disease progression. Despite this, the study had not yet been able to predict acute pancreatitis death following treatment with tocilizumab. The specific risk factors that contribute to these AEs had not yet been pinpointed. Nonetheless, if these factors can be identified early in clinical practice, it holds the potential to facilitate prompt and well-thought-out interventions that could enhance patient outcomes.
The objective of this study was to evaluate AEs related to IL-6 inhibitors (tocilizumab, sarilumab, and siltuximab) through analysis of postmarketing AEs reported in FAERS. In addition, the study aimed to identify risk factors for mortality by developing a nomogram.
2. Method
2.1. Data Source
FAERS is a database used for spontaneously reporting AEs and medication errors, as well as monitoring the postmarket safety of drugs and therapeutic biological products. The data structure complies with the International Conference on Harmonization (ICH) guidelines for international safety reporting, and AEs are encoded in preferred terms (PTs) according to the Medical Terminology Dictionary for Regulatory Activities (MedDRA, Version 26.0). The FDA publishes FAERS data folders to the public on a quarterly basis and provides two formats (ASCII/XML) that can be downloaded from its website. In each ASCII format data package, there are seven datasets: patient demographics and management information (“DEMO”), drug/biological information (“DRUG”), adverse event (“REAC”), event result (“OUTC”), report source (“RPSR”), drug treatment start and end dates (“HER”), and diagnosis (“INDI”).
2.2. Data Processing
IL-6 inhibitors, including tocilizumab, sarilumab, and siltuximab, were used as keywords to obtain report data of AEs from the FAERS database from the drug launch to the fourth quarter of 2023, with these drugs being identified as the primary suspect drug (role code = “PS”). We have included all data and imported these datasets into the SQL server to create a local database. The following data will be excluded: duplicate data with the same field values for PRIMARY ID, CASE ID, and FDA_DT in the DEMO table. We followed the deduplication method recommended by the FDA, and rules were as follows: the reports were sorted in the order of CASE ID, FDA_DT, and PRIMARY ID. For reports with the same CASE ID, the one with the highest FDA_DT value was retained. In cases where reports had identical CASE ID and FDA_DT values, the report with the highest PRIMARY ID value was selected.
AEs were systematically classified and characterized using the PTs and system organ classification (SOC) as outlined in the Medical Dictionary for Regulatory Activities (MedDRA, Version 26.0). Defined as the time interval between the onset time of AEs and the start date of medication, we also excluded incorrectly entered (reports where EVENT_DT precedes START_DT) or inaccurate date entries.
2.3. Signal Detection
Safety signals were identified through disproportionality analysis methods, specifically the reporting odds ratio (ROR), proportional reporting ratios (PRR), and the Multi-Item Gamma Poisson Shrinker (MGPS) algorithm. The calculation formulas and signal criteria for each method are detailed in Supporting Table 1. A signal was considered present only when all three methods indicated its presence. The formula utilized for conducting the disproportionality analysis can be found in Supporting Table 2.
2.4. Statistical Analysis
The chi-square test was utilized for comparing categorical variables between groups, while the Wilcoxon test was employed to assess the statistical significance of continuous variables. A significance level of p < 0.05 was considered statistically significant. Predictive models were constructed to assess the risk of mortality in IL-6 inhibitors. We excluded reports with missing age and weight and standardized the units to age in years and weight in kg. In addition, we retained only reports from physicians and pharmacists. The model’s discriminatory ability was assessed using area under the curve (AUC) values, which range from 0.5 to 1.0. A value of 0.5 represents randomness, while 1.0 signifies a perfect fit. AUC values exceeding 0.7 were considered to provide reliable estimations [14]. Calibration curves were used to assess the concordance between predicted probabilities and actual outcomes. Clinical utility was evaluated using decision curves. Data manipulation and statistical analysis were performed using a PostgreSQL database (Version 15.3) and R software (Version 4.2.2).
3. Results
3.1. Basic Data of ADE Report
From drug launch to the fourth quarter of 2023, a total of 52,020 reports of tocilizumab as the main suspected drug were recorded, 11,186 reports of sarilumab as the main suspected drug, and 239 reports of siltuximab as the main suspected drug. As shown in Table 1, AEs reports using IL-6 inhibitors were collected in the FAERS database. Among patients of known age, patients aged 18–64 accounted for the largest proportion, followed by patients over 64 years old. In the known gender of patients, the proportion of AEs for males in tocilizumab was 20.9% and for females was 69.6%. For sarilumab, the proportion for males was 14.0% and for females is 74.7%. For siltuximab, the proportion for males is 17.5% and for females is 12.5%. The countries with the highest number of reports included the United States, Canada, Japan, and various European countries. AEs were predominantly reported by health professionals and consumers.
| Tocilizumab (52,020) | Sarilumab (11,186) | Siltuximab (239) | |
|---|---|---|---|
| Age | |||
| < 18 | 1307 (2.5%) | 7 (0.1%) | 3 (1.2%) |
| 18 ≤ age ≤ 64 | 15,755 (30.2%) | 5002 (44.7%) | 33 (13.8%) |
| > 64 | 10,270 (19.7%) | 1841 (16.4%) | 12 (5.0%) |
| NA | 24,688 (47.4%) | 4336 (38.7%) | 191 (79.9%) |
| Gender | |||
| Male | 10,913 (20.9%) | 1575 (14.0%) | 42 (17.5%) |
| Female | 36,227 (69.6%) | 8367 (74.7%) | 30 (12.5%) |
| NA | 4880 (9.3%) | 1244 (11.1%) | 167 (69.8%) |
| Reporter country (top 5) | |||
| America (24,182; 46.4%) | America (10,127; 90.5%) | America (148; 61.9%) | |
| Canada (8957; 17.2%) | Japan (490; 4.3%) | Italy (13; 5.4%) | |
| Japan (3640; 6.9%) | Canada (166; 1.4%) | Germany (11; 4.6%) | |
| United Kingdom (1654; 3.1%) | United Kingdom (104; 0.9%) | Spain (11; 4.6%) | |
| Germany (1434; 2.7%) | France (59; 0.5%) | Korea (11; 4.6%) | |
| Reporter | |||
| Physician (19,530; 37.5%) | Consumer (5271; 47.1%) | Physician (102; 42.6%) | |
| Consumer (17,131; 32.9%) | Physician (5118; 45.7%) | Health professional (53; 22.1%) | |
| Health professional (6460; 12.4%) | Health professional (317; 2.8%) | Pharmacist (38; 15.8%) | |
| Other health- professional (4843; 9.3%) | Pharmacist (271; 2.4%) | Consumer (27; 11.2%) | |
| Pharmacist (2733; 5.2%) | Other health professional (196; 1.7%) | Other health professional (19; 7.9%) | |
3.2. Time of Onset of Adverse Reactions to IL-6 Inhibitors
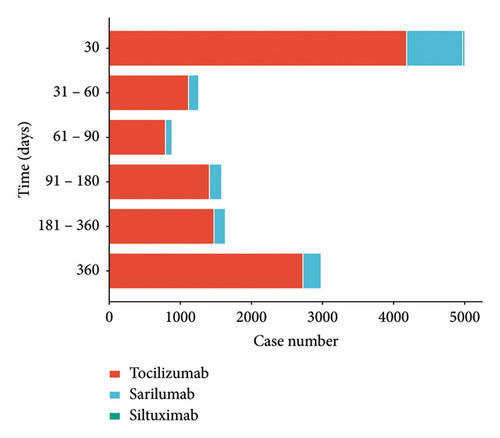
The median time to AEs with IL-6 inhibitors was 74 days (interquartile range [IQR]: 10-311), with the majority of reported cases occurring within one month after the start of medication (Figure 1).
3.3. Comparative Analysis
A total of 10,288 AEs signals of the three targeted IL- 6 drugs mined in this study were obtained. Among them, 7373 were tocilizumab, 2583 were sarilumab, and 332 were siltuximab. After excluding signals unrelated to drug treatment, such as product problems, social environment, surgeries, medical operations, medication errors, and primary disease-related AEs, signals that meet the threshold criteria are evaluated according to the MedDRA system. The SOC classification involved a total of 23 SOCs, as illustrated in Figure 2.
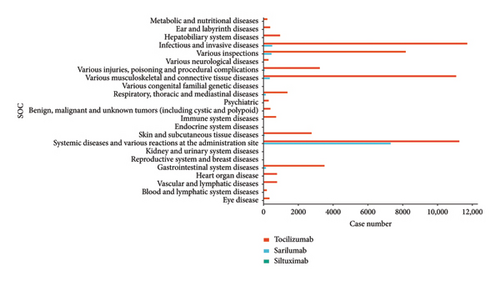
The abovementioned AEs were ranked and the top 10 AEs were screened for further analysis. We further excluded adverse reactions caused by operational problems (including overdosage, product omission, and product preparation errors), and the final results are shown in Table 2 (only 8 AEs for siltuximab after screening). The results found major AEs symptoms associated with tolizumab, including drug intolerance, infection, and peripheral swelling. Patients treated with sarilumab exhibited symptoms such as pain, condition aggravated, and peripheral swelling. Conversely, siltuximab was correlated with AEs symptoms including disease progression, thrombocytopenia, and ascites.
| PT | n | ROR (95%CI) | PRR (95%CI) | MGPS (95%CI) |
|---|---|---|---|---|
| Tocilizumab | ||||
| Drug intolerance | 1616 | 4.72 (4.49–4.96) | 4.70 (4.47–4.93) | 4.61 (4.39–4.84) |
| Infection | 1580 | 3.23 (3.07–3.39) | 3.21 (3.06–3.38) | 3.18 (3.02–3.34) |
| Peripheral swelling | 1579 | 2.78 (2.65–2.92) | 2.77 (2.64–2.91) | 2.74 (2.61–2.88) |
| Nasopharyngitis | 1494 | 2.30 (2.19–2.42) | 2.30 (2.18–2.42) | 2.28 (2.17–2.40) |
| Swelling | 1459 | 3.58 (3.40–3.77) | 3.56 (3.38–3.75) | 3.51 (3.34–3.70) |
| Treatment failure | 1309 | 4.59 (4.34–4.84) | 4.57 (4.32–4.82) | 4.48 (4.24–4.74) |
| Synovitis | 1173 | 20.47 (19.27–21.74) | 20.37 (19.19–21.63) | 18.54 (17.46–19.69) |
| Musculoskeletal stiffness | 1055 | 3.34 (3.14–3.55) | 3.33 (3.13–3.53) | 3.29 (3.09–3.49) |
| Hepatic enzyme increased | 1010 | 4.65 (4.37–4.95) | 4.64 (4.36–4.94) | 4.55 (4.28–4.85) |
| Sinusitis | 945 | 2.56 (2.40–2.73) | 2.55 (2.39–2.72) | 2.53 (2.37–2.70) |
| Sarilumab | ||||
| Pain | 908 | 3.20 (2.99–3.41) | 3.13 (2.94–3.34) | 3.12 (2.92–3.34) |
| Condition aggravated | 872 | 3.49 (3.18–3.82) | 3.45 (3.15–3.77) | 3.44 (3.14–3.77) |
| Peripheral swelling | 194 | 2.41 (2.09–2.77) | 2.40 (2.08–2.76) | 2.40 (2.08–2.76) |
| Infection | 190 | 3.14 (2.72–3.62) | 3.13 (2.71–3.60) | 3.12 (2.70–3.60) |
| White blood cell count decreased | 166 | 3.56 (3.05–4.14) | 3.54 (3.04–4.12) | 3.53 (3.03–4.12) |
| Illness | 147 | 3.66 (3.12–4.31) | 3.65 (3.11–4.29) | 3.64 (3.10–4.28) |
| Swelling | 146 | 3.48 (2.96–4.10) | 3.47 (2.95–4.08) | 3.46 (2.94–4.07) |
| Sinusitis | 107 | 2.63 (2.18–3.18) | 2.63 (2.17–3.17) | 2.62 (2.17–3.17) |
| Hepatic enzyme increased | 97 | 3.55 (2.91–4.34) | 3.54 (2.90–4.32) | 3.53 (2.89–4.32) |
| Injection site pruritus | 97 | 23.18 (18.94–28.35) | 23.11 (18.90–28.25) | 22.60 (18.47–27.64) |
| Siltuximab | ||||
| Disease progression | 13 | 13.15 (7.60–22.78) | 12.91 (7.54–22.11) | 12.91 (7.45–22.35) |
| Thrombocytopenia | 7 | 7.56 (3.59–15.91) | 7.48 (3.58–15.64) | 7.48 (3.55–15.76) |
| Ascites | 4 | 15.64 (5.85–41.80) | 15.55 (5.85–41.30) | 15.54 (5.82–41.55) |
| Leukopenia | 3 | 7.10 (2.28–22.08) | 7.07 (2.29–21.87) | 7.07 (2.27–21.99) |
| C-reactivw protein increased | 3 | 9.65 (3.10–29.99) | 9.61 (3.11–29.71) | 9.60 (3.09–29.86) |
| Night sweats | 3 | 10.68 (3.43–33.20) | 10.63 (3.44–32.89) | 10.63 (3.42–33.06) |
| Liver function test increased | 3 | 13.03 (4.19–40.52) | 12.98 (4.20–40.14) | 12.97 (4.17–40.34) |
| Diffuse large B-cell lymphoma | 3 | 46.70 (15.02–145.25) | 46.49 (15.03–143.83) | 46.46 (14.94–144.48) |
3.4. Treatment Outcomes of IL-6 Inhibitors
In order to analyze the prognosis of patients receiving IL-6 inhibitors treatment, we calculated the proportion of outcomes (death, disability, initial or long-term hospitalization, life-threatening, and other serious diseases that require intervention) after receiving IL-6 inhibitor treatment, as shown in Supporting Table 3. Of all the outcomes, the largest proportion was other serious diseases that require intervention, followed by initial or long-term hospitalization and death.
3.5. Comparison of General Data on Survival and Death Patients After the Use of IL-6 Inhibitors
Clinical data were analyzed for 5519 patients (3916 females and 1603 males) who were prescribed IL-6 inhibitors from the time of drug launch until the fourth quarter of 2023. These patients were categorized into a survival group (n = 4835) and a death group (n = 684) for the study. The differences in demographic and clinical characteristics between survival and death patients were shown in Supporting Table 4. The results showed that there were significant differences in sex, age, vitamin, corticosteroids, monoclonal antibody, antimalarial drug, bone medicine, acylated anilines, antihistamines, musculoskeletal and connective tissue disorders, surgical and medical procedures, vascular disorders, infections and infestations, metabolism and nutrition disorders, gastrointestinal disorders, nervous system disorders, neoplasm, respiratory thoracic and mediastinal disorders, cardiac disorders, immune system disorders, and renal and urinary disorders (p < 0.05).
3.6. Construction and Validation of Nomogram
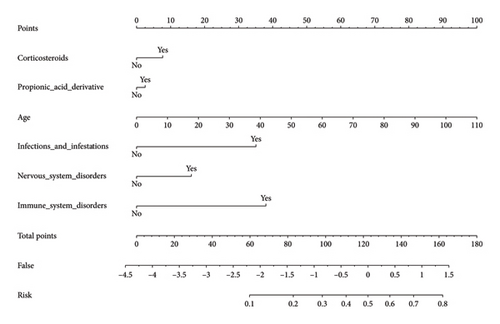
After screening, there are 5519 reports describing the occurrence of adverse reactions after IL-6 inhibitor use were extracted. Reports were randomized 8:2 into training and validation sets. We included gender, age, weight, concomitant medications, and concomitant diseases in the logistic regression model. Univariate logistic regression showed that age, corticosteroids, vascular disorders, infections and infestations, metabolism and nutrition disorders, gastrointestinal disorders, nervous system disorders, neoplasm, respiratory thoracic and mediastinal disorders, cardiac disorders, immune system disorders, and renal and urinary disorders were risk factors for patient mortality. Multifactorial logistic regression analysis showed that age, corticosteroids, propionic acid derivative, infections and infestations, nervous system disorders, and immune system disorders were independent risk factors for patient death (OR > 1, p < 0.05) (Supporting Table 5).
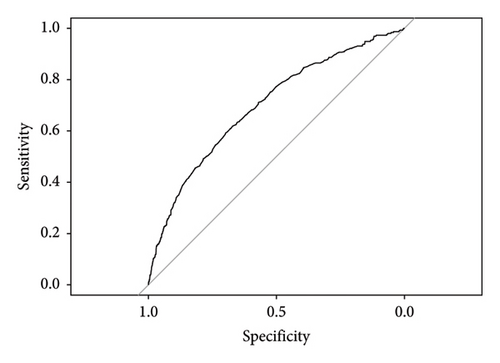
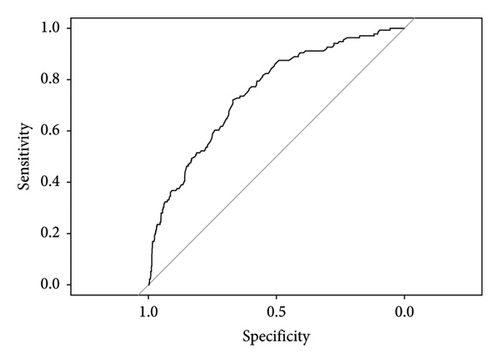
A column-line diagram model was constructed using the six independent risk factors (corticosteroids, propionic acid derivative, AGE, infections and infestations, nervous system disorders, and immune system disorders) screened in the logistic regression model (Figure 3). The column-line diagram was validated in the training and validation sets. Analysis of the resulting ROC curves showed an AUC of 0.6968 (95% CI: 0.6728–0.7208) and 0.7502 (95% CI: 0.7074–0.7930) for the training and validation sets, respectively, demonstrating good model discrimination (Figures 4(a) and 4(b)). Bootstrap self-sampling methods were used to validate the models. The results of the 2000 self-sampling and internal validation procedures showed that the mean absolute error between the predicted risk of death and the actual risk of death occurring from IL-6 inhibitor coadministration and concomitant reactions was 0.01 in the training set and 0.008 in the validation set, demonstrating good agreement (Supporting Figures 1A and 1B). At the probability thresholds in the training and test sets, the column plots showed higher net benefits compared with the “all survive” or “all die” scenarios (Supporting Figures 2A and 2B).
4. Discussion
To the best of our knowledge, our study reveals for the first time the occurrence of adverse reactions related to IL-6 inhibitors in the real world. Risk factors for death were also illuminated and presented in visualized graphics. Furthermore, in the population using IL-6 inhibitors, we developed a mortality risk prediction model, which showed good discrimination (training set AUC = 0.6968 and validation set AUC = 0.7502) and calibration performance with potential clinical value, providing guidance for the rational clinical application of this type of drug.
Overall, from the drug launch to the fourth quarter of 2023, there were 63,445 reports in the FAERS database containing age and sex data describing IL-6 inhibitors reported. From the reported baseline data, reported AEs for tolizumab and sarilumab were greater in females than males. While in cetolizumab, the number of AEs reported was greater in males than in females. Among all age groups reporting AEs of IL-6 inhibitors, the 18–64 age group had the highest proportion. Since some IL-6 inhibitors were used for the treatment of RA and studies have reported that the majority of RA patients are middle-aged women (typically > 70% in any RA cohort), this was consistent with our findings, which may be related to the significant difference in the prevalence of RA between genders [15, 16]. Second, the risk of AEs was also higher in elderly patients over 65 years of age, which may be related to the underlying disease in elderly patients [16]. For the small population of individuals under 18 years old, particularly children with special needs, it is important to consider that their organ function may not be fully developed or may be declining. Due to the higher risk of AEs and the lack of sufficient evidence regarding its safety and efficacy in this age group, the clinical use of this treatment is expected to be relatively uncommon.
The significant increase in the number of AE reports from 2019 to 2023 compared with 2017 may be attributed to the surge in clinical demand for the novel coronavirus following its sudden outbreak in 2019. This increased demand likely led to a higher probability of AE occurrence. Approximately 54.31% of these reports originated from the United States, with fewer cases reported in other countries. This discrepancy could be linked to factors such as the establishment of the FAERS in the US, the drug’s country of origin, and the timing of its launch in different regions. Data suggest that consumers and physicians are the primary sources of postmarketing AE reports. Reporting AEs through healthcare professionals and patients plays a crucial role in postmarketing monitoring, overcoming limitations of premarketing drug studies. This approach can help reduce the recurrence of AEs, enhance rational drug use, and provide valuable data for drug regulation, selection, and adjustment. Continual enhancement of the adverse drug event reporting system is essential for timely identification, assessment, and control of potential medication risks. Critical information missing from reports, such as patient age, gender, and drug dosage, limits the ability to conduct in-depth analysis of AEs. Optimizing adverse drug event reporting systems is essential for improving data quality, ensuring patient safety, and facilitating informed drug regulatory decision-making. By simplifying the process, providing enhanced training, utilizing technical support, and committing to continuous improvement, the occurrence of incomplete information and errors can be significantly reduced. This enhances the accuracy and reliability of the reported data, which not only improves the efficiency of domestic drug supervision but also contributes valuable insights to global drug safety oversight.
In this study, ROR, PRR, and EBGM methods were used for statistical analyses and they showed good agreement in terms of specificity and signal detection ability [17]. The safety of tocilizumab in rheumatoid arthritis (RA) patients has been assessed in numerous phase 3 and phase 4 randomized controlled trials. The most common infections reported include nasopharyngitis, upper respiratory tract infections, pneumonia, and cellulitis, which align with the findings of our research [18, 19]. The analysis of sarilumab revealed AEs related to inflammation, such as decreased white blood cell count and elevated liver enzymes. These findings are consistent with the AEs reported by Heissigerová et al. who also observed neutropenia and increased levels of aspartate aminotransferase (AST) associated with sarilumab use [20]. The AEs of thrombocytopenia analyzed with cetuximab is consistent with the study reported by Karkhur et al. [21]. Due to its relatively specific indications, the scope of use of siltuximab is relatively small, which may be the reason for the low number of ADE reports. Nonetheless, most reported ADEs are consistent with the risk information in the drug package inserts. The instructions specifically emphasize the risks of abnormal liver function test indicators, leukopenia, and thrombocytopenia but do not include increased C-reactive protein levels and other possible AEs. In addition, other potential adverse effects such as cytokine release syndrome and night sweats are not covered by the labeling [22].
We also observed that the median time to onset of IL-6 inhibitor-associated AEs was 74 days, whereas the majority (46.88%) of the reported cases occurred within 1 month of dosing. In a previous study, data from COVID-19 patients obtained from the FAERS database showed that AEs occurred predominantly within 2 days of tocilizumab treatment compared with 4 days after sarilumab treatment. The difference in time of onset was also statistically significant (p = 0.0292) [11]. As can be seen, most of our results are similar in the early stages of drug therapy suggesting that IL-6 inhibitors may increase the risk of adverse effects.
The construction of a nomogram model for severe AEs caused by IL-6 inhibitors has not been reported yet. A nomogram is a graphical representation of clinical predictive models that calculates scores based on the values of individual predictive variables and then calculates the risk of a certain event or survival probability based on the scores. Compared with other predictive models, it has the advantages of being intuitive and easy to use. The results of ROC curve and DCA curve tests show that this model has good calibration and can quantitatively and concisely evaluate the corresponding risks of different risk factors, providing a basis for prognosis judgment and precise medical treatment of patients using IL-6 inhibitors.
Despite the advantages of the real-world large-sample study and data-mining techniques in this study, there are still some limitations; first, as the FAERS database is voluntary reporting, including public reporting, there are problems such as underreporting, which may lead to overestimation or underestimation of some ADE signals and prevent more detailed analyses of some items with missing information. Second, although the analyses showed a statistical correlation between drugs and AEs, the actual causality still needs to be further verified. Finally, confounding factors were difficult to control for, for example, if a COVID-19 patient was also treated with other medications at the time of tolizumab therapy and if the patient had other primary conditions that could have influenced the occurrence of AEs.
5. Conclusion
This study is centered around the FAERS database and employs various statistical methods to extract and examine AE data related to IL-6 inhibitors. The analysis reveals that tocilizumab and sarilumab primarily present risk signals of infection, peripheral swelling, and increased hepatic enzymes, whereas siltuximab is associated with disease progression and thrombocytopenia. Factors such as age, corticosteroids, propionic acid derivatives, infections, infestations, nervous system disorders, and immune system disorders were identified as independent risk factors for deaths linked to IL-6 inhibitor usage. Consequently, we recommend heightened clinical monitoring, particularly during the initial phase of treatment, to promptly detect and address potential issues such as infection and peripheral swelling.
Disclosure
Meilin Fang, Jinglin Li, Boyang Zhuang, and Weijie Liang are co-first authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Meilin Fang: conceived and designed the study, performed data acquisition, and wrote the manuscript.
Jinglin Li: contributed to the acquisition of data and revised the manuscript critically for important intellectual content.
Boyang Zhuang: during the revision process, Boyang Zhuang played a decisive role in improving the quality of the manuscript with his outstanding data analysis skills and in-depth insights into the research results and was, therefore, promoted to co-first author.
Weijie Liang: analyzed and interpreted the data and contributed to the writing of the manuscript.
Ling Wang: designed the study, performed statistical analysis, and provided critical feedback on the manuscript.
Cunze Wang: assisted in the collection of data and provided technical support.
Wujin Chen: contributed to the preparation of response letters to the reviewers, ensuring that our responses were comprehensive and clear.
Fangqing Cai: provided critical revisions of the manuscript and contributed to the final approval of the version to be published.
Zhuiliang Huang: provided critical insights that helped refine the discussion section and supervized the statistical reanalysis of our data to ensure the most accurate interpretation of the results.
Junshan Ruan: involved in the acquisition of data and provided input on the final draft of the manuscript.
Yishun Jin: conducted a reanalysis of the data to address the reviewers’ concerns regarding the research data and assisted in thoroughly revising the manuscript to ensure that all feedback was addressed clearly and accurately.
Zhuliang Huang and Yishun Jin have been designated as corresponding authors due to their key leadership and communication roles during the revision process. Meilin Fang, Jinglin Li, Boyang Zhuang, and Weijie Liang contributed equally to this work.
Funding
This project was supported by the Fujian Provincial Natural Science Foundation Project (grant numbers 2023J011188 and 2024J011032), Training Program for Young and Middle-Aged Backbone Talents of Fujian Provincial Health Care Commission (grant number 2022GGA001), Fujian Provincial Health Commission Science and Technology Innovation Joint Funding Project (grant number 2023Y9330), Fujian Provincial Hospital in-hospital supporting projects (grant number 0080072220), and Yan Xiaohua Nationally Famous Traditional Chinese Medicine Expert Inheritance Studio.
Supporting Information
Supporting Table 1: Summary of major algorithms used for signal detection. Supporting Table 2: 2 × 2 Contingency table for disproportionality analysis. Supporting Table 3: Treatment outcomes of IL-6 inhibitors. Supporting Table 4: Comparison of clinical data between survival and death groups. Supporting Table 5: Baseline characteristics of the patients within the training and internal validation sets.
Supporting Figure 1: The calibration curve of the nomogram used to predict the risk of death. (A) The calibration curve of the training dataset and (B) the calibration curve of the validation dataset.
Supporting Figure 2: The decision curve of the nomogram for predicting the risk of death. (A) The decision curve of the training dataset and (B) the decision curve of the validation dataset.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




