Effect of Diesel Fuel Oil Exposure on Morphology, Behavior, and Internal Organ of Freshwater Fish, Nile Tilapia (Oreochromis niloticus L. 1758)
Abstract
Water pollution resulting from petroleum derivatives, notably diesel fuel oil, has emerged as a significant environmental concern recently. The experiment was conducted to find out the impact of diesel oil on morphology, behavior, and changes in internal organs i.e., heart, intestine, kidney, and liver in Nile tilapia (Oreochromis niloticus). There were controls and three groups of Nile tilapia (Oreochromis niloticus) exposed to 0 mL/L (control), 0.5 mL/L, 1.0 mL/L, and 1.5 mL/L of diesel for 15 days. Several morphological changes such as a broken fin, rough scale, increased mucous, and discolored gill were recorded. The treated fish displayed erratic swimming patterns, rapid operculum movements, and hovering in the water column, displaying signs of weakness whereas the untreated (control) remained calm and normal. Probit analysis revealed that the mortality rates were increased significantly (p < 0.05) with the increase of diesel concentrations. Multiple histopathological abnormalities were found and they were fragmentation of myocardial muscle fiber and formation of degenerative vacuoles in the heart; degeneration of epithelium, submucosa, and hemorrhage in the mucosa of the intestine; hemorrhages of glomerulus and dilation of tissues in the kidney; and enlargement of central vein, degeneration of hepatocyte, dilation of the sinusoid, hemorrhage, vacuolization of hepatocytes, necrosis, and thickening of the hepatocytic cells in the liver. This study also concluded that the dissolved oxygen levels decreased with the increase of oil concentrations, which led to poor water quality and had a significant effect on morphology, behavior, histology, and mortality. This study will grow social awareness and people will handle commercial diesel oil properly during transportation. Thus, the present findings might be helpful to save aquatic animals as well as aquatic environments.
1. Introduction
The fisheries sector is one of the major sources of economic, social, and physiological development in Bangladesh. Fish are regarded as a highly nutritious food, providing high-quality animal proteins at a relatively low cost and often more available and affordable than other animal protein sources [1]. Unfortunately, some natural causes and also anthropogenic activities can pollute water which endangers fish habitat and reduces fish productivity. Among various types of pollutants, petroleum products are particularly significant in aquatic ecotoxicity [2]. Nowadays, due to urbanization and industrialization and the rapid rise in vehicles, petroleum-related activities have become widespread. The primary sources of petroleum pollution in aquatic environments include oil transport pipelines, storage tanks, collisions involving oil-carrying vehicles, and oil shale mining [3]. Petroleum hydrocarbons absorbed by aquatic organisms can accumulate in tissues at concentrations 10–100 times higher than in water [4]. One notable example of these petroleum products is diesel oil, which contains a complex mixture of benzene, toluene, and other mono and polyaromatic hydrocarbons (PAHs) that are highly toxic to aquatic organisms. The introduction of diesel into water sources alters the water’s quality and condition, causing stress to fish [5]. Diesel can easily dissolve in water and is highly toxic to fish [6]. Aromatic hydrocarbons in diesel solutions are highly lipophilic and can accumulate in various body tissues which leads to respiratory dysfunction, osmotic imbalance, immune system weakening, growth retardation, and genetic mutations [7, 8].
The Nile tilapia (Oreochromis niloticus) stands as one of the most extensively cultured freshwater fish globally [9] and the most extensively studied fish species. The Nile tilapia is a freshwater exotic fish extensively cultured in our country to meet the demand for fish. Due to rapid growth, ease of management, omnivorous diet, robustness, tolerance to low dissolved oxygen levels, and pleasant taste, this fish became popular. In addition, Nile tilapia is a prevalent model organism in ecotoxicology, making it suitable for monitoring toxic-induced alterations in fish health [10]. Numerous authors have documented the impact of diesel and various lubricant oils on oxidative stress, histopathological tissue alterations, growth, and biotransformation enzymes in fish [11]. However, information on petroleum hydrocarbon exposure in freshwater fish or aquatic organisms is limited. Therefore, the present study focused on investigating the effects of diesel fuel oil exposure on the morphology, behavior, and internal organs of Nile tilapia (Oreochromis niloticus), a freshwater fish species commonly used as a model organism in ecotoxicological research. The objectives include (1) assessing morphological changes, such as external deformities induced by diesel fuel oil exposure; (2) analyzing behavioral modifications, including swimming patterns, feeding habits, and respiratory activity, as indicators of stress or neurotoxicity; and (3) evaluating histopathological changes in vital internal organs like the heart, intestine, kidney, and liver to understand the systemic impacts of hydrocarbon toxicity. By addressing these objectives, the study aims to provide comprehensive insights into the ecological and physiological risks posed by diesel pollution in aquatic ecosystems.
2. Materials and Methods
2.1. Experimental Fish
Healthy Nile tilapia fishes with good physical appearance were collected from local fishermen. The average body weight of the tilapia was 35 g and the mean length was 11.0 cm. The collected fish were initially kept in the laboratory for 15 days under natural photoperiod conditions in fresh glass aquaria containing 35 L of water containing 10 fish each to acclimatize them to the laboratory environment. The experiment involved utilizing a semicirculatory aquarium (45 cm × 30 cm × 30 cm). During this period, they were fed commercial fish feed thrice daily, at 8:00 a.m., 11:00 a.m., and 4:00 p.m., at a rate of 5% of their total body weight.
2.2. Exposure Assessment and Observation
The experimental fishes were treated with three different doses with 0.5, 1.0, and 1.5 mL/L of diesel solution with a control treatment (0 mL/L). The experimental fishes were kept under observation for around 15 days. The behavior of the fish was observed daily for 10 minutes using a stopwatch after administering the dose in both the treatment and control groups. The mortality rate among the treated fishes with different doses was observed. The dead fishes were immediately preserved at −20°C in a freezer. At the end of exposure, the surviving and dead fishes were used for histopathological study. A control aquarium was also maintained under the laboratory conditions.
2.3. Histopathological Procedures
Histopathological studies involved examining sections of the heart, intestine, liver, and kidney tissues from both control and treated fishes. The tissues were processed following the standard microtome techniques as described by [12, 13]. Furthermore, the procedure was succinctly explained as follows: selected organ tissues were cut into tiny pieces and preserved in alcoholic Bouin’s solution. After that, they were dehydrated by going through a succession of alcohol grades ranging from 70% to 100%, and the alcohol was eliminated using xylene. The tissues were then submerged in the melted paraffin wax. A rotator microtome was used to segment the wooden blocks at 6 μm after they were attached to the microtome chuck and the paraffin block was cut to reveal imbedded tissues. Hematoxylin and eosin were then used to stain the sections of tissues. A compound microscope was used to examine the slices in detail.
2.4. Data Analysis
The data were analyzed by correlation test, regression equation, and significance test (χ2) to evaluate the relation between the mortality rate and dose of concentration through Microsoft Excel 2013. Diesel oil LC50 values were performed through probit analysis. Microsoft GW-Basic 3.23 (1983) was used for probit analysis.
3. Results
This study was conducted to investigate the impact of diesel oil on the morphology, behavior, and histopathology of Nile tilapia (Oreochromis niloticus).
3.1. Morphological Changes
The experimental fish was shown different morphological features in different concentrations of diesel, which is displayed in Table 1.
| Experimental parameter | Control | Treatment | ||
|---|---|---|---|---|
| 0 mL/L | 0.5 mL/L | 1.0 mL/L | 1.5 mL/L | |
| Fins | No change | Compressed | Compressed | Broken |
| Scales | No change | Unsmooth | Rough | Rough |
| Eyes | No change | Swelled | Swelled | Swelled |
| Gill | No change | Fade | Fade | Discolored |
| Operculum | Normal | Less smooth | Less smooth | Less smooth |
| Mucous | Normal | Increased | Increased | Increased |
| Body color | Normal | Whitish | Whitish | Whitish |
3.2. Behavioral Changes
The behavioral changes included rapid opercula movement, weakness, hanging in the water column, and restlessness. These changes were more pronounced at higher diesel concentrations compared with lower ones. At 0.5 mL/L, fishes showed increased opercula movement; at 1.0 mL/L, fishes became weak and hung in the water column and moribund swam; and at 1.5 mL/L, fishes were in extreme weakness, lost their balance, and died. No similar changes were observed in the control group.
3.3. Mortality Observation
There was no recorded mortality in fish during the acclimatization period and in the control group during toxicity tests. Because the behavioral and swimming patterns of the fishes were normal in the case of the control group. After the exposure to diesel, various changes and mortality were observed. The effect of different concentrations and exposure periods of diesel treatments represented by cumulative percentages of mortality with relative percentages of survival (RPS) of Oreochromis niloticus is shown in Table 2.
| Dose concentration (mL/L) | Number of fishes | Cumulative mortality | |||||
|---|---|---|---|---|---|---|---|
| Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | RPS (%) | ||
| 0 | 10 | 10/0 (0%) | 10/0 (0%) | 10/0 (0%) | 10/0 (0%) | 10/0 (0%) | 100 |
| 0.5 | 10 | 10/1 (10%) | 10/1 (10%) | 10/1 (10%) | 10/2 (20%) | 10/4 (40%) | 60 |
| 1.0 | 10 | 10/1 (10%) | 10/2 (20%) | 10/2 (20%) | 10/4 (40%) | 10/7 (70%) | 30 |
| 1.5 | 10 | 10/2 (20%) | 10/3 (30%) | 10/4 (40%) | 10/7 (70%) | 10/10 (100%) | 0 |
- Note: According to [14], relative percentage of survival (RPS) = 1 − (mortality/control) × 100%.
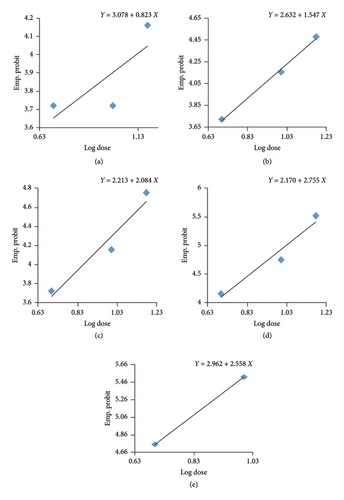
The lethal concentration values of diesel oil for Nile tilapia were determined to range from 0.5 mL/L, 1 mL/L, to 1.5 mL/L for 3–15 days. No mortality was observed in the control group after 15 days of exposure. Probit analysis was performed based on the number of dead fish after 15 days of exposure to different concentrations of diesel oil (Table 3).
| Exposure (day) | LC50 (mL/L) | 95% confidence limits | Regression equations | χ2 values (df) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| 3 | 17.930 | 5.543 | 5805.76 | Y = 3.078 + 0.823X | 0.178 |
| 6 | 3.356 | 0.341 | 32.912 | Y = 2.632 + 1.547X | 1.030 |
| 9 | 2.147 | 0.682 | 6.756 | Y = 2.213 + 2.084X | 0.152 |
| 12 | 1.079 | 0.716 | 1.625 | Y = 2.170 + 2.755X | 0.289 |
| 15 | 0.634 | 0.370 | 1.085 | Y = 2.962 + 2.558X | 1.514 |
The probit analysis reveals that the toxicity of diesel oil to Oreochromis niloticus (tilapia) increases significantly with prolonged exposure, as indicated by the decreasing LC50 values from 17.930 mL/L on Day 3 to 0.634 mL/L on Day 15. The regression equations show steeper slopes over time (e.g., 0.823 on Day 3 to 2.558 on Day 15), indicating a stronger correlation between diesel oil concentration and mortality with longer exposure. The linear transformation of the mortalities percentage of Nile tilapia against the log concentration of diesel oil is shown in Figure 1. The χ2 values suggest the models fit well, and the graphical representation confirms a leftward shift in mortality curves, illustrating higher sensitivity to lower diesel oil concentrations over time (Figure 1). These findings highlight the severe environmental risk posed by prolonged exposure to diesel oil, even at low concentrations.
3.4. Histological Changes
Histology of heart, intestine, liver, and kidney tissues of Oreochromis niloticus at different concentrations of diesel oil exposure were also studied.
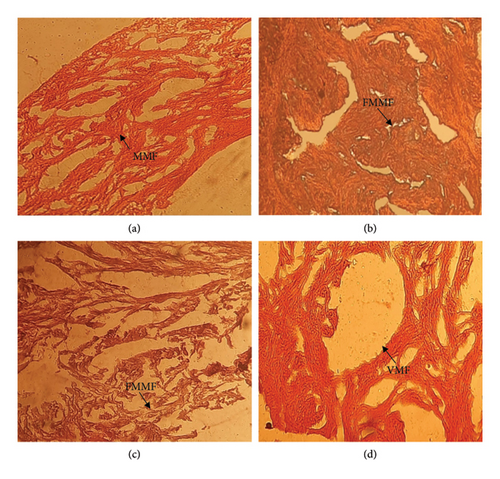
3.4.1. Heart
The section of the heart (control) showed normal structure with the normal arrangement of myocardial muscle fibers (Figure 2(a)). After treatment (0.5, 1.0, and 1.5 mL/L), the heart tissues showed the following alteration of myocardial muscle fibers (fragmentation and formation of degenerative vacuoles), as shown in Figures 2(b), 2(c), and 2(d).
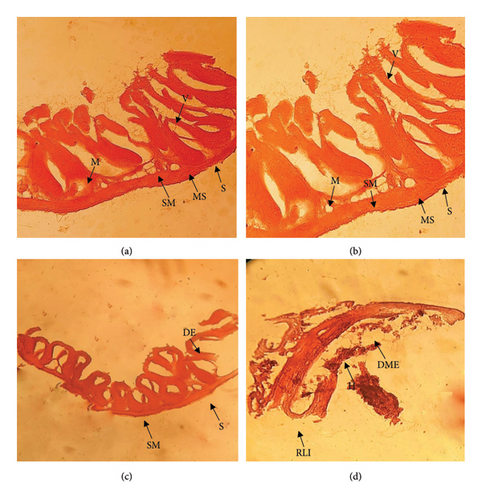
3.4.2. Intestine
The control intestine showed normal histological structures (Figure 3(a)). Degeneration of epithelium, mass depletion of epithelium, degeneration of submucosa, a rupture in a layer of the intestine, and hemorrhage in the mucosa of the intestine were shown in different concentrations of diesel oil exposure as presented in Figures 3(b), 3(c), and 3(d).
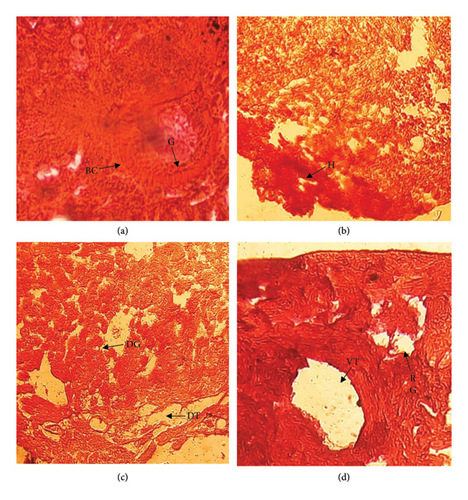
3.4.3. Kidney
Normal kidney showed normal arrangement of the glomerulus and epithelial lining of the proximal and distal tubules (Figure 4(a)). Different histological changes were observed in treated fish such as hemorrhage at 0.5 mL/L diesel exposure (Figure 4(b)), disorganization of glomerulus and dilation of tissue at 1.00 mL/L diesel exposure (4C), and rupture in glomerulus and vacuolization in tissue in large scale at 1.5 mL/L diesel exposure as displayed in Figure 4(d).
3.4.4. Liver
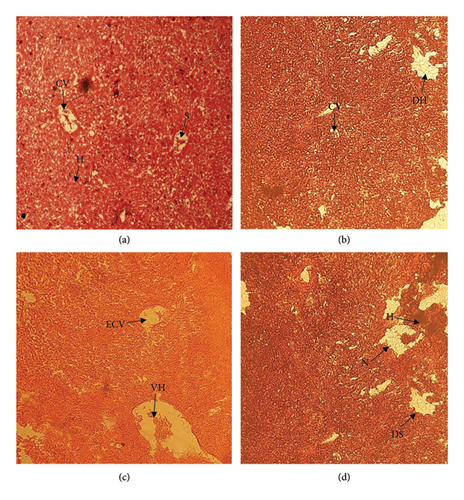
The liver was observed normal (Figure 5(a)) and compared with low- and high-dose treatment groups. Treated fish (Oreochromis niloticus) showed the following disruption in the liver tissue enlargement of the central vein, degeneration of hepatocyte (Figure 5(b)), dilation of the sinusoid, hemorrhage, vacuolization of hepatocyte (5C), and necrosis and thickening of the hepatocyte cell in different concentration of oil exposure as shown in Figure 5(d).
4. Discussion
Diesel, a derivative of crude oil, exhibits high toxicity to fish due to its harmful compounds, including aromatic hydrocarbons, heavy metals, and sulfur content [15], which can seriously damage the vital organs of aquatic creatures and hamper growth, reproduction rate, and survival rate [16]. Several morphological changes occur in different concentrations of diesel oil exposure shown in Table 1, which is in line with the study performed on Oreochromis niloticus by [17]. The authors in [18] denoted that discolored skin, opacity, and irregular swimming were found in 100% of oiled fish, resulting in 100% death.
The present study revealed that the acute toxicity of diesel to experimental fishes showed several behavioral and physiological changes such as not taking food, frequent jumping, erratic swimming, hanging on the water column, convulsion and tendency to escape from the aquaria, loss of energy and orientation, loss of balance, and finally, fall in death. This type of behavior resulted from the high concentration of diesel in water bodies where the volume of dissolved oxygen decreased. These findings are in agreement with [16, 19, 20].
In the present study, the susceptibility of Oreochromis niloticus to the toxic effects of diesel is directly proportional to the concentration and exposure time. If the concentration of diesel was increased, the rate of mortality also increased which was consistent with the results of [16]. Again, no mortality was found here in the control group (0 mL/L), which is consistent with previous findings of [21], where the fish was Clarias gariepinus. We found 100% mortality at higher concentrations (1.5 mL/L) of diesel oil. The authors in [16] reported 100% mortality of Clarias gariepinus at a concentration of 0.75 g/L of diesel oil. The authors in [22] recorded 50% mortality rates in rohu (Labeo rohita), catla (Catla catla), and mrigal (Labeo mrigala) fingerlings at diesel oil concentrations of 0.35, 0.30, and 0.3625 ppm, respectively.
There were several histopathological abnormalities in various organs. The major changes observed in the heart exposed to diesel at different concentrations were degeneration of myocardial cells with the formation of degenerative vacuoles. The authors in [23] reported that significant alterations in heart tissue caused by exposure to industrial effluents combined with diesel are consistent with the current findings. Research conducted by [24] revealed that Pacific herring exposed to crude oil had comparable cardiac problems, such as lower heart rate and arrhythmias. The authors in [7] reported that PAHs cause abnormalities in the heart’s development, as well as edema and arrhythmia in fish. Experiments on fish by [25] have shown that exposure to crude oil affects cardiac development by disrupting excitation–contraction coupling, leading to abnormal calcium cycling in heart cells.
The treated fishes showed severe degenerative and necrotic changes in the intestinal mucosa and submucosa, atrophy in the muscular and submucosa, and aggregations of inflammatory cells in the mucosa and submucosa with edema between them in the present study. The disintegration of the mucosal fold and epithelium might cause severe problems with the absorption of food materials. Also, necrosis, or the complete dissolution of intestinal cells, causes acute problems in the digestion of food. Thus, the present findings are similar to the impairment of food absorption [26] and degenerative changes in the intestine after exposure to several toxins [27, 28].
The kidney is one of the internal organs that are affected by contaminants in any aquatic environment. In fish, however, the kidney only serves an osmoregulatory function, the excretion of nitrogenous waste occurs at the gill where ammonia is excreted as quickly as it is produced. The kidney tissue plays the dominant role in body homeostasis and works synergistically with the liver to complete the process of detoxification [29]. Kidneys of the treated fishes showed disorganization of the glomerulus, degeneration of the epithelial lining of the proximal and distal tubules, vacuolar degeneration in tubular cells, tubular necrosis, loss of tubular structure, and organization, which is consistent with the findings of [30] in Cyprinus carpio. The authors in [31] demonstrated that tissue lesions in the kidney increased with higher concentrations of water-soluble hydrocarbon compounds. The authors in [32] found that tissue damages included tubular edema, epithelial cell vacuolation, hyaline droplets, and glomerular and tubular disorganization in the kidneys of tilapia larvae exposed to soluble crude oil.
The liver plays a crucial role in detoxification and biochemical transformation and the abnormalities in liver cells may result from pollutants present in the water [33, 34]. The present study showed vacuolar degeneration in the hepatocytes, focal vacuolar degeneration in the hepatocytes, focal area of necrosis, thrombosis formation in central veins, dilation and congestion in blood sinusoids, and fibrosis. More or less similar histopathological abnormalities such as vacuolization, necrosis, patchy degeneration, blood congestion, hemorrhage, and hypertrophy nucleus were found in Oreochromis niloticus fish exposed to diesel oil by [10]. The authors in [35] recorded deterioration of endothelial lining cells, inflammation of hepatocytes diffuse necrosis, and hemolysis within the blood vessels observed in the liver of Channa punctatus exposed to diesel fuel. The authors in [36] reported dilated central veins, multifocal degeneration, and necrosis in some hepatocytes of red tilapia fish exposed to petroleum crude oil. In another study, nuclear hypertrophy, cellular hypertrophy, vacuolation, and blood congestion were reported in the liver of Prochilodus lineatus exposed to diesel oil [37]. The authors in [18] observed that lesions included degenerative and necrotic alterations in renal and hepatic tissues of Sciaenops ocellatus, with the most pronounced damage seen at elevated doses and extended exposure periods.
5. Conclusion
The aquatic ecosystem is continuously being contaminated with toxic chemicals from domestic industrial and agricultural activities. Diesel is one of the major classes of toxic compounds used in engine boats, trawlers, etc. From these, vehicles diesel mixed with the aquatic ecosystem and injured fish health. The present experimental study could reveal that diesel has a severe effect on the behavior, morphological, and histopathological changes of tissue and internal organs of a fish and can lead to extreme stress. It can damage different organs after high concentrations of toxins and expanded exposure time. The observed histopathological changes in the heart were degeneration of myocardial cells, lesions including degenerative, hemorrhage, and necrotic alterations in intestinal, renal, and hepatic tissues. These changes can result in economic losses to fish culturists due to fish mortality and retarded growth and reproduction. Therefore, the introduction of diesel into aquatic environments should be controlled to mitigate its effects on aquatic life. In order to address the issue of diesel pollution, more stringent regulatory measures must be established concerning the storage, transportation, and disposal of diesel fuel. This should encompass the obligatory utilization of double-hulled tankers as well as the routine auditing of facilities that manage diesel operations. It is essential to implement real-time monitoring systems to identify contamination at an early stage, especially in sensitive water bodies located near industrial and transportation centers. Encouraging the embrace of clean energy technologies and offering financial incentives to industries shifting from diesel to sustainable alternatives can significantly mitigate pollution levels. Public awareness campaigns ought to enlighten communities and industries regarding the detrimental impacts of diesel pollution and promote proactive initiatives. Ultimately, the necessity of regional and international collaboration cannot be overstated when tackling transboundary pollution and advancing exemplary practices. These recommendations offer a pragmatic structure to mitigate diesel pollution, safeguard aquatic species, and uphold the integrity of freshwater ecosystems, thereby ensuring environmental sustainability for future generations.
Ethics Statement
All sampling, animal maintenance, and experimental procedures of this study involving fish were performed following the ethical guidelines of the Animal Ethical Review Board, Faculty of Biological Science, University of Chittagong, Reference Number: AERB-FBSCU-20250225-(1).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
There was no external funding source for this research.
Acknowledgments
The authors are thankful to the concerned authority of the fisheries research laboratory, Department of Zoology, University of Rajshahi, for providing their laboratory facilities.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




