Study on the Life History and Critical Habitat Identification of Coilia nasus From the Yellow River Based on Microchemical Characteristics of Otolith
Abstract
To reconstruct the life history of Coilia nasus in the Shandong section of the Yellow River, an electron probe microanalyzer (EPMA) was employed to analyze the Sr and Ca values in the otoliths of 12 individuals collected from Pangkou Bay. The results indicated that the life history of C. nasus is complex and diverse, the individuals in this study can be categorized into five distinct patterns. Pattern I includes P1, P2, P3, and P4, whose life history tracks indicate hatching in brackish water near the Yellow River estuary; after approximately 1 year, they begin to migrate along the Yellow River to Pangkou Bay for spawning. Pattern II encompasses P9, P12, and P13, which hatch in freshwater habitats; as these individuals grow, there is an abrupt change from Stage 1 to Stage 2, but their life history does not extend to marine habitats with high Sr/Ca values, and after about 1 year, they migrate along the Yellow River to Pangkou Bay for spawning. Pattern III includes P6, P8 and P10, which also hatch in freshwater habitats and experience abrupt changes; however, the mapping analysis reveals a large area of red and yellow concentric rings, indicating a history of seawater habitat and an extension of their life history to Laizhou Bay. Pattern IV is represented by P11, whose living environment was relatively stable, with no significant abrupt changes and no migratory life history characteristic, which presumed to be a freshwater settlement type that passively entered the Yellow River due to the flood season of Dongping Lake. Pattern V is exemplified by P7, whose analysis results show two trips to freshwater and brackish water habitats, both of which produced significant pattern changes which indicates that it conducted a reproductive migration at the first instar, swam to the Yellow River estuary after spawning, and migrated to Pangkou during the reproductive period of the following year to lay eggs until it was captured. This study demonstrates that most individuals in Pangkou Bay are of the migratory type. Compared with the sexual maturity of individuals in other waters of the Yangtze River basin, Pangkou Bay is identified as an important spawning ground for the migratory type of C. nasus.
1. Introduction
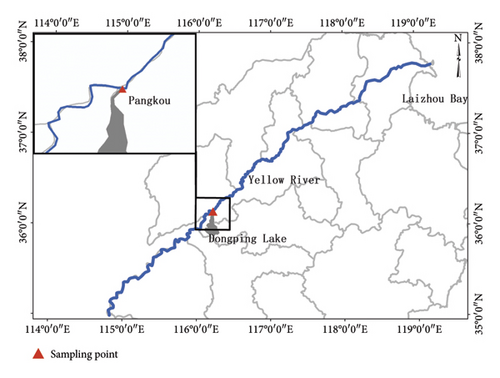
The Yellow River, located in northern China, is the fifth-longest river in the world and the second-longest river in China. It flows through nine provinces from west to east,spanning a total length of approximately 5464 km. Originating in Qinghai Province, it empties into the Bohai Sea. Shandong Province is the final region where the Yellow River flows into the sea, with a section of 628 km, accounting for 11.5% of the total length. Shandong is the only river–sea confluence area and an important lake–river connection area in the Yellow River Basin, with Dongping Lake being the sole lake in the lower reaches of the Yellow River.
The Tapertail anchovy (Coilia nasus (Temminck and Schlegel), Clupeiformes, Engraulidae) is an extremely valuable migratory fish species listed among the key protected economically important aquatic animal and plant resources by the Chinese government. C. nasus can be categorized into migratory and freshwater settlement types, each with distinct life histories. The migratory type, known for its higher economic value, serves as a crucial umbrella species in the lower Yellow River region, meaning its habitat requirements encompass those of numerous other species, thus protecting this species indirectly safeguards other ecosystem components [1]. Historical records [2] indicate that adult fish migrate to Dongping Lake via the Yellow River from April to May each year, spawn in June to July, and enter Laizhou Bay at the beginning of the following year after hatching. Historically, the Shandong section of the Yellow River was a vital migration channel for C. nasus,with Dongping Lake serving as the primary spawning ground and the Yellow River Estuary as a feeding area, as documented in the 1980s [2]. However, due to the impact of water conservancy projects and the interruption of the Yellow River, the spawning ground in Dongping Lake was severely damaged, and C. nasus had not been observed for nearly 30 years until our investigation in 2015.
Otolith microchemistry can help understand fish migration pathways from birth to capture, as otoliths grow continuously and absorb trace elements from ambient water that reflects the surrounding environment [3, 4]. Microchemical analysis of otoliths can effectively reconstruct the life history of migratory fish and changes in strontium and calcium deposits in otoliths serve as “fingerprints” to study conditions in habitats with different salinities [5, 6]. The advantage of this method is that it provides an objective representation of habitat correlation and even a single sample can efficiently represent the habitat state [7]. Yang [8] analyzed the microchemical characteristics of C. nasus in Poyang Lake and Ganjiang River, firstly confirmed the existence of anadromous C. nasus in the Ganjiang River. Jia et al. [9] studied the life history types of the Japanese eel (Anguilla japonica) in the Pearl River and found that Japanese eel in the Pearl River system has the ability to adapt to different habitats and salinities and exhibits flexible migration strategies between different water bodies.
In May 2023, during the monitoring of fishery resources in the Yellow River, we observed a large number of C. nasus in the breeding period gathered at the confluence of Dongping Lake and the Yellow River, known as Pangkou Bay. Whether these C. nasus in this area are migratory has become an important research topic. This study, based on Electron Probe Microanalysis (EPMA) technology, we conducted otolith microchemical analysis of C. nasus from the Yellow River to explore its life history type and provide feasible suggestions for the conservation of C. nasus resources and habitat protection.
2. Materials and Methods
2.1. Study Area and Data Collection
Fish samples were collected from Pangkou Bay by Gillnet (Figure 1) in May 2023. A total of 12 individuals were obtained (Table 1), which were frozen at −20°C for later use. After dissection, the otolith was removed. In order to reduce the error, the left otolith was uniformly used for analysis and the impurities on the surface of the otolith were cleaned with deionized water.
| Sample code | Sampling date | Maturity of fish gonad | Male (♂)/female (♀) | Body length (cm) | Body mass (g) |
|---|---|---|---|---|---|
| P1 | 2023.5.15 | V | ♀ | 18.9 | 23.3 |
| P2 | 2023.5.15 | V | ♀ | 18.9 | 21.2 |
| P3 | 2023.5.15 | V | ♀ | 17.9 | 23.5 |
| P4 | 2023.5.15 | V | ♀ | 18.4 | 22.3 |
| P6 | 2023.5.15 | V | ♂ | 18.9 | 23.5 |
| P7 | 2023.5.15 | V | ♀ | 20.5 | 32.5 |
| P8 | 2023.5.15 | V | ♀ | 19.8 | 28.2 |
| P9 | 2023.5.15 | V | ♂ | 18.5 | 25.1 |
| P10 | 2023.5.15 | V | ♂ | 18.8 | 23.1 |
| P11 | 2023.5.15 | V | ♀ | 19.2 | 22.7 |
| P12 | 2023.5.15 | V | ♀ | 19.5 | 24.1 |
| P13 | 2023.5.15 | V | ♂ | 18.6 | 21.8 |
The otolith sample was fixed with epoxy resin (Epofix, Struers) embedding to make a resin block and then the resin block was fixed on the carrier fragment. After solidification, the excess resin was cut off. The otolith was roughed with Discoplan-TS grinding machine until the core of otolith was just about to be exposed and then finely ground with 1200 mm sandpaper until the core of the otolith was completely exposed. Finally, it was polished with Roto Pol-35 grinding machine and polishing liquid until the surface of the otolith was free of scratches. The treated otolith was ultrasonic cleaned in ultrapure water for 5 min and dried overnight in the oven at 38°C.
2.2. Microchemical Analysis of Otolith
Referring to the method of Yang et al. [10], X-ray electron probe microanalyzer (JXA-8100 EPMA, Nippon Electronics Co., LTD.) was used to analyze otolith samples. Line transect analysis was performed from the core along the longest diameter to the edge of the otolith. The acceleration voltage and electron beam current of EPMA were 15 kV and 2 × 10−8A, respectively. The beam spot diameter was 5 μm and the dwell time of each point was 15 s. Calcium carbonate (CaCO3) and strontium titanate (SrTiO3) were used as standard samples. After line transect analysis, mapping analysis of otolith samples was performed with the acceleration voltage and electron beam current of 15 kV and 5 × 10−7A respectively. The beam spot diameter was 5 μm, the pixel was 7 μm × 7 μm, and the dwell time of each point was 30 ms.
2.3. Data Processing
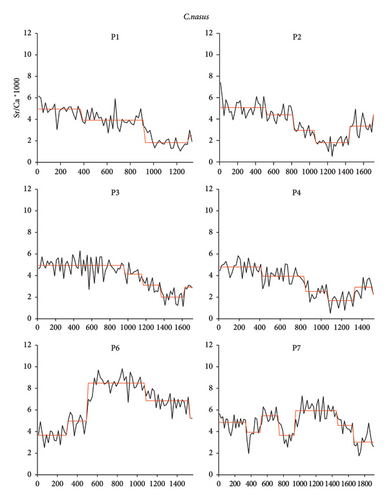
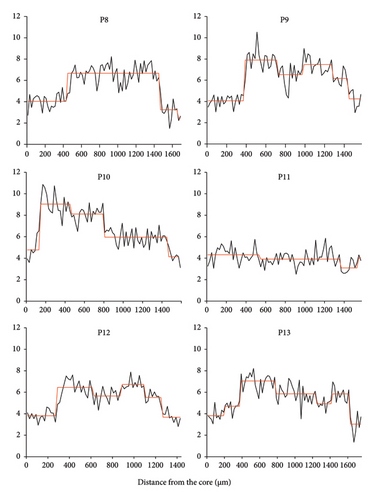
Excel2017 was used for data processing and SPSS17.0 was used for nonparametric test (Mann–Whitney U-test). The Sequential T-test Analysis of Regime Shifts (STARS) method was used for analysis of regime shifts,which means when more than one consecutive point changes significantly (based on the data variance and p value of the T-test), it is considered a “shift” and then generate a new moving average [11, 12]. In this paper, the truncation length was set to 10, Huber weight was set to 1, confidence P was set to 0.1 and the Sr/Ca value was normalized to Sr/Ca × 1000 according to the convention.
3. Results
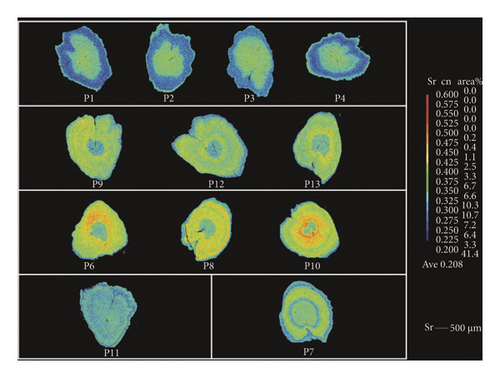
Line transect analysis showed (Table 2 and Figure 2) that the life history of C. nasus was different among individuals. In this study, the Sr/Ca value could be divided into distinct life history stages,with significant differences observed between adjacent stages (p < 0.05, the Mann–Whitney U-test) (Table 2). The results of the mapping analysis clearly delineate the habitat history of C. nasus (Figure 3). The core areas of P1, P2, P3, and P4 are initially green and progressively transition to blue as the individuals mature. In contrast, the core regions of P9, P12, and P13 are predominantly blue, while their peripheral zones remain green. The core regions of P6, P8, and P10 exhibit blue areas with low Sr values, and over time, distinct yellow and red rings form around the otoliths, signifying a shift to an environment with higher Sr values. Individual P11 displays a consistent blue coloration, whereas individual P7 shows alternating blue and yellow hues.
| Code | Significant fluctuation phases | Distance from the core | Detected points | Sr/Ca value × 103 |
|---|---|---|---|---|
| P1 | 1 | 0–880 | 46 | 4.38 ± 0.82a |
| 2 | 880–1320 | 22 | 2.04 ± 0.62b | |
| P2 | 1 | 0–800 | 41 | 4.87 ± 0.42a |
| 2 | 800–1700 | 46 | 2.60 ± 0.69b | |
| P3 | 1 | 0–1200 | 62 | 4.70 ± 0.86a |
| 2 | 1200–1700 | 25 | 2.45 ± 0.61b | |
| P4 | 1 | 0–860 | 44 | 4.31 ± 0.95a |
| 2 | 860–1500 | 32 | 2.23 ± 0.70b | |
| P6 | 1 | 0–480 | 25 | 4.16 ± 0.99a |
| 2 | 480–1360 | 44 | 8.02 ± 1.78b | |
| 3 | 1360–1540 | 15 | 5.48 ± 0.92c | |
| P7 | 1 | 0–480 | 25 | 4.73 ± 2.19a |
| 2 | 1120–1440 | 32 | 4.84 ± 0.76b | |
| P8 | 1 | 0–460 | 24 | 4.09 ± 1.29a |
| 2 | 460–1440 | 49 | 6.68 ± 0.76b | |
| 3 | 1440–1680 | 12 | 3.11 ± 1.16c | |
| P9 | 1 | 0–360 | 18 | 4.09 ± 0.95a |
| 2 | 360–1400 | 52 | 7.16 ± 1.23b | |
| 3 | 1400–1560 | 8 | 4.12 ± 0.83c | |
| P10 | 1 | 0–80 | 5 | 4.30 ± 1.33a |
| 2 | 80–780 | 33 | 8.57 ± 2.01b | |
| 3 | 780–1580 | 40 | 5.61 ± 1.21c | |
| P11 | 1 | 0–1560 | 78 | 4.01 ± 1.63 |
| P12 | 1 | 0–320 | 17 | 4.07 ± 1.37a |
| 2 | 320–1180 | 43 | 6.28 ± 1.21b | |
| 3 | 1180–1420 | 12 | 4.11 ± 1.02c | |
| P13 | 1 | 0–360 | 19 | 4.23 ± 1.22a |
| 2 | 360–1040 | 34 | 6.55 ± 1.13b | |
| 3 | 1040–1740 | 35 | 4.90 ± 1.92c | |
- Note: Phases in one otolith sample having the different letters indicate significant differences (p < 0.05).
4. Discussion
Changes in the ratio of strontium and calcium concentrations in otoliths have been extensively utilized in studies documenting the life history of fish [13–18]. However, there is limited literature specifically addressing C. nasus from the Yellow River. Historical records [2] indicate that the life cycle of C. nasus in the Yellow River was relatively straightforward: adult fish migrated to Dongping Lake via the Yellow River in April and May each year, spawned in June and July, and returned to the Yellow River estuary at the beginning of the following year after hatching. This suggests that the Shandong section of the Yellow River historically served as a crucial migration route for C. nasus, with Dongping Lake being the primary spawning ground. Recently, C. nasus has re-emerged in the Shandong section of the Yellow River; however, scholarly opinions remain divided regarding whether these populations exhibit migratory behavior. Some researchers argue that the destruction of spawning grounds has eliminated migratory populations in the lower reaches of the Yellow River. From the perspective of otolith microchemistry, the findings of this study provide an objective and accurate reflection of the habitat conditions of C. nasus in the Yellow River, offering a theoretical foundation for understanding the diverse life histories of C. nasus in this region. The results of this study demonstrate that the life history of C. nasus in Pangkou Bay is characterized by complexity. According to Yang [16], the Sr/Ca × 103 values in freshwater, brackish water and seawater differ significantly, with values < 3 (blue), 3–7 (yellowish green), and > 7 (yellowish red). The average Sr/Ca × 103 values were 8.3 ± 4.5 in seawater, 5.6 ± 1.1 in estuaries, and 2.7 ± 1.5 in freshwater [14–16]. However, Brown [18] reported that the Sr/Ca × 103 value in seawater was relatively stable (8.17–8.87), while the values in rivers (0.27–19.18) and freshwater lakes (0.20–5.02) fluctuated significantly. Microchemical analysis of three freshwater settlement types of C. nasus in the Yellow River Basin revealed that the otolith microchemical characteristics of C. nasus in the Yangtze River Basin and the Yellow River Basin differ markedly in response to environmental conditions. Specifically, the microchemical analysis of the freshwater settlement type in the three streams indicated that the Sr/Ca values in freshwater habitats were < 6 [17]. Therefore, this study redefined the otolith microchemical characteristics of C. nasus from the Yellow River Basin: freshwater habitats < 6, brackish water 6-7, and seawater > 7. These differences are attributed to regional water transfer projects, such as the east route of the South-to-North Water Diversion Project and the Water and Sediment Diversion Project, which have caused significant fluctuations in the water environment, leading to substantial differences in calcium and strontium content compared with the Yangtze River Basin.
The life history of C. nasus in Pangkou Bay was analyzed using mapping techniques, revealing five distinct patterns. P1, P2, P3, and P4 belong to Pattern I, characterized by hatching in brackish water near the Yellow River estuary, followed by migration along the Yellow River to Pangkou Bay for spawning after about 1 year. P9, P12, and P13 belong to Pattern II, where individuals hatch in freshwater habitats and undergo abrupt changes from Stage 1 to Stage 2. Studies [18] have shown that such abrupt changes with a clear trend are a primary feature of migratory types, but their life history does not extend to marine habitats with high Sr/Ca values; after about 1 year, they migrate along the Yellow River to Pangkou Bay to spawn. P6, P8, and P10 belong to Pattern III, similar to Pattern II, but with a significant presence of red and yellow concentric rings, indicating a history of seawater habitat and extension to Laizhou Bay. P11 belongs to Pattern IV, characterized by a relatively stable living environment with no obvious abrupt changes, suggesting a freshwater settlement type that may have entered the Yellow River passively during the flood season of Dongping Lake. P7 belongs to Pattern V, showing two trips to freshwater and brackish water habitats, both producing clear pattern changes,this indicates that it conducted a reproductive migration around the first year, swam to the Yellow River estuary after spawning, and migrated to Pangkou Bay during the reproductive period of the following year to lay eggs until capture.
In this study, the microchemical characteristics of Sr and Ca elements in C. nasus from Pangkou Bay were investigated using electron microprobe microanalysis technology. The results indicate that most individuals in Pangkou Bay are migratory types, with gonadal maturity at Stage V. Comparing sexual maturity with other waters in the Yangtze River Basin, Pangkou Bay is identified as an important spawning ground for the migratory type of C. nasus.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Xuri Cong: conceptualization, investigation, and writing the original draft; Guancang Dong: methodology development, otolith grinding and preparation, supervision, and project administration; Junpeng Wang: funding acquisition, resource allocation for otolith processing, and personnel coordination; Xiuqi Li: technical validation, formal analysis, and reviewing and editing; Yang Li: visualization and Figure 1 design and production; Yanan Wang: field sampling, specimen collection, and Figure 3 development; Chunmei Leng, Yunfang Gao, Xiuli Chen, Lufeng Sun, Qingqing Wang, and Zhaoming Gao: specimen acquisition, otolith extraction and preparation, and data curation; Yiping Ren and Tao Jiang: analytical methodology design, instrumentation provision and calibration, and experimental validation.
Funding
This work was supported by the Shandong Natural Science Foundation (ZR2024QC057), Shandong Natural Science Foundation (ZR2020MC193), Special project of the Ministry of Agriculture and Rural Affairs on the Yellow River (HHDC-2022-0403), Key Research and Development Program of Shandong (2021LZGC029), and Shandong Provincial Key Laboratory of Eco-Environmental Science for Yellow River Delta.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




