Stocking and Harvesting Patterns Influence Age Structure, Growth, and Body Condition of Bighead Carp Populations From Two Large Subtropical Reservoirs
Abstract
Understanding the density-dependent effects is essential for sustainable fishery management. Stocking and harvesting activities directly influence fish abundance and associated density-dependent responses. In this study, we compared the body size, age structure, growth patterns, and body condition of bighead carp (Hypophthalmichthys nobilis) from two large subtropical reservoirs in China: Shanmei Reservoir (SMR) (n = 161) and Liuxihe Reservoir (LXHR) (n = 257), which have different stocking and harvesting patterns. In SMR, annual stocking and harvesting maintain a lower bighead carp biomass (catch per unit effort, 0.62 ± 0.03 g/m2/24 h), whereas in LXHR, annual stocking without harvesting results in higher fish biomass (0.84 ± 0.01 g/m2/24 h). Plankton biomass and abundance, particularly zooplankton, were significantly higher in SMR than in LXHR. The SMR population exhibited a more stable age structure, faster growth, and better body condition, whereas the LXHR population showed the opposite trends under higher fish density. Both populations exhibited isometric growth and included individuals aged one to five, but dominant age groups differed. The SMR population had a larger inflection age and greater standard length (SMR: 4.7 years and 591 mm; LXHR: 3.5 years and 456 mm). In addition, total mortality, fishing mortality, and exploitation rates were higher in SMR than in LXHR. These findings highlighted the role of stocking and harvesting in shaping fish density and hence inducing density-dependent effects. A balanced fisheries strategy integrating both practices is crucial for sustainable fish population management.
1. Introduction
Density-dependent effects are a common phenomenon in natural populations, critically influencing population growth and persistence [1]. Fluctuations in population density affect individual survival and growth primarily through changes in resource availability, such as food or habitat [2, 3]. Density-dependent effects are influenced by multiple factors, primarily including the interaction between human activities [4], biological factors (e.g., resource availability and competition [5]), and environmental factors (e.g., climate change and water quality [6]). Human activities, especially stocking and harvesting, directly influence fish density in aquatic ecosystems [7]. Overharvesting or inappropriate stocking can lead to imbalances in population density, affecting the growth and competition dynamics [8, 9]. Overstocking may lead to high fish density and intensify competition for resources, resulting in slower growth rate and poorer body condition [10]. Conversely, overfishing can significantly reduce population density, simplify age structure, destabilize population dynamics, and threaten long-term sustainability [11, 12]. Environmental changes can also affect resource availability and modulate the strength of density-dependent effects [13]. For example, increased water temperature promotes fish metabolism, intensifying resource competition and enhancing negative density dependence in high-density populations, while potentially fostering growth and weakening these effects in low-density populations [14]. Decreased water levels may compress habitat space, intensifying competition [15]. Extreme weather events may disrupt habitats, increase mortality, and further complicate density-dependent effects [16]. In conclusion, a comprehensive understanding of density-dependent effects is crucial for sustainable fisheries management [4].
Bighead carp (Hypophthalmichthys nobilis) belong to the order Cypriniformes and the family Cyprinidae [17]. As one species of the four major Chinese carp [18], bighead carp had a total production of 3.3 × 106 tons in China, contributing 12% to the country’s total freshwater fishery output in 2022 [19]. Bighead carp inhabit pelagic zones and primarily feed on zooplankton and phytoplankton [20]. They are commonly stocked to increase fishery outcomes and control algal blooms in lakes and reservoirs [21, 22]. Due to their inability to spawn in lentic reservoir ecosystems, the population densities of bighead carp are mainly managed through stocking and harvesting activities [23]. Therefore, understanding and accounting for density-dependent effects is essential for optimizing fisheries management strategies. Despite the economic and ecological importance of bighead carp, current research primarily focuses on their age and growth [24, 25], whereas the effects of fisheries management strategies on their biological traits remain poorly understood.
China possesses the highest number of reservoirs globally (24,089; [26]). Among the most widely applied fishery models in these reservoirs are enhanced fisheries, particularly for silver carp (H. molitrix) and bighead carp. However, the stocking and management strategies vary significantly across different reservoirs [27]. Shanmei Reservoir (SMR) and Liuxihe Reservoir (LXHR), both large subtropical reservoirs located in southeastern China, illustrate two different stocking and harvesting patterns for bighead carp. In the SMR, bighead carp are annually stocked and harvested [28]. In contrast, bighead carp are also stocked annually, but harvesting has been thoroughly prohibited in the LXHR since 2017 due to its designation as a national aquatic germplasm resource conservation area for the endangered fish species Spinibarbus hollandi. The different management strategies make them ideal systems for analyzing how the biological traits of bighead carp respond to different stocking and harvesting patterns.
In this study, we compared the body size, growth patterns, age structure, body condition, mortality, and exploitation rate of bighead carp populations in the SMR and LXHR. We primarily focused on exploring the effects of stocking and fishing activities, as well as plankton resources, on the growth of bighead carp from both top-down (fishing pressure) and bottom-up (food resources) perspectives. We hypothesized that the bighead carp population in SMR with regular stocking and harvesting would have a lower density, faster growth, better body condition, and more stable population dynamics according to the theory of density dependence.
2. Materials and Methods
2.1. Study Area
SMR and LXHR are located in subtropical areas of China (Figure 1). Both reservoirs have large capacities and areas and are vital drinking water sources for downstream residents, but they differ in fisheries stocking and harvesting patterns (Table 1). In the SMR, bighead carp are annually stocked and harvested, while in the LXHR, bighead carp are likewise stocked annually, but not harvested. Due to the fishing ban in LXHR, our sampling was approved by the management office. Similarly, sampling in SMR was carried out with authorization from the reservoir management authority.
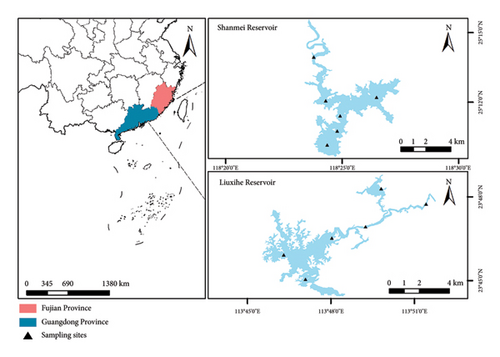
| Reservoir | Total volume (×108m3) | Water-spread area (km2) | Mean depth (m) | Stocking (×104 ind.) | Harvesting (t) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2021 | 2022 | 2018 | 2019 | 2020 | 2021 | 2022 | ||||
| SMR | 6.55 | 23.75 | 28 | 63.9 | 77.3 | 40.2 | 65.3 | 70.0 | 355.7 | 218.1 | 150.9 | 128.8 | 145.0 |
| LXHR | 3.25 | 15.25 | 21 | 83.5 | 24.4 | 22.0 | 0.60 | 0.15 | 0 | 0 | 0 | 0 | 0 |
2.2. Fish Sampling and Data Collection
According to official records, the initial body weight (BW) of the bighead carp stocked was approximately 150–250 g in both reservoirs (unpublished data). From April to July 2022, we conducted monthly fish sampling using the same experimental gillnets in both reservoirs. The gillnets, measuring 50 m in length and 5 m in height, consisted of four different mesh sizes (8, 10, 12, and 14 cm, stretched). At each of the six designated sampling sites per reservoir, we deployed four gillnets (totaling 200 m in length) for 12 h each night (18:00–19:00 to 6:00–7:00). To improve our capture efficiency, this sampling procedure was repeated at each site over the next 2 days, resulting in a cumulative sampling duration of 36 h per site. Catch per unit effort (CPUE) was estimated based on the biomass (BPUE, g/m2/24 h) and abundance (NPUE, ind./m2/24 h), representing the total biomass and number of individuals captured per unit effort, respectively.
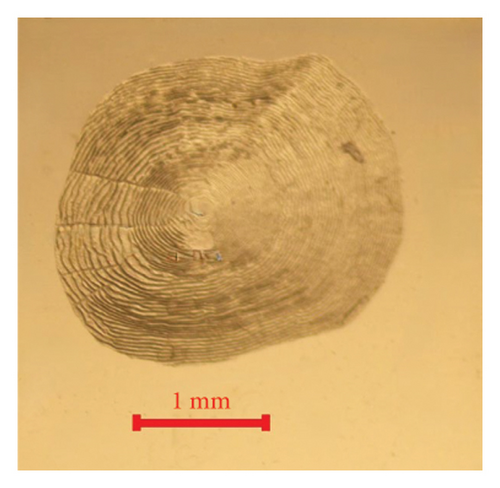
We measured the standard length (SL) and BW of all freshly caught fish with precision to the nearest 1 mm and 1 g, respectively. Then, all samples were euthanized by immersion in about 250–350 mg/L of tricaine methanesulfonate (MS-222). In addition, we collected 5–10 scales below the pectoral fin, which were stored in scale clips for subsequent analysis. The scales were soaked in a 50 mL 10% NaOH solution for approximately 5 min to remove any adhering tissues and then rinsed with distilled water. Using a dissecting microscope (Olympus CX33), we determined the fish ages by examining the cleaned scales (Silva and Stewart, 2006). We read at least three scale samples from each individual, and each scale was read twice to ensure the accuracy of the results. We randomly dissected subsamples of 88 and 150 individuals to obtain the eviscerated BW (WE) from the SMR and LXHR Reservoirs, respectively. In addition, at each sampling site, we used a YSI Pro Plus multiparameter water quality analyzer to record the water temperature (T, °C) at a depth of 1 m around midday. We also collected samples of zooplankton and phytoplankton at each site, determining their respective densities and biomasses according to the methods described by Zhang and Huang [29].
2.3. Data Analysis
We applied the Shapiro–Wilk test to assess data normality and Levene’s test to evaluate the homogeneity of variances. We used nonparametric tests (Mann–Whitney U test) for variables that did not meet the normality assumptions to reduce bias, and parametric tests (t-test) for variables that were normally distributed or became normally distributed after transformation. Bonferroni correction was applied for multiple comparisons. To compare the differences in CPUE, SL, and growth rate between the two reservoirs, we first used the Shapiro–Wilk test to assess data normality. Since the data did not follow a normal distribution, we conducted the Mann–Whitney U test. Then, we assessed the similarity of length frequency distributions between the two populations with the Kolmogorov–Smirnov test. We also applied chi-square tests to evaluate the differences in age structures of the bighead carp populations. Since there was only one sample for the 5-year-old group in SMR, we analyzed SL differences for the remaining four age groups using the Mann–Whitney U test. We log-transformed (log [x + 1]) the plankton biomass and density data to achieve a normal distribution, and then compared the means between the reservoirs using an independent samples t-test. A significance level of 0.05 was used.
We established the length–weight relationship using an exponential regression equation (BW = aSLb [30]), where a is the proportionality constant and b is the exponent of the power. To determine the growth pattern, we analyzed the b values according to Ricker [30] and conducted a t-test to assess whether these values significantly deviated from three [31]. We estimated the growth constant (K) and the asymptotic SL (L∞) based on SL-at-age data collected over 4 months using FAO-ICLARM Fish Stock Assessment Tools (FiSAT II), Version 1.2.2 [32]. We determined t0 using the formula, ln(−t0) = −0.3922 – 0.2752ln (L∞) − 1.038ln (K), proposed by Pauly [33], and it fitted the von Bertalanffy growth equation (Lt = L∞[1–e−K(t − t0)]), where K is the growth factor and Lt is the predicted SL of t-aged fish [34]. We calculated the growth rates and growth acceleration equations for the two bighead carp populations [35]. In addition, we calculated the condition factor (CF) using CF = 100WE/(SL/10)3 [36], where WE is the eviscerated BW.
We employed the length-converted catch curves method to estimate the total mortality coefficient (Z, [37]) using FiSAT II. We calculated the natural mortality constant (M) by the empirical formula: ln (M) = −0.0066 − 0.279ln (L∞) + 0.6543ln (K) + 0.6463ln (T) [38]. In the equation, T denotes the average water temperature of the two reservoirs during our sampling (SMR: 25.6°C; LXHR: 26.2°C). The fishing mortality constant (F) and the population exploitation rate (E) were calculated by F = Z – M and E = F/Z, respectively.
We performed all statistical analyses in R (V.4.0.2, [39]) using packages, including MASS (t-test, [40]) and stats (chi-square tests, [39]). All figures were created using the ggplot2 package [41].
3. Results
3.1. Fish Abundance and Plankton Availability
The NPUE of bighead carp in the SMR (0.004 ± 0.0006 ind./m2/24 h) was significantly lower than that in the LXHR (0.007 ± 0.001 ind./m2/24 h, Mann–Whitney U test, W = 594, p < 0.05). Similarly, the BPUE of bighead carp in the SMR (0.62 ± 0.03 g/m2/24 h) was significantly lower than that in the LXHR (0.84 ± 0.01 g/m2/24 h, W = 796, p < 0.001). In contrast, both the density and biomass of phytoplankton and zooplankton were significantly higher in the SMR compared to the LXHR, respectively (Table 2).
| Reservoir | Zooplankton | Phytoplankton | ||
|---|---|---|---|---|
| Biomass (mg/L) | Density (ind./L) | Biomass (mg/L) | Density (× 106 cells/L) | |
| SMR | 3.33 ± 1.63 | 3229.1 ± 1362.1 | 2.56 ± 0.42 | 20.2 ± 0.28 |
| LXHR | 0.41 ± 0.02 | 1137.6 ± 170.2 | 1.38 ± 0.30 | 7.39 ± 0.21 |
| t = 4.60 | t = 2.12 | t = 2.45 | t = 4.37 | |
| p < 0.001 | p < 0.05 | p < 0.05 | p < 0.05 | |
3.2. Length Frequency Distribution and Age Structure
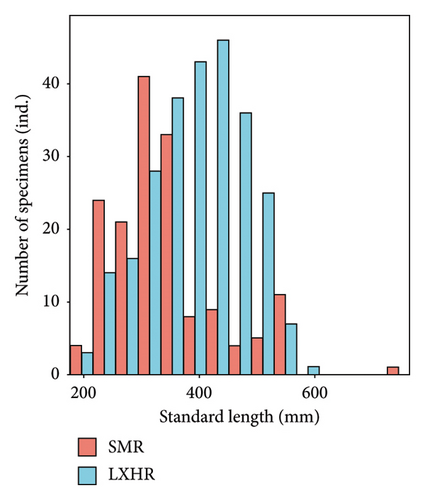
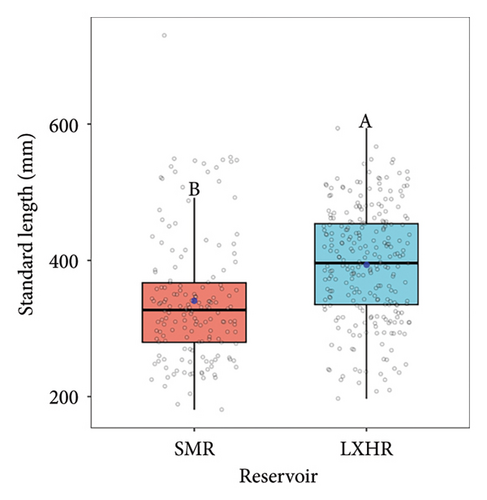
We measured 161 and 257 bighead carp individuals in SMR and LXHR, respectively. In the SMR, the SL of bighead carp ranged from 181 to 730 mm, with the dominant length class (250–450 mm) comprising 73.3% of the total. In the LXHR, the SL ranged from 197 to 594 mm, with the dominant length class (300–500 mm) accounting for 75.5% of the total (Kolmogorov–Smirnov test, D = 0.3, p < 0.001; Figure 2(a)). The mean SL of bighead carp in the SMR (341 ± 7 mm) was significantly smaller than that in the LXHR (394 ± 5 mm, Mann–Whitney U test, W = 12892, p < 0.001; Figure 2(b)).
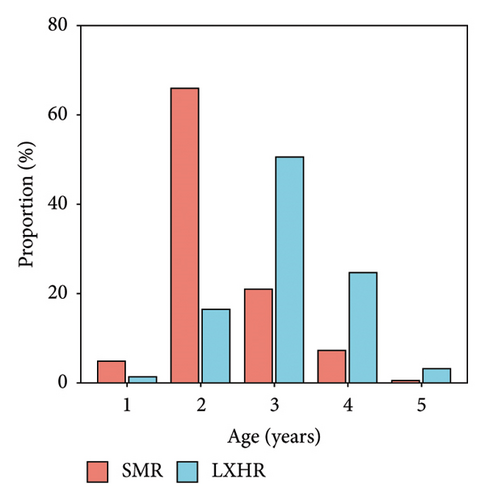
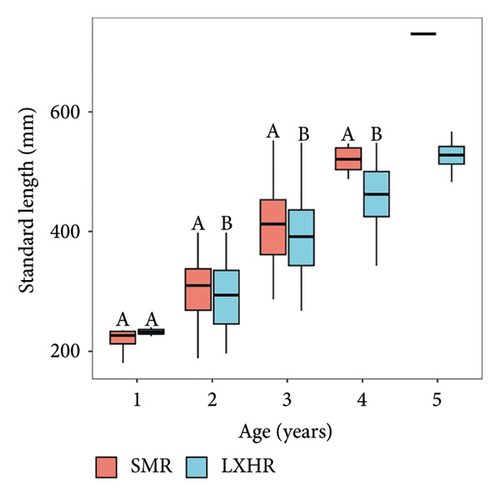
The age structures of both bighead carp populations consisted of ages 1–5 (Figure 3). In the SMR population, the two-year-old age group was the most dominant one, accounting for 65.8% of the population, whereas in the LXHR population, the three-year-old age group was the most dominant, comprising 51.3% (X2 = 114.25, p < 0.001; Figure 4(a)). The mean SL of most age groups were significantly higher in the SMR compared to the LXHR (Figure 4(b)).

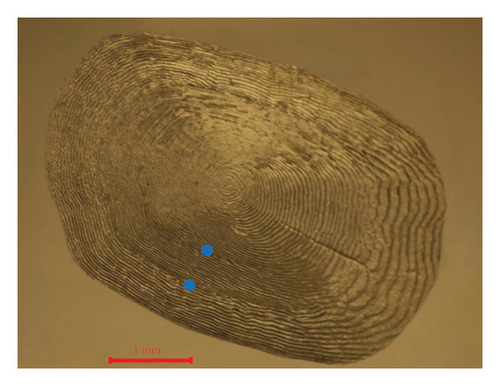
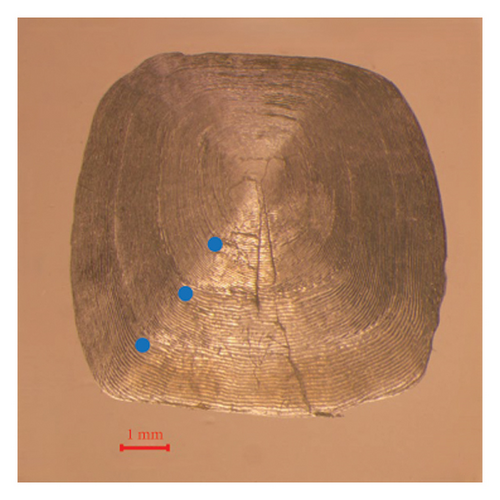
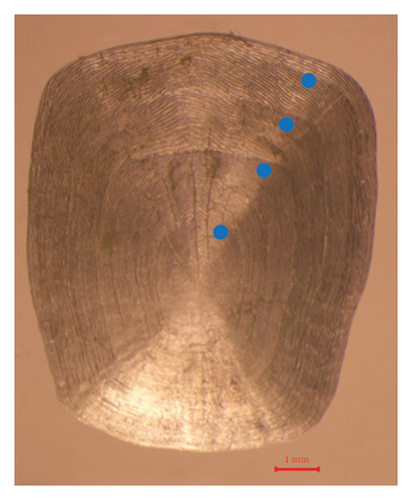
3.3. Growth Pattern and Body Condition
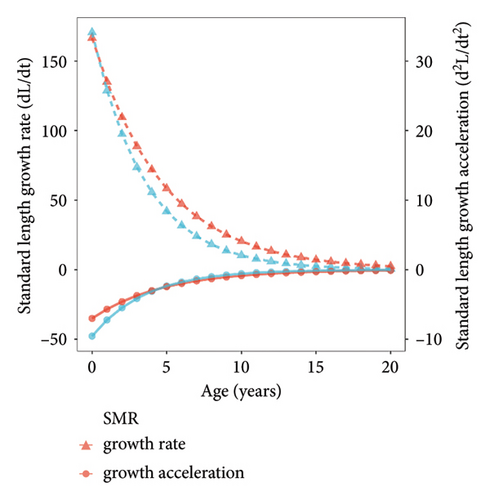
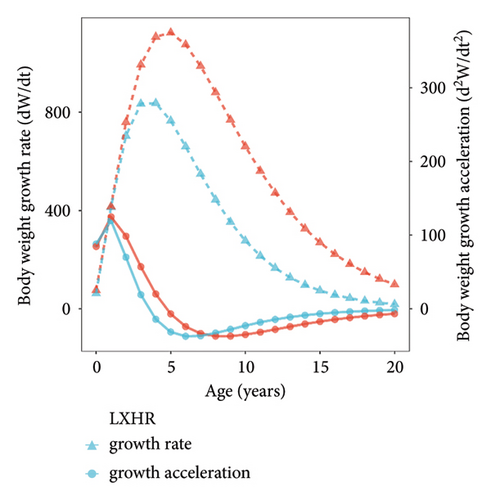
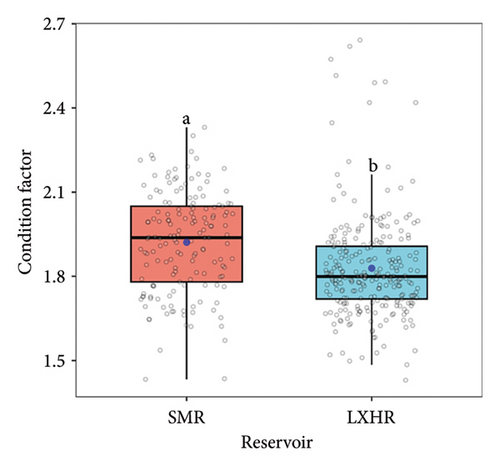
We summarized the SL–BW relationship and the von Bertalanffy growth equation of both populations (Table 3). The b values of the SL–BW relationship were close to three in both reservoirs (SMR: b = 2.98, t = 0.51 < t(0.05, 159); LXHR: b = 3.01, t = 0.24 < t(0.05, 255)). The mean SL growth rate was significantly higher in the SMR population compared to the LXHR population (Mann–Whitney U test, W = 1613, p < 0.05; Figure 5). In addition, in the SMR, the age and SL at the inflection point of the BW were 4.7 years and 591 mm, respectively, and both values were higher than those in the LXHR (3.5 years and 456 mm). The Z, F, and E values of the SMR population were 1.18, 0.94, and 0.80, respectively, and the three metrics also were higher than those of the LXHR population (0.48, 0.17, and 0.35; Table 4). The mean CF of the SMR population (1.76 ± 0.20) was significantly higher than that of the LXHR population (1.66 ± 0.14) (Mann–Whitney U test, W = 8863, p < 0.001; Figure 6).
| Reservoir | Length–weight relationship | VBGF equation | Standard length growth rate | Standard length growth acceleration |
|---|---|---|---|---|
| SMR | BW = 2 × 10−4SL2.98 (R2 = 0.9697) | Lt = 886.78[1–e−0.21(t+0.53)] | dLt/dt = 186.22e−0.21(t+0.53) | d2Lt/dt2 = −39.11e−0.21(t+0.53) |
| LXHR | BW = 2 × 10−4SL3.01 (R2 = 0.9618) | Lt = 684.96[1–e−0.28(t+0.42)] | dLt/dt = 191.79e−0.28(t+0.42) | d2Lt/dt2 = −53.70e−0.28(t+0.42) |
| Biological parameters | SMR | LXHR |
|---|---|---|
| Asymptotic length (L∞, mm) | 886.8 | 685.0 |
| Growth factor (K) | 0.21 | 0.28 |
| Age at the inflection point of BW | 4.7 | 3.5 |
| SL at the inflection point of BW (mm) | 591.1 | 456.4 |
| Total mortality (Z/year) | 1.18 | 0.48 |
| Natural mortality (M/year) | 0.24 | 0.31 |
| Fishing mortality (F/year) | 0.94 | 0.17 |
| Population exploitation rate (E/year) | 0.80 | 0.35 |
- Abbreviations: BW, body weight; SL, standard length.
4. Discussion
Negative density dependence is primarily caused by high population density, which limits the availability of food resources for each individual, thereby impacting growth, age structure, and body condition [1]. Our study analyzed the differences in body size, age structure, growth pattern, and body condition of bighead carp populations from two large subtropical reservoirs with different fishery management strategies. We found that different stocking and harvesting patterns affected fish density and food availability and further influenced age structure, growth rate, and body condition through triggering density-dependent effects. Specifically, the population at lower density with abundant food resources exhibited a more stable age structure, faster growth rate, and better body condition compared to the population at a higher density with fewer food supplies (Figure 7).
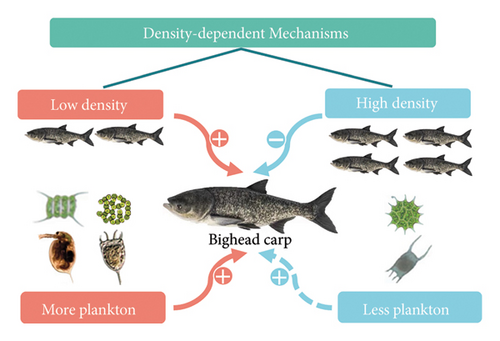
Our study suggested that stocking and harvesting patterns play a critical role in mediating density-dependent effects on fish growth and body condition. Both bighead carp populations exhibited isometric growth, consistent with other studies in Chinese reservoirs, such as Yankou Reservoir [42] and Chitan Reservoir [43]. However, significant differences were observed in the growth rate and body condition between the two reservoirs. In SMR, the bighead carp population exhibited a faster growth rate and better body condition, aligning with the findings from Qingcaosha Reservoir [44]. In SMR, regular harvesting may reduce population density and alleviate intraspecific competition, which likely creates favorable conditions for rapid growth. Moreover, higher plankton densities, particularly zooplankton, certainly favored by lower fish densities, may further enhance growth in SMR [45]. In contrast, the fishing ban in the LXHR resulted in a higher population density, similar to the Jinshahe Reservoir (high stocking density, [46]). High fish density increases intraspecific competition, leading to a decline in growth rate and body condition [47]. Although we observed a faster growth rate in SMR, the average SL and growth factor (K) of bighead carp were lower compared to those in LXHR. The smaller size of individuals in SMR was likely due to the high fishing pressure, reflected by the fishing mortality rate of 0.94. This high fishing pressure likely contributed to the miniaturization of the population [48, 49]. The growth factor (K) of the fish was likely shaped by various factors, including water temperature, food availability, water quality, population density, fishing pressure, and habitat conditions, acting independently or interactively [50]. In SMR, intensive fishing reduced the number of large individuals, resulting in a population dominated by smaller individuals. This shift limited the overall growth potential, leading to a smaller growth factor [51].
Fish growth varies significantly across regions and water bodies, driven by hydrological conditions, nutrient dynamics, food availability, population density, and fishing pressure [25, 52]. We conducted a comparative study on the growth of bighead carp in different reservoirs and lakes located in different regions in China (Table 5, [22, 42–44, 46, 53–59]). When compared to populations in northern reservoirs, bighead carp in southern reservoirs usually grow faster, reach their growth inflection points earlier, and have a younger inflection age. These differences are largely attributed to variations in latitude and climate [60]. In northern reservoirs, higher latitudes and lower water temperatures slow the growth rates of bighead carp. In this study, both SMR and LXHR are located in southern China, where food resource availability plays a crucial role in growth [61]. Abundant food, especially in eutrophic waters with rich plankton resources, enhances growth and results in larger asymptotic lengths [62]. The higher plankton density and biomass in SMR, particularly the abundant zooplankton, contributed to the larger asymptotic length of bighead carp compared to LXHR. Furthermore, bighead carp in lakes tend to have higher inflection point ages than those in reservoirs. This may be due to the commonly higher plankton density and biomass in lakes, which provide more stable and abundant food sources for bighead carp [25].
| Reservoir/lake | N | K | L∞ (mm) | t | b | Growth pattern | Age structure (/year) | Age identification materials | Trophic state | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Wudalianchi1 | 223 | 0.14 | 976 | 7.6 | 2.90 | Isometric | 4–9 | Scale | Mesotrophic | [53] |
| Hongqipao Reservoir1 | 160 | 0.18 | 812 | 6.2 | 2.89 | Isometric | 1–6 | Scale | — | [54] |
| Biliuhe Reservoir1 | 693 | 0.25 | 941 | 5.7 | 2.88 | Isometric | 2–8 | Scale + fin ray | — | [55] |
| Mengjiaduan Reservoir1 | 43 | 0.16 | 810 | 5.4 | 2.69 | Isometric | 2–10 | Scale + fin ray | Oli-mesotrophic | [56] |
| Nihe Reservoir1 | 278 | 0.09 | 1494 | 2.94 | Isometric | 1–3 | Scale | Eutrophic | [57] | |
| Qingcaosha Reservoir2 | 59 | 0.17 | 921 | 5.0 | 2.95 | Isometric | 1–11 | Scale + vertebrae | Mesotrophic | [44] |
| Yankou Reservoir2 | 37 | 0.32 | 733 | 3.3 | 3.07 | Isometric | 2–7 | Scale | — | [42] |
| Jinshahe Reservoir2 | 53 | 0.40 | 685 | 3.0 | 2.87 | Allometric | 2–6 | Scale | Oli-mesotrophic | [46] |
| Chitan Reservoir2 | 62 | 0.19 | 684 | 4.9 | 2.97 | Isometric | 2–6 | Scale | Mesotrophic | [43] |
| Shanmei Reservoir2 | 161 | 0.21 | 887 | 4.7 | 2.98 | Isometric | 1–5 | Scale | — | This study |
| Liuxihe Reservoir2 | 257 | 0.28 | 685 | 3.5 | 3.01 | Isometric | 1–5 | Scale | — | This study |
| Jili Lake1,∗ | 68 | 0.14 | 1207 | 8.1 | 2.96 | Isometric | 1–8 | Scale | — | [22] |
| Ge Lake2,∗ | 153 | 0.16 | 1095 | 5.7 | 2.94 | Isometric | 2–9 | Scale | Eutrophic | [58] |
| Hongfeng Lake2,∗ | 243 | 0.13 | 966 | 7.1 | 2.70 | Isometric | 1–4 | Scale | — | [59] |
- Note: N, number of specimens; K, growth factor; L∞, asymptotic length; t, growth inflexion point age; b, growth ratio. The reservoirs and lakes in the table are arranged in order of latitude, from north to south areas.
- ∗Lakes.
- 1Reservoirs located in northern China.
- 2Reservoirs located in southern China.
Both bighead carp populations comprised of only five age groups. However, the two-year-old group was the most dominant in SMR, while the three-year-old group predominated in LXHR, which also has a higher proportion of older individuals. Some previous studies found that bighead carp populations exhibit more complex age structures in other subtropical reservoirs [44, 46]. Such a difference may be related to the use of fishing gear. Other reservoirs combined various gear types and also employed light trapping, which could have influenced the distribution of the age structure of fish populations. The inflection age, which marks the point where the fish growth rate reaches its peak [35], was higher in SMR (4.7 years) compared to LXHR (3.5 years). This suggested that the SMR population may possess a more stable age structure despite its higher fishing pressure. Compared to other reservoirs, the inflection ages of bighead carp in SMR and LXHR are lower than those in Biliuhe Reservoir (5.7 years, [55]) and Mengjiadun Reservoir (5.4 years, [56]) but higher than those in Jinshahe Reservoir (3.0 years, [46]). These differences may reflect variations in fishing pressure, population density, locations, and food availability [46, 55, 56]. In this study, the majority of bighead carp individuals are younger than the growth inflection ages in both reservoirs. In SMR, this is likely due to the high fishing pressure, while in LXHR, the fishing ban likely led to the high population density, intensifying competition for food and space and limiting individual growth [63]. A more complex and balanced age structure is essential for population stability and sustainable development. Optimizing stocking and harvesting patterns would improve fisheries management and foster long-term sustainability.
Density-dependent effects play a crucial role in shaping population dynamics, particularly mortality and exploitation rates [64, 65]. We found that the total mortality and fishing mortality of the bighead carp population in SMR were higher than those in the LXHR. Specifically, the fishing mortality in SMR was 0.94/year, accounting for nearly 80% of the total mortality, indicating that fishing is the primary driver of fish mortality [66]. This was also consistent with the long-term and regular harvesting practices implemented over the years in SMR. In contrast, despite the implementation of a fishing ban in LXHR, the bighead carp population had a fishing mortality rate of 0.17/year due to the inevitability of some illegal fishing activities. The natural mortality in SMR was observed to be lower than that in LXHR. Such discrepancy likely emerges because fishing activities in SMR reduce population density, alleviating density-dependent effects, which subsequently decreases competition for resources and reduces natural mortality rates [66]. In LXHR, the higher population density intensified competition for food and habitat, leading to a higher natural mortality rate [10]. According to Peter [67], an optimal exploitation rate (E) of 0.5 is applied as a key benchmark for sustainable resource utilization. The population exploitation rates (E) of 0.80 in SMR and 0.35 in LXHR reflect different levels of population exploitation, with SMR experiencing a higher exploitation. The elevated exploitation rate in SMR suggested that continued intensive harvesting may threaten the long-term sustainability of bighead carp populations [8]. Conversely, the lower exploitation rate in LXHR indicates a lower sustainability risk, allowing the population to maintain itself more effectively [68]. However, despite the comprehensive fishing ban in the LXHR maintaining a low exploitation rate, high population density has increased interspecific competition, negatively affecting fish growth and survival [69]. Thus, continuous monitoring and effective management are crucial for maintaining the sustainability of the populations in both reservoirs.
Our results demonstrated that different stocking and harvesting patterns can significantly affect the age structure, growth pattern, and body condition of the bighead carp population. These effects are primarily driven by variations in fish abundance and food resource availability, further highlighting the important role of density-dependent effects in fisheries management. This is particularly relevant when formulating stocking and harvesting strategies for economically important fish species. Based on our findings, we recommend that reservoir managers implement adaptive stocking and harvesting strategies to optimize density-dependent effects and ensure the sustainability of fish populations. Specifically, for SMR, it is advisable to reduce fishing pressure, particularly on larger individuals, to prevent population miniaturization. For LXHR, adjusting stocking levels to avoid excessive population density and alleviate intraspecific competition is recommended. Regular monitoring of population density, age structure, and body condition is crucial for timely adjustments in stocking densities and fishing. Balancing stocking and harvesting patterns to optimize the density-dependent effects is essential for the sustainable management of reservoir ecosystems.
In our study, we primarily focused on exploring the effects of stocking and fishing activities, as well as plankton biomass, on the growth of bighead carp from both top-down (fishing pressure) and bottom-up (food resources) perspectives. The productivity of reservoirs is influenced by multiple factors, such as climate conditions, reservoir topography, catchment area runoff, and nutrients, which can further impact the age structure and growth of fish. In addition, the relatively short sampling period likely affected the results. Future research incorporating more influencing factors and extending the sampling period are needed to improve the comprehensiveness and reliability of the findings.
Ethics Statement
We confirm that the research did not involve endangered or protected species. All the procedures described in this study were in accordance with ethical standards (Guidance options on experimental animals) of the Ministry of Science and Technology of the People’s Republic of China. The Institute of Hydrobiology, Chinese Academy of Sciences, reviewed and approved the study (approval file order: IHB-LL-2022047).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors read and approved the final manuscript. Xuemei Chen: writing–original draft, methodology, formal analysis, data curation, and conceptualization. Lei Yang, Hang Zhang, and Xiang Ji: investigation and data curation. Chuansong Liao, Tanglin Zhang, Chuanbo Guo, and Jiashou Liu: writing, review, editing, methodology, supervision, and conceptualization. Thomas Mehner: writing, review, editing, and methodology. Chuansong Liao and Jiashou Liu: supervision and funding acquisition.
Funding
This study was funded by the National Key Research and Development Program of China (2023YFD2400900), the Earmarked Fund for China Agriculture Research System (CARS-45), and the Reservoir Fishery Resources Survey and Water Purification Fishery Development Planning Project of Quanzhou Shanmei Reservoir Water Resources Deployment Center (I-2019-11-2).
Open Research
Data Availability Statement
Data are available from the authors upon request.




