Size Frequency, Length–Weight Relationships, and Condition Factors of the Four Introduced Fish Species in Geray Reservoir, Northwest Ethiopia
Abstract
Studying size frequency, size relationships, and the conditions of fish is vital for managing the resources properly. Some biological aspects of the fishes in Geray Reservoir were studied from October 2017 to August 2018. Fish were sampled monthly by overnight setting of gillnets. Fishers’ catch was also used. Fish were dissected and sexed. Biological parameters were measured following standard procedures. Four fish species (Oreochromis niloticus, Tilapia rendalli, Carassius auratus, and Cyprinus carpio) belonging to the Cichlidae and Cyprinidae families were identified. From the total 570 fish specimens collected, 426 (74.7%), 66 (11.6%), 44 (7.7%), and 34 (6.0%) were O. niloticus, T. rendalli, C. auratus, and C. carpio, respectively. In Geray Reservoir, most of the sampled fish specimens were found in length intervals of 24–27, 18–24, 24–32, and 41–49 cm for O. niloticus, T. rendalli, C. auratus, and C. carpio, respectively. Length–weight relationships of O. niloticus, T. rendalli, C. auratus, and C. carpio were curvilinear and statistically significant (p < 0.05). For all introduced fish species, b values were less than 3, negative allometry growth. The mean Fulton condition factor of O. niloticus, T. rendalli, C. auratus, and C. carpio was > 1 and showed significant variation (p > 0.05) among months. In Geray Reservoir, all four economically important fish species are fully established and contribute significantly to local livelihoods. However, in this sampling scheme, the number of catches of T. rendalli, C. auratus, and C. carpio deserve due attention for the management of the exotic fish species. The data presented in this work could be vital for further biological investigations, helping researchers to assess the status of the introduced fishes in the reservoir and for local fish managers to select appropriate management strategies.
1. Introduction
Ethiopia is the water-tower of East Africa and has many inland water bodies meant for its socio-economic development. The major lakes, reservoirs, and small water bodies cover an area of 7,740, 1,447, and 4450 km2, respectively [1]. In addition, rivers cover 8065 km in total length (TL) [1].
Ethiopian inland freshwater bodies have a rich diversity of ichthyofauna [2–4]. The fish species contain a mixture of Nilo-Sudanic, East African, and Endemic of the Ethiopian highland fish fauna [2, 5]. In addition to the indigenous fishes, about 11 exotic fish species have been introduced to different water bodies, particularly in the reservoirs [1], contributing a lot to food security and means of income for local communities. In this regard, exotic fish species such as Carassius auratus, Cyprinus carpio, Tilapia zillii, Tilapia rendalli, and Oncorhynchus mykiss have been introduced in different years, mainly to increase fish production in the nation [2, 6].
According to Tesfaye and Wolff [1], the potential yield of the nation is 94,500 tons/year (39,262 tons from lakes, 7,879 tons from reservoirs, 25,996 tons from small water bodies, and 21,405 tons from riverine systems). However, the nation’s per capita consumption is very low (i.e., about half of a kilo) as compared to the rest of the world and Africa. Therefore, boosting the fisheries sector is one of the key agendas of the nation as well as the region through fish farming and introductions. In this regard, the ever-increasing reservoirs have great potential to increase quality animal food production.
In Ethiopia, many investigations on fish ecology have been conducted on lakes and rivers, but less attention is given to reservoirs, particularly the exotic fish species. Several reservoirs and weirs have been built to divert and store water for irrigation and hydropower generation. Although these infrastructures are barriers to the free movement of fish, they create wonderful opportunities for new fisheries via the indigenous stocks in the system or introductions. Thus, more food production will be available to ensure the nutritional security of the ever-increasing human population.
A cheap source of protein is urgently required to combat food and nutritional insecurities, especially stunting in the country. Aquatic resources offer one of the best solutions to these gaps in the area and country. Reservoir fisheries will, thus, receive the necessary amount of attention to improve local livelihoods and support the country’s socioeconomic development. Furthermore, reservoirs could be used for comparative socioeconomic and biological studies due to their variability in their features and geographical distributions.
Geray Reservoir is a relatively old reservoir mainly built for irrigation in the 1970s by the then Ethiopian government [7]. There is a great interest in integrating irrigation with fish harvest as the reservoir is situated in good agro-climatic conditions for fish production. For such intent and long years before, multiple fish introductions of indigenous and exotic origin have been carried out in the Sebeta National Fishery and Aquatic Life Research Center to boost fish production. Consequently, fishing began a few years ago, and the fish that were caught were used for household consumption. More recently, fish cooperatives have been formed, selling their harvest to local markets like Finote Selam and Jiga towns. The reservoir has also been used as a source for fingerling production for the region’s agricultural extension programs. Despite the introduction of fish species and ongoing fisheries, limited reports have been made to assess the status of the existing fish stocks in the reservoir.
Studies on the biology of fishes are vital to making wise decisions, i.e., sound management of the existing resources. Information on length–weight relationships and condition factors can help obtain weight/biomass estimates, growth, survival, maturity, reproduction, and health conditions; identify adaptation strategies; and model the population dynamics, i.e., stock assessment. However, such information is limited in the Geray Reservoir. Determining the length–frequency distribution, length–weight relationships, and condition factors of introduced fishes in the Geray Reservoir was the aim of this study. This study is the first attempt to explore the details of some biological aspects of introduced fishes in Geray Reservoir.
2. Materials and Methods
2.1. Description of the Study Area
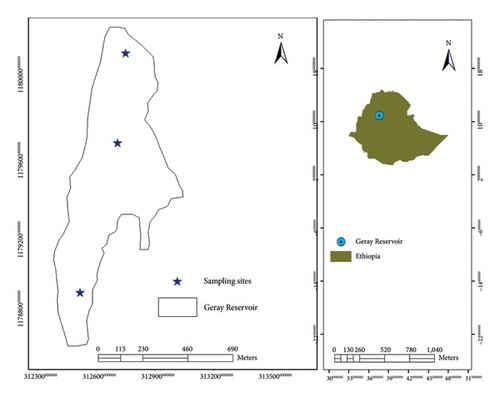
Geray Reservoir is a small manmade impoundment in Northwest Ethiopia. It was formed by impounding the Geray Stream in the 1970s (Figure 1). It is 365 km, 190 km, and 5 km away from Addis Ababa, Bahir Dar, and Finote Selam cities, respectively. It lies at 10°60′ N, 37°36′ E, and 1802 m a.s.l. [8]. The reservoir has 10 ha area and 4 m mean depth with 8 m maximum [8].
2.2. Site Selection
Fish specimens were collected from three sampling sites (inlet, open water, and outlet), representing the reservoir. The nature of the habitats, vegetation cover, gillnet suitability, water velocity, and depths were considered when selecting the specific sampling sites.
2.3. Sampling
Fish specimens were collected monthly (first week of the month) from October 2017 to August 2018, by setting multifilament gillnets with 6, 8, 10, 12, and 14 cm stretched bar mesh, 25 m length, and 1.5 m depth parallel to the shoreline. In addition, fishers’ catch was also included to get enough samples of different species and sizes. Due to the small size of the reservoir, all the fish specimens caught were pooled.
All fish specimens were sorted out and identified at the species level using keys developed by different authors [4, 9, 10].
2.4. Length–Frequency and Length–Weight Relationships
All the specimens were measured for TL, fork length (FL), standard length (SL), total weight (TW), and gonad weight (GW) using a measuring board to the nearest centimeters (cm) and a sensitive balance to the nearest 0.1 g (g) precision of lengths and weight, respectively. Each fish specimen was dissected and sexed.
In addition, the 95% confidence limits a and b of the regression model were reported [14, 15].
2.5. Fulton’s Condition Factor (FCF)
The well-being of the existing fish was studied using FCF [12, 13].
2.6. Data Analysis
Data was analyzed using SPSS version 23 and Microsoft Excel. Some descriptive statistics were used to summarize and analyze the primary data collected through monthly sampling. The coefficient of determination (r2) was used to assess the goodness of fit of a regression model of the length–weight relationship. One-way ANOVA was used to assess the statistical significance of the regression model. A t-test at p < 0.05 was employed to assess whether the value of b equals 3 or not. The nonparametric Mann–Whitney U test was used to test the FCF variations between sexes. In addition, the FCF values among the different months were compared for the introduced fish species, having more than three sample sizes using both one-way ANOVA (p < 0.05) and the Kruskal–Wallis test, followed by post hoc Dunn’s test.
3. Results
3.1. Types and Length–Frequency Distribution of the Introduced Fish Species
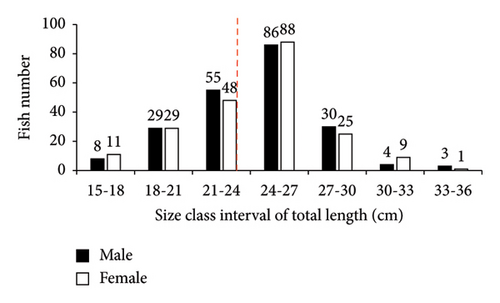
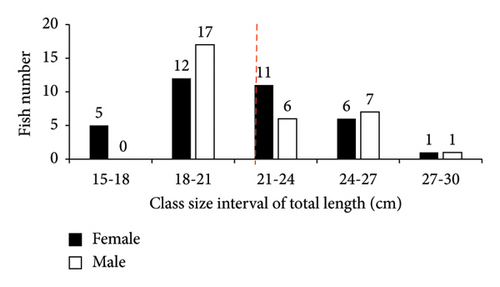
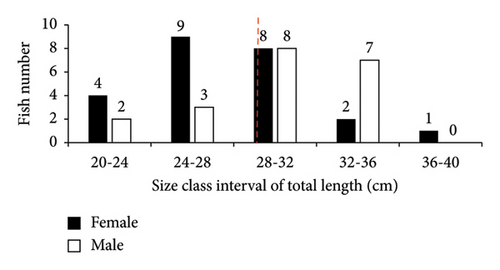
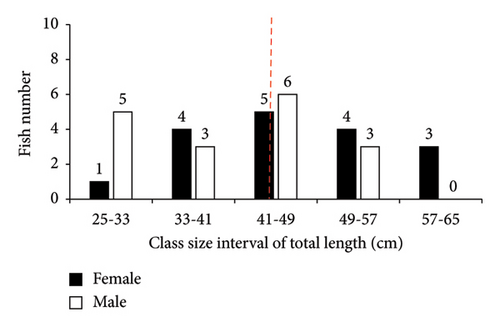
In this study, only two families (Cichlidae and Cyprinidae), each with two species (O. niloticus, T. rendalli or Coptodon rendalli, C. auratus, and C. carpio) were identified as well-established fish species in Geray Reservoir since the 1980s. A total of 570 fish specimens were collected. Among these, O. niloticus, T. rendalli, C. auratus, and C. carpio constituted 426 (74.7%), 66 (11.6%), 44 (7.7%), and 34 (6.0%), respectively. The length frequency distribution of the existing fish species is presented in Figures 2 and 3. In Geray Reservoir, the relatively dominant O. niloticus and T. rendalli had a TL (mean and standard error) of 23.99 ± 0.22 cm for females and 23.79 ± 0.24 for males (overall mean 23.89 ± 0.16) and 21.01 ± 0.51 cm for females and 21.26 ± 0.52 cm for males (overall mean 21.13 ± 0.36), respectively. For the two cichlids, the males and females had almost similar mean TLs. The less abundant C. auratus had a TL (mean ± standard error) of 27.42 ± 0.87 cm for females and 29.78 ± 0.92 cm for males, with an overall mean of 28.49 ± 0.65 cm. The least abundant C. carpio had a TL of 45.97 ± 2.42 cm for females and 39.94 ± 2.07 cm for males, with an overall mean of 42.96 ± 1.65 cm. For C. auratus, males had a higher mean TL, but the vice versa was true for C. carpio.
3.2. Length–Weight Relationships
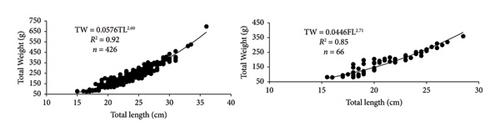
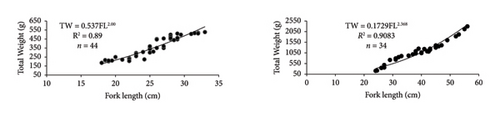
In this study, the minimum and maximum TL and TW of females O. niloticus and T. rendalli were 16 and 36 cm, 77 and 697 g, and 15.5 and 27 cm, 82 and 320 g, respectively. For males, the minimum and maximum TL and TW of O. niloticus and T. rendalli were 15 and 33 cm, 75.2 and 510 g, and 18 and 28.5 cm, 81 and 360 g, respectively. For cyprinids, the minimum and maximum TL and TW of female C. auratus, and C. carpio were 20 and 36 cm, 185 and 527.5 g, and 26 and 60 cm, 215 and 2300 g, respectively. The minimum and maximum FL and weight of male C. auratus, and C. carpio were 21 and 35.5 cm, 202 and 512 g, and 25.5 and 50.5 cm, 206.3 and 1360 g, respectively.
The relationship between length and weight was curvilinear, and the slope ‘b’ combined sexes were 2.6, 2.7, 2.0, and 2.5 for O. niloticus, T. rendalli, C. auratus, and C. carpio, respectively. The length–weight relationships for O. niloticus, T. rendalli, C. auratus, and C. carpio are indicated in Table 1 and Figure 4. Generally, the fitted regression was curvilinear and statistically significant (p < 0.001, ANOVA Table) for the four introduced species (Table 1). All four introduced fish species showed a negative allometric growth type, indicating that growth in length had been faster than weight and resulted in a more elongated appearance (Table 1).
| Species | n | Mean TL (cm) ± SE | Min–max TL (cm) | Mean TW (g) | Min–max TW (g) | a | 95% CL of a | b | 95% CL of b | r2 | Growth |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oreochromis niloticus | 426 | 23.89 ± 0.16 | 15–36 | 231.49 | 73.5–697 | 0.058 | 0.045–0.073 | 2.601 | 2.525–2.678 | 0.9134 | Allomeric (−) |
| Tilapia rendalli | 66 | 21.13 ± 0.36 | 15.5–28.5 | 180.59 | 80–360 | 0.045 | 0.0188–0.106 | 2.706 | 2.423–2.989 | 0.8509 | Allomeric (−) |
| Carassius auratus | 44 | 28.49 ± 0.65 | 20–36 | 355.52 | 185–527.5 | 0.538 | 0.263–1.098 | 2.001 | 1.779–2.222 | 0.8859 | Allomeric (−) |
| Cyprinus carpio | 34 | 42.96 ± 1.63 | 25.5–60 | 1089.51 | 206.3–2300 | 0.173 | 0.064–0.464 | 2.367 | 2.097–2.638 | 0.9083 | Allomeric (−) |
- Note: n = number of fish, min = minimum, Max = Maximum.
- Abbreviations: CL = confidence limit, SE = standard error, TL = total length, and TW = total weight.
3.3. FCF
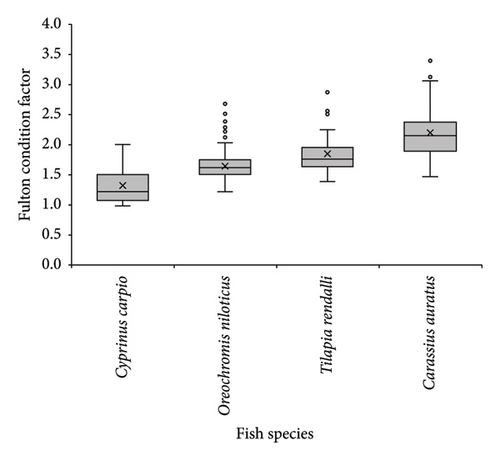
The mean FCF (mean ± SD) values of the four introduced fishes showed an increasing order of C. carpio (1.3 ± 0.32), O. niloticus (1.6 ± 0.23), T. rendalli, (1.9 ± 0.32), and C. auratus (2.2 ± 0.45) (Figure 5 and Table 2). In Geray Reservoir, larger specimens of the introduced fish species had low FCF values (Figure 5). This had also been reflected in b values, as seen before.
| Species | Sex | n | FCF (mean ± SD) | p-value |
|---|---|---|---|---|
| Oreochromis niloticus | Female | 215 | 1.65 ± 0.2236 | 0.8493ns |
| Male | 211 | 1.64 ± 0.2305 | ||
| Total | 426 | 1.64 ± 0.2305 | ||
| Tilapia rendalli | Female | 35 | 1.79 ± 0.2179 | 0.5485ns |
| Male | 31 | 1.92 ± 0.4026 | ||
| Total | 66 | 1.85 ± 0.3222 | ||
| Carassius auratus | Female | 24 | 2.29 ± 0.4688 | 0.06148ns |
| Male | 20 | 2.09 ± 0.4098 | ||
| Total | 44 | 2.20 ± 0.4501 | ||
| Cyprinus carpio | Female | 17 | 1.29 ± 0.3654 | 0.77182ns |
| Male | 17 | 1.32 ± 0.2839 | ||
| Total | 34 | 1.31 ± 0.3225 | ||
- Note: n = number of fish. (p > 0.05).
- Abbreviations: FCF = Fulton condition factor, ns = not significant, SD = standard deviation.
In all the introduced fish species, there was no significant difference (p > 0.05; Mann–Whitney U test) in FCF between the sexes in Geray Reservoir (Table 2). However, the mean FCF of females was higher than males for O. niloticus and C. auratus. The Vice versa was true for T. rendalli and C. carpio. In terms of seasons, higher values were observed during October, November, December, and January, which seem to coincide with the early breeding season of the fish species. In this study, there was a significant difference in FCF values among most of the months for all introduced fish species (both the ANOVA, p < 0.05 and the Kruskal–Wallis test, followed by post hoc Dunn’s test). Interestingly, the FCF values were > 1 for all introduced fish species (Table 3), indicating the availability of an enabling environment for better conditions of the introduced fish species.
| Months | Fulton condition factor (mean ± SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| O. niloticus | n | T. rendalli | n | C. auratus | n | C. carpio | n | |
| October | 1.53 ± 0.03 | 38 | 1.50 ± 0.12 | 4 | 2.16 ± 0.17 | 6 | 1.78 ± 0.00 | 1 |
| November | 1.73 ± 0.03 | 39 | 2.2 ± 0.00 | 1 | 1.56 ± 0.04 | 3 | 1.74 ± 0.01 | 3 |
| December | 1.73 ± 0.06 | 38 | 2.1 ± 0.032 | 3 | 2.27 ± 0.00 | 2 | 1.68 ± 0.15 | 4 |
| January | 1.75 ± 0.06 | 38 | 1.69 ± 0.22 | 5 | 2.27 ± 0.00 | 2 | 1.68 ± 0.00 | 2 |
| February | 1.69 ± 0.34 | 33 | 1.99 ± 0.10 | 8 | 2.33 ± 0.08 | 4 | 1.19 ± 0.00 | 2 |
| March | 1.60 ± 0.05 | 25 | 1.75 ± 0.22 | 18 | 2.25 ± 0.09 | 4 | 1.22 ± 0.09 | 3 |
| April | 1.67 ± 0.03 | 40 | 1.75 ± 0.51 | 6 | 2.06 ± 0.07 | 4 | 1.14 ± 0.17 | 4 |
| May | 1.59 ± 0.04 | 32 | 1.8 ± 0.35 | 6 | 1.95 ± 0.00 | 1 | 1.18 ± 0.08 | 7 |
| June | 1.58 ± 0.02 | 42 | 1.83 ± 0.55 | 5 | 2.07 ± 0.12 | 4 | 1.21 ± 0.03 | 5 |
| July | 1.57 ± 0.01 | 51 | 1.58 ± 0.13 | 4 | 1.84 ± 0.15 | 4 | 1.22 ± 0.00 | 1 |
| August | 1.66 ± 0.03 | 50 | 2.16 ± 0.14 | 6 | 2.59 ± 0.22 | 10 | 1.08 ± 0.00 | 2 |
| Mean | 1.64 ± 0.23 | 426 | 1.85 ± 0.32 | 66 | 2.21 ± 0.07 | 44 | 1.31 ± 0.06 | 34 |
- Note: n = number of fishes.
- Abbreviations: C. auratus = Carassius auratus, Cyprinus carpio = Cyprinus carpio, O. niloticus = Oreochromis niloticus, T. rendalli = Tilapia rendalli, and SD = standard deviation.
4. Discussion
In the current study, we collected four species, belonging to two families. However, previous reports indicated that there are five species: three cyprinids (Varicorhinus beso, C. carpio, and C. auratus) and two cichlids (O. niloticus and Tilapia rendalli) [16]. Here, it would be good to mention Varicorhinus beso as Labeobarbus beso and Tilapia rendalli as Coptodon rendalli, following the later taxonomic revision by Beshera et al. [17] and Dunz and Schliewen [18], respectively.
During our long sampling months of the current study, we never sampled V. beso. The local experts claim that there is no V. beso at all in the reservoir but instead 3 cichlids. Recently, Enawgaw et al. [19] have reported some biological aspects of V. beso in Geray Reservoir. It appears that there is confusion in identifying this species and its related species. The authors believe that there is no L. beso in Geray Reservoir since this species is not normally introduced into the reservoir unless it is accidently introduced or occurred naturally before the reservoir was built. Thus, further assessment and confirmation is needed.
The mean, minimum, and maximum body sizes for both sexes showed almost similar values for O. niloticus and T. rendalli. However, the mean, minimum, and maximum body sizes of males were high as compared to females for C. auratus but reversed for C. carpio. In the recently built Ribb Reservoir, O. niloticus had higher body sizes for both sexes, reaching a maximum of 44.00 cm in TL and 1450 g TW as compared to the 36 cm TL and 697 g TW in the current study [20]. However, higher catch sizes (⁓65%–67%) were found between 20 and 27 cm TL in both reservoirs. Higher body sizes (⁓46 cm maximum TL and 1500 g maximum TW) were reported from the Ribb Reservoir [21]. Furthermore, the maximum TL (36 cm) of O. niloticus in Geray Reservoir was lower than those reports from other reservoirs in Ethiopia such as Tekeze Reservoir (38.5 cm) [22], Fincha Reservoir (42.0 cm) [23], Gilgel Gibe Reservoir (50 cm [24], but it was higher than Koka Reservoir [35.2 cm and 29.9 cm] [25, 26]), and Amerti Reservoir (35.5 cm) [27].
In the current study, the maximum TL (60 cm) and TW (2300 g) of C. carpio were higher than Enawgaw et al. [28], who reported 44 cm TL and 2400 g TW. Surprisingly, the maximum length in the current study recorded a lower TW, indicating the possibility of growth changes. Degefu et al. [23] in Fincha Reservoir reported a maximum TL of 60 cm, which is similar to the current study. However, in the same study, a higher proportion of the sampled fish were in the ranges of 16–24 cm, which is lower than the current study. Hailu [29] reported a maximum FL 49 cm and 1535.8 TW in Amerti Reservoir, which is lower than the current study.
In the current study, the max TL and TW for T. rendalli and C. auratus were 28.5 cm and 360.0 g, 36 cm and 527.5 g, respectively. There is no published biological information for these species in Ethiopian reservoirs and inland water bodies, making it difficult to undertake a comparison. From FishBase, T. rendalli and C. auratus can attain 45.0 cm TL and 2.5 kg TW [30], and 48.0 cm TL [31] and 1.6 kg [32], respectively. Therefore, our size reports are far below the information available from FishBase for both species.
In Geray Reservoir, the majority of the fish specimens were found in length ranges of 24–27, 18–24, 24–32, and 41–49 cm for O. niloticus, T. rendalli, C. auratus, and C. carpio, respectively. Except for higher and lower length ranges, most fish species showed normal length distribution in the reservoir, indicating good recruitment and the presence of a dynamic fish population [11]. However, the length–frequency data could be influenced by sex, gear type and efficiency, sampling period and frequency, fish behavior, and sampling sites. In this study, the relatively small sample sizes of some introduced fish species in the reservoir may not indicate the true structure of the population. Thus, the low sample sizes for the catches of T. rendalli, C. auratus, and C. carpio are of great concern.
In Geray Reservoir, all the introduced fish species showed a curvilinear length–weight relationship. The regression coefficient (b) was 2.6, 2.7, 2.0, and 2.5 and significantly different from the cube value for O. niloticus, T. rendalli, C. auratus, and C. carpio, respectively. In Geray, all the introduced fish species showed b values within the recommended ranges (2–4) for fishes generally, but C. auratus fell short of the recommended ranges for tropical fishes (2.5 < b < 3.5) [13, 14]. Of course, Carlander [33] stated that b values of less than 2.5 are found in fish specimens with narrow size ranges or class intervals.
All the introduced fish species showed negative allometric growth (b < 3). This (b < 3) could mean the increase in fish length was faster than its body weight, i.e., large fish had a more elongated body shape or smaller fish were in better nutritional condition during the sampling period [34]. In other words, the population of the introduced species in Geray might have a heterogeneous group with body weights varying differently with the cube of length. In Geray Reservoir, it seems that there are less favorable environmental conditions where food energy may be allocated for survival in lieu of weight gain.
The curvilinear relationship of O. niloticus agrees with other studies in some reservoirs of Ethiopia. The calculated b (2.6) value for O. niloticus was higher than the report of Mequanent et al. [21] in Ribb Reservoir (b = 2.5), but it was lower than the report of Gebru [22] and Teame et al. [35] from Tekeze Reservoir (b = 3.1 and 2.9), Degefu et al. [23] from Fincha Reservoir (b = 3.2), Wakjira [24] from Gilgel Gibe Reservoir (b = 2.7), Asmamaw et al. [26] from Koka Reservoir (b = 3.17), and Asres et al. [20] from Ribb Reservoir (b = 2.8). Like the present study, most of the previously mentioned reports showed a negative allometric growth pattern, i.e., slender body [36].
For C. carpio, the b value (b = 2.5) in the current study, Enawgaw et al. [28] reported higher b values (3.97), showing positive allometric growth as opposed to negative allometric growth in Geray Reservoir. Similarly, Degefu et al. [23] and Hailu [29] reported higher b values of 3.0 and 2.9 from Fincha and Amerti Reservoirs, respectively. From the current study, it is clear that there might be unfavorable environmental conditions for C. carpio as opposed to the isometric and positive allometric growth in C. carpio from the same reservoir and others in Ethiopia. In addition, T. rendalli and C. auratus showed a negative allometric growth pattern, implying that fish length growth occurs faster than weight gain. For all species of concern, the negative allometric fish growth pattern may suggest suboptimal ecological conditions for introduced fish species.
The variations in b values for different reservoirs of the nation and the negative allometric growth pattern might occur due to several factors related to environmental conditions or habitat/land use changes and other changes, including water temperature, salinity, availability and quality of food, stress due to intense fishing and others, water mismanagement, recession farming, overfishing, water quality, sex, age, and resource competition. Stage of maturity, egg density, number of specimen, sampling season, productivity, destruction of wetlands around the reservoir, morphology and fatness pose a risk on overall health of the reservoir and its biodiversity [13, 14, 34, 37–42]. It is also affected by diet, stomach fullness, feeding rate, season, local habitat, health and parasites, pollution, maternity type, and preservation techniques as well as season and habitat [36, 43–47]. As mentioned before, sample size may affect the obtained b values, where more samples may represent the true population growth type. However, low sample sizes are still acceptable [14].
The overall mean FCF (mean ± SD) obtained for O. niloticus, T. rendalli, C. auratus, and C. carpio in this study was 1.64 ± 0.23, 1.85 ± 0.32, 2.20 ± 0.45, and 1.31 ± 0.32, respectively (Table 1). In Geray Reservoir, the FCF of all introduced fish species was above 1, indicating good condition as opposed to the b values. The FCF of O. niloticus in Geray was higher than some reservoirs, but it was lower than some reservoirs and most lakes in Ethiopia (Table 4).
| Mean FCF | Waterbody | References |
|---|---|---|
| 1.64 | Geray Reservoir | Current study |
| 1.71 | Ribb Reservoir | Asres et al. [20] |
| 1.65 | Ribb Reservoir | Mequanent et al. [21] |
| 1.78 | Fincha Reservoir | Degefu et al. [23] |
| 1.66 | Koga Reservoir | Asmamaw et al. [26] |
| 1.44 | Tekeze Reservoir | Gebru [22] |
| 1.87 | Tekeze Reservoir | Tsegay et al. [48] |
| 2.13 | Lake Babogaya | Hirpo [49] |
| 2.33 | Lake Tana | Tadesse [50] |
| 1.82 | Lake Ziway | Asnake [51] |
| 1.84 | Lake Ziway | Tesfaye and Tadesse [52] |
| 1.77 | Lake Langano | Temesgen [53] |
| 2.13 | Lake Shala | Wagaw et al. [54] |
| 1.80 | Lake Hayq | Tessema et al. [35] |
| 1.81 | Lake Hayq | Worie and Getahun [55] |
| 2.13 | Lake Beseka | Hirpo [56] |
- Abbreviation: FCF = Fulton condition factor.
In this study, the average FCF values of C. carpio (1.31 ± 0.32) agreed with the recent report of Enawgaw et al. [28] from the same reservoir (b = 1.33). In addition, the mean FCF was more than the report of Hailu [29] from Amerti Reservoir (1.22 ± 0.14). However, it was lower than the report of Degefu et al. [23] from Fincha Reservoir who reported b = 1.97. Like the present study, the FCF showed no significant variation between sexes in Fincha Reservoir. There is a scarcity of data to compare the FCF values of T. rendalli and C. auratus in Ethiopia. However, the mean FCF values (2.20 ± 0.45) of C. auratus in Geray Reservoir were more than those observed (b = 1.92) in East Hammar Marsh (Iraq) [57]. In this study, the b and FCF values of cyprinids did seem to show inverse relationships.
In Geray Reservoir, for all introduced fish species, the sampled specimens showed FCF above one, indicating that the population of these introductions is in good condition. This is because a higher body condition is correlated with high energy content, adequate food availability, reproductive potential, and favorable environmental conditions [58].
The FCF expresses the relative plumpness or degree of well-being or “fitness” of the fish. It also indicates the nutritional level and status of the fish over time. Hence, indirectly measures the well-being of fish [13]. Generally, higher conditions are associated with higher energy content, adequate food availability, reproductive potential, and favorable environmental conditions [58, 59].
In this study, most of the introduced fish species in Geray Reservoir FCF did not show variation between sexes but seasons and species. These variations could be due to several interacting factors. Some of them are the reproductive cycle of the fish, food availability, stress, pollution, species, habitat, season, age, and specific conditions [13, 21, 22, 60, 61].
Finally, in this study, bias was minimized by sampling for 11 months and collecting fish specimens using fishery-independent and -dependent data. Therefore, the results of the current study could be used to make wise management decisions in the reservoir. On the other hand, the ever-increasing number of reservoirs are severely impacted by anthropogenic pressures from time to time. To sustainably utilize the well-established exotic aquatic resources, management decisions shall be based on the best and updated scientific data on the status of the fish population. For this, management tools like close season and area, mesh size regulation, limiting the number of fishers and boats, buffer zone delineation, protecting the share vegetation, weed control, banning illegal fishing gears, regular monitoring, awareness creation and empowering fishers and local institutions, developing management plans and policies, and implementation of adaptive management approaches are needed in Geray Reservoir.
Ethics Statement
This study conformed to the guidance of animal ethical treatment for the care and use of experimental animals. For this study, no ethical clearance is obtained from the institution.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
M.M.: conceptualization, investigation, final analysis, methodology, writing, supervision, and reviewing; Y.Y.: conceptualization, investigation, data entry, methodology, and initial writing. All authors read and approved the final manuscript.
Funding
No funding was received for this research.
Acknowledgments
The authors are grateful to Geray Fish Cooperative members who supported them during the data collection. Dr. Dagnew Mequanent was acknowledged for developing the map of the Geray Reservoir.
Open Research
Data Availability Statement
The data from this study’s findings are available from the corresponding author, upon reasonable request.




