Total Length–Weight Relationships and Condition Factor of Invasive Procambarus clarkii (Girard, 1852) in Caohai Lake, China
Abstract
Caohai Lake, hailed as a gem on the Yunnan–Guizhou Plateau, is currently being faced with serious biological invasion threats. Among the most influential invasive species, the Procambarus clarkii (Girard, 1852) is causing various adverse effects on the lake’s ecosystem. However, there has been little attention paid to this issue for a long time. In light of this, this study conducted a sample collection in August 2022 to investigate the total length–weight relationships (LWRs) and condition factors (K) of the target species with the aim of providing fundamental data for the upcoming ecological restoration in Caohai Lake. The results are as follows: (1) P. clarkii was distributed throughout the lake, with females exhibiting larger body sizes than males; (2) the LWRs were expressed as: W♂ = 2 × 10−6 TL3.66 (R2 = 0.90 and n = 227); W♀ = 4 × 10−6 TL3.39 (R2 = 0.95 and n = 259), both showing positive allometric growth pattern and indicating, the weight gain rate of males is significantly faster than that of females during the growth period; (3) the K was 0.27 ± 0.04%, indicating robustness in P. clarkii in the lake. These findings underscore that urgent scientific measures should be taken to handle the rapid invasion of P. clarkii in Caohai Lake.
1. Introduction
As global climate change continues and global economic integration deepens, biological invasions have become an ever-increasing challenge [1]. Biological invasion refers to the process that a species formed breeding populations in new habitats which are beyond the biogeographical barrier by natural or human assistances [2]. Generally, after the successful invasion, the alien species will alter the structure and function of the local ecosystem, cause the decline of local biodiversity, and therefore trigger severe ecological crises, as well as lead to huge economic losses [3, 4]. According to incomplete statistics, over 660 invasive species have been detected in China [5]. Among them, freshwater species are the most head-scratching, especially the red swamp crayfish Procambarus clarkii (Girard, 1852), an extremely infamous invasive aquatic invertebrate in China [6].
P. clarkii, commonly known as the freshwater crayfish, belongs to Arthropoda, Crustacea, Decapoda, and Cambaridae. It is native to southern United States and northern Mexico [7]. This species has a diverse diet: aquatic plants, benthic organisms, and occasional cannibalism in specific circumstances. The adaptability of P. clarkii is prominent, with optimal water pH requirement ranging between 6.5 and 8.5, ideal dissolved oxygen (DO) level above 1.5 mg/L, salinity below 15 ppt, and a temperature tolerance span of 4°C–35°C [8]. Furthermore, the crayfish exhibits remarkable resilience, being capable of surviving harsh environments and accumulating and transferring heavy metals (e.g., Cd, Cu, and Pb) or other pollutants (e.g., antibiotics, pesticides, and algal toxins) to subsequent trophic levels [9–11]. Notably, P. clarkii follows an r-type breeding strategy, characterized by high fecundity, with adult females laying over 100–700 eggs at a time [12, 13]. In short, this species possesses exceptional ecological plasticity. Introduced to Nanjing, China, in the 1930s, the species gradually spread into the middle and lower reaches of the Yangtze River, now ubiquitously inhabits various water bodies such as rivers, lakes, and marshes [14].
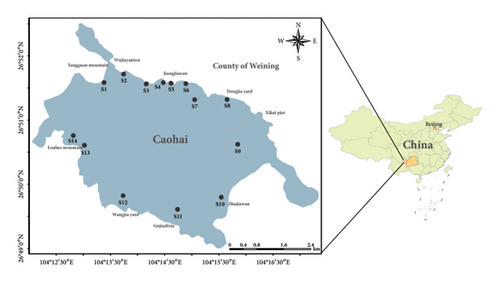
Caohai Lake (26°49′–26°53′ N, 104°12′–104°18′ E) is located in the hinterland at the apex of the Wumeng Mountains in the Yunnan–Guizhou Plateau in Guizhou Province, near the southwestern corner of Weining Yi, Hui, and Miao Autonomous County of the Yangtze river basin [15]. With a water surface area of 25 km2 and an average depth of 2.4 m, Caohai Lake is the largest, most intact, and quintessential highland karst lake in Guizhou Province [16] and is one of China’s three major plateau freshwater lakes [17]. Situated within a subtropical monsoonal climate region [18], it serves as a critical wintering habitat for rare avian species such as Grus nigricollis (Przevalski, 1876), Grus grus (Linnaeus, 1758), and Anser indicus (Latham, 1790) [19]. Moreover, the lake nurtures diverse aquatic plants, including Potamogeton wrightii Morong, Hydrilla verticillata (L.f.) Royle, and Sagittaria trifolia L., alongside indigenous fish species such as Yunnanilus caohaiensis Ding 1992, Ctenogobius giurinus (Rutter, 1897), and Oryzias latipes (Temminck and Schlegel, 1846) [15]. All in all, Caohai Lake plays a pivotal role in sustaining and regulating local climatic conditions and biodiversity. However, in recent decades, intense anthropogenic activities such as artificial drainage, land reclamation, and wastewater discharges have dramatically damaged the ecological integrity of the lake, rendering it very susceptible to the invasion of alien species [15, 20]. Not unexpectedly, P. clarkii was documented in Caohai Lake in 2010 [21].
Although knowledge on individual biology can help understand the invasion of this alien species, in which the length–weight relationships (LWRs) play an important role in its morphological biology and fisheries management [22], as well as the Fulton’s condition factors (K) are closely associated with the LWRs and can reflect the physiological, nutritional status, and habitat environment of individual organisms [23]; reports on the LWRs and K of P. clarkii are relatively scarce by far.
Hence, a comprehensive lake-wide sampling was carried out in Caohai Lake with the following targets to (1) gain the current distribution and invasion situation of P. clarkii in the lake; (2) delineate the growth pattern of P. clarkii through its LWRs; and (3) calculate the K of P. clarkii. Relevant information can help us formulate more effective management strategies for this problematic species in Caohai Lake.
2. Materials and Methods
2.1. Samples Collection
A specialized survey on P. clarkii at 14 sampling sites (Figure 1) was implemented in Caohai Lake from August 11th to 17th, 2022. To collect P. clarkii samples, rapeseed meal was employed as bait in crayfish cages (dimension approximately 5.00 m × 0.30 m × 0.25 m; mesh size around 7.00 mm). During the sampling period, several physicochemical water quality indicators were also recorded, including water temperature (WT), DO, electrical conductivity (EC), pH, ammonia nitrogen (NH3-N), nitrate nitrogen (NO3-N), water depth (Depth), and transparency (Trans) (Table 1). These data provided a foundation for further analysis to determine which environmental factors predominantly influence the growth patterns of P. clarkii.
| Sample site | WT (°C) | DO (mg/L) | EC (μS/cm) | pH | NO3-N (mg/L) | NH3-N (mg/L) | Depth (cm) | Trans (cm) |
|---|---|---|---|---|---|---|---|---|
| S1 | 24.6 | 5.4 | 411.5 | 8.18 | 0.43 | 0.03 | 95.0 | 35.0 |
| S2 | 24.5 | 5.8 | 416.3 | 8.20 | 0.45 | 0.05 | 92.0 | 25.3 |
| S3 | 24.6 | 5.9 | 430.9 | 7.97 | 0.45 | 0.03 | 79.0 | 35.8 |
| S4 | 30.3 | 5.3 | 515.0 | 8.32 | 0.48 | 0.08 | 40.0 | 32.0 |
| S5 | NA | NA | NA | NA | NA | NA | NA | NA |
| S6 | 25.0 | 5.9 | 436.8 | 8.05 | 0.45 | 0.07 | 60.0 | 40.5 |
| S7 | NA | NA | NA | NA | NA | NA | NA | NA |
| S8 | 25.3 | 6.5 | 460.6 | 8.17 | 0.49 | 0.08 | 68.0 | 57.5 |
| S9 | 24.5 | 6.9 | 423.5 | 8.27 | 0.45 | 0.07 | 67.0 | 55.8 |
| S10 | 25.9 | 6.5 | 418.7 | 8.52 | 0.46 | 0.09 | 64.0 | 57.0 |
| S11 | 23.9 | 6.0 | 389.0 | 8.29 | 0.56 | 0.07 | 90.0 | 72.5 |
| S12 | 25.0 | 7.2 | 384.7 | 8.37 | 0.49 | 0.06 | 54.0 | 54.0 |
| S13 | 25.7 | 6.2 | 396.1 | 8.32 | 0.48 | 0.07 | 65.0 | 48.0 |
| S14 | 25.2 | 6.2 | 393.0 | 8.28 | 0.55 | 0.05 | 75.0 | 44.0 |
| ± SD | 25.38 ± 1.58 | 6.15 ± 0.54 | 423.01 ± 34.85 | 8.25 ± 0.14 | 0.48 ± 0.04 | 0.06 ± 0.02 | 70.75 ± 15.65 | 46.45 ± 12.91 |
- Note: NA represents missing data.
2.2. Morphological Measurement and Sex Identification
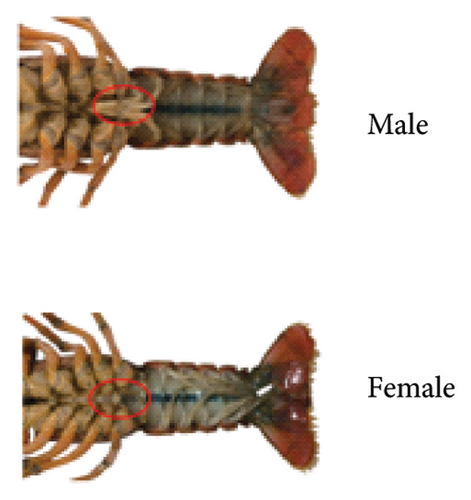
Intact P. clarkii samples were selected for measuring according to standard morphological measurements in their fresh state. Total length (TL) was obtained using a digital caliper with an accuracy to 0.01 mm, while weight (W) was determined using a high-precision electronic scale with an accuracy to 0.01 g. Genders were identified based on external morphological characteristics of the crayfish (males have tubular appendages on the first and second pairs of pleopods, whereas females have the first pair of pleopods specialized as sperm receptacles [24]), as illustrated in Figure 2.
2.3. Data Analysis
In equations (1) and (2), W represents weight (g), TL represents total length (mm), a is a constant, b is allometric growth factor, and K indicates the Fulton condition coefficient.
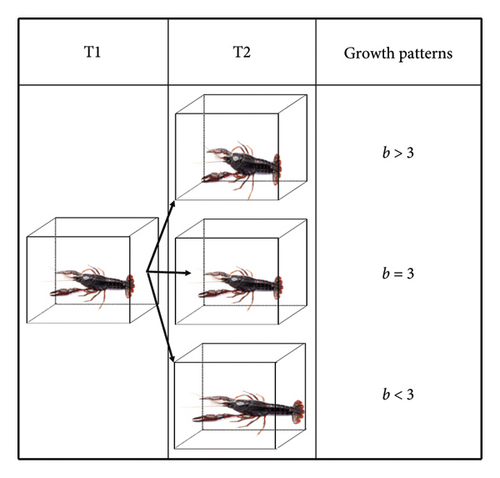
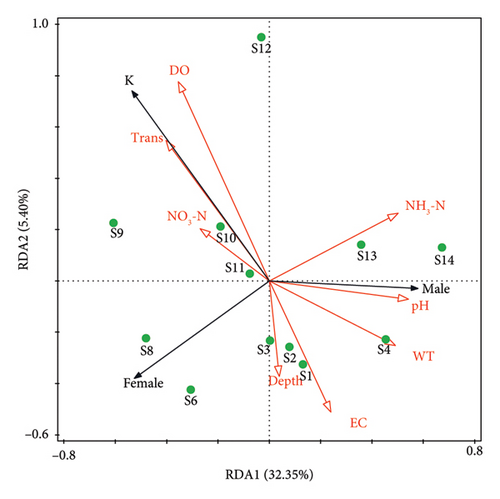
The Kolmogorov–Smirnov test was adopted to assess the normality of the total length and weight distributions of P. clarkii. The Chi-square test was used to check whether the male–female ratio in P. clarkii is 1:1. Independent samples t-test was conducted to analyze whether there were significant differences in total length and weight between males and females of P. clarkii populations. One sample t-test was applied to ascertain whether b and three varied significantly, which ultimately delineates the growth pattern of P. clarkii. Specifically, that b > 3 indicates positive allometric growth, that b = 3 indicates isometric growth, and that b < 3 indicates negative allometric growth (Figure 3). Analysis of covariance (ANCOVA) was employed to assess the difference in the LWRs between different sexes of P. clarkii. In addition, to investigate the relationship between water environment variables and the growth and distribution of P. clarkii, detrended correspondence analysis (DCA) was first applied to the water quality indicators. The results showed that the gradient length of the first axis was 0.07 (< 3), thus redundancy analysis (RDA) was chosen for further fitting.
The original data sorting was fulfilled using Excel 2016. Statistical analysis was performed using SPSS 27.0. Graphical representations were created using ArcGIS 10.8 and Origin 2021. Both DCA and RDA were conducted using Canoco 5.0. Unless otherwise specified, all statistical values in this study are presented as mean ± standard deviation ( ± SD). The significance level for all statistical tests was set to 0.05.
3. Results
3.1. Sex Ratio
A total of 486 samples were collected in the study (Table 2), with 227 males and 259 females (the gender ratio is 1:1.14). Through the chi-square test, it was found that there was no significant difference between the observed gender ratio and the theoretical ratio 1:1 (p > 0.05), which indicated a relatively balanced male–female ratio of the population. However, at certain sampling sites, there were significant differences in gender ratios. For instance, at sampling sites S6 (p < 0.05) and S9 (p < 0.05), females predominated, while at sampling site S13 (p < 0.05), males predominated.
| Sample site | Male | Female | Ratio (M:F) | χ2 | p |
|---|---|---|---|---|---|
| S1 | 13 | 11 | 1:0.85 | 0.149 | 0.700 |
| S2 | 15 | 17 | 1:1.13 | 0.108 | 0.743 |
| S3 | 22 | 33 | 1:1.50 | 1.729 | 0.189 |
| S4 | 24 | 22 | 1:0.92 | 0.071 | 0.790 |
| S5 | 24 | 28 | 1:1.17 | 0.244 | 0.621 |
| S6 | 7 | 20 | 1:2.86 | 5.533 | 0.019 |
| S7 | 70 | 88 | 1:1.26 | 1.149 | 0.284 |
| S8 | 6 | 6 | 1:1 | 0 | 1 |
| S9 | 0 | 5 | NA | 4.811 | 0.027 |
| S10 | 4 | 4 | 1:1 | 0 | 1 |
| S11 | 3 | 7 | 1:2.33 | 1.524 | 0.217 |
| S12 | 4 | 1 | 1:0.25 | 1.756 | 0.185 |
| S13 | 25 | 11 | 1:0.44 | 4.630 | 0.031 |
| S14 | 10 | 6 | 1:0.60 | 0.926 | 0.336 |
| Total | 227 | 259 | 1:1.14 | 0.616 | 0.433 |
3.2. Total Length and Weight Distribution
As shown in Figure 4, the total length range of P. clarkii samples was 40.53–116.18 mm, with an average of 84.20 ± 11.65 mm. The dominant total length group was 75.00–95.00 mm, accounting for 63.17% of the samples. Among the samples, the male population has a total length range of 50.80–106.73 mm, showing a unimodal left-skewed distribution, with an average of 82.33 ± 9.97 mm. The dominant total length group for males was 70.00–95.00 mm, representing 78.85% of the male samples. The female population has a total length range of 40.53–116.18 mm, also showing a unimodal left-skewed distribution, with an average of 85.84 ± 12.72 mm. The dominant total length group for females was 75.00–100.00 mm, accounting for 73.36% of the female samples. Independent samples t-test indicated a significant difference in total length between males and females (p < 0.05).
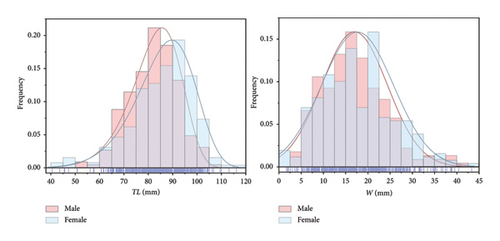
The weight range of P. clarkii samples was 1.70–43.33 g, following a normal distribution, with an average weight of 17.36 ± 7.76 g. The dominant weight group was 10.00–22.50 g, accounting for 58.85% of the samples. Specifically, the male population had a weight range of 2.54–40.41 g, with an average of 17.00 ± 7.37 g, and the dominant weight group was 7.50–20.00 g, accounting for 60.67% of the male sample. The female population had a weight range of 1.70–43.33 g, with an average of 17.67 ± 8.07 g, and the dominant weight group was 10.00–22.50 g, accounting for 57.53% of the female samples. Independent samples t-test indicated that there was no significant difference in weight between males and females (p > 0.05).
3.3. LWRs
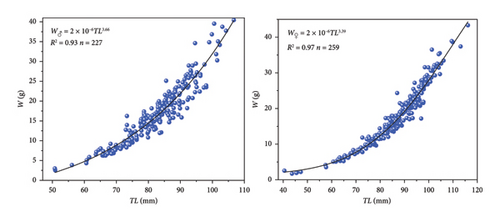
As depicted in Figure 5, the LWRs for males and females of the P. clarkii samples were as follows: W♂ = 2 × 10−6 TL3.66 (R2 = 0.90, n = 227); W♀ = 4 × 10−6 TL3.39 (R2 = 0.95, n = 259). ANCOVA revealed a significant difference in the LWRs between males and females (p < 0.05), which indicated that the two groups should not be combined. One sample t-test showed there were significant differences between both the exponents in the power function equations (male: b = 3.66; female: b = 3.39) and three (male: p < 0.05; female: p < 0.05), which indicated that P. clarkii exhibits allometric growth pattern.
3.4. K Value
The K range of the P. clarkii samples was 0.18%–0.41%, with a mean value of 0.27 ± 0.04% (Table 3). There was no significant difference in K between males (0.26 ± 0.03%) and females (0.28 ± 0.04%) (p > 0.05).
| Sample site | Number | Range (%) | ± SD (%) |
|---|---|---|---|
| S1 | 24 | 0.21–0.33 | 0.26 ± 0.03 |
| S2 | 32 | 0.21–0.34 | 0.26 ± 0.03 |
| S3 | 55 | 0.19–0.34 | 0.27 ± 0.04 |
| S4 | 46 | 0.18–0.36 | 0.26 ± 0.04 |
| S5 | 52 | 0.19–0.38 | 0.25 ± 0.04 |
| S6 | 27 | 0.21–0.33 | 0.26 ± 0.03 |
| S7 | 158 | 0.20–0.41 | 0.27 ± 0.04 |
| S8 | 12 | 0.26–0.35 | 0.30 ± 0.03 |
| S9 | 5 | 0.26–0.28 | 0.28 ± 0.01 |
| S10 | 8 | 0.26–0.37 | 0.33 ± 0.04 |
| S11 | 10 | 0.27–0.35 | 0.30 ± 0.03 |
| S12 | 5 | 0.24–0.39 | 0.30 ± 0.05 |
| S13 | 36 | 0.20–0.40 | 0.30 ± 0.04 |
| S14 | 16 | 0.21–0.41 | 0.30 ± 0.05 |
| Total | 486 | 0.18–0.41 | 0.27 ± 0.04 |
4. Discussion
P. clarkii, as one of the most serious invasive species on the globe, has shown high reproductive rates and strong dispersal capabilities during its spread [25]. Investigations have revealed that since its first record in 2010 [21], the mass release of the crayfish near Jiangjiawan in 2012 further accelerated its spread. By 2017, this species had expanded to Xihai pier, Wujiayantou, and other locations [26]. This study indicates that this species is now present throughout Caohai Lake, which is consistent with Han et al. [20]. However, unlike previous research, our sample size was several times larger. This disparity is likely attributed to the crayfish’s rapid reproduction [27], similar occurrences were also observed in other lakes such as Poyang Lake, Dongting Lake, and Tai Lake [28, 29]. Furthermore, the RDA analysis (Figure 6) revealed the significant influence of physicochemical water quality indicators on the distribution of P. clarkii, particularly highlighting the spatial differences between male and female individuals. More specifically, the results indicated that P. clarkii tends to be in the areas with higher WT and pH value. Previous studies have demonstrated that male individuals exhibit stronger competitiveness and adaptability in warmer waters, a phenomenon which has been widely observed in various environments such as lakes and aquaculture systems [30]. Byeon and Lee in a comparative study of the growth and behavior of male and female individuals found that pH significantly influences the growth of male individuals [31]. Meanwhile, this study also found that areas with higher Trans are more conducive to the distribution of female P. clarkii. In lakes and wetlands with high Trans, clear water and abundant sunlight promote the growth of aquatic plants, providing females with rich food sources and suitable habitats, thereby enhancing their health and reproductive capacity [32]. These findings suggest that the favorable environmental conditions of Caohai Lake will promote the further spread of P. clarkii, thus pose a severe threat to the biodiversity and ecological integrity of the lake. Therefore, real-time monitoring on the distribution of this species in Caohai Lake is paramount so as to provide a scientific basis for comprehensive prevention and control of invasive species in the area.
Individual morphological characteristics are crucial for studying crustacean biology and ecology [33]. According to statistical data on the length and weight distribution of P. clarkii, the average total length (85.84 ± 12.72 mm) and average weight (17.67 ± 8.07 g) of females were both greater than males (82.33 ± 9.97 mm; 17.00 ± 7.37 g). The result means that females generally have larger body sizes than males, which was commonly observed in other invasive species as well [34], and served as an important guarantee for large progeny size and a key factor for the successful invasion into new environments. On the whole, the crayfish’s average total length is 84.20 ± 11.65 mm, and its average weight is 17.36 ± 7.76 g. Compared with the previous research, the result by Zhang et al. (average total length 86.20 ± 5.50 mm) [35] is significantly longer (p < 0.05) than this study. This preliminarily indicates that there was a declining trend in body length over time for P. clarkii in Caohai Lake, which is consistent with the studies of He and Zhang [29, 36]. The decline in total length may be attributed to various factors, including environmental stress, food competition, and population density, all of which are key contributors to this trend [37]. In recent years, Caohai Lake has faced challenges of overfishing and water quality deterioration, which have severely disrupted the ecological balance of the lake. To change the situation, a strict fishing ban was implemented in 2019 [38]. Following this policy, fishery resources gradually recovered, with fish species increasing from 7 to 14 and biomass rising from 14.16 t km−2 to 25.81 t km−2. However, compared to preban levels, the coverage of submerged plants decreased by 65%, and the total ecosystem biomass declined by 60% [39]. Therefore, it is speculated that intense food competition is the primary cause for the total length decline of P. clarkii. Compared with total length, body weight is often overlooked. In the previous research on P. clarkii in Caohai Lake, although body weight was mentioned, no specific data were reported. However, body weight holds significant information in reproductive biology and fisheries resource assessment [40, 41]. Therefore, this study filled a gap in this field.
The LWRs of males and females of P. clarkii in Caohai Lake follow the pattern W = aTLb and manifest high correlations (male: R2 = 0.90; female: R2 = 0.95). The LWRs suggest that P. clarkii exhibit positive allometry growth patterns with males (b = 3.66) growing faster than females (b = 3.39) and indicate significant sexual dimorphism in growth patterns (p < 0.05) which evolved from their prolonged adaptation to the environment [42]. This finding is consistent with the findings of Ivanov and Krylov [43] and Mazlum et al. [44] and similar with other crustaceans such as Scylla serrata (Forskål, 1775) [45]. Furthermore, compared with females, males possess stronger claws so they have an advantage in predation behavior to acquire more energy for growth [46]. In short, the variation of the allometric growth factor b in the LWRs is influenced by various factors, such as different growth stages, reproductive behaviors, seasons, and environmental conditions [47].
The K is widely used to evaluate the growth status of animals [48, 49]. In this study, the K of males was slightly lower than females. The average K of P. clarkii was 0.27 ± 0.04%, which is comparatively lower than Zhang (0.29 ± 0.06%) [35]. With the rapid reproduction of P. clarkii, the competition pressure among individuals intensifies, which leads to a likely decrease in the K [50]. Overall, the K of P. clarkii in Caohai Lake is relatively high. The suitable temperature, sufficient precipitation, and long sunshine duration in the lake are conducive to the growth of aquatic plants, which therefore provides abundant food sources for the crayfish. August (sampling time of this study) is the breeding season for P. clarkii, during which females’ food intake increases, which may result in a higher K value.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was founded by the Program Foundation for Talents of Guizhou University (Nos. [2021]65 and [2021]15), the Guizhou Provincial Science and Technology Projects (No. Yiban 104 2023 Qiankehe Jichu-ZK), the National Natural Science Foundation of China (No. 32102808), and the Basic Program of Guizhou University (No. [2023]14).
Acknowledgments
We would like to express our sincere appreciations to those anonymous reviewers who made contributions to this work for their valuable observations, comments, and recommendations, leading the huge improvement of the earlier draft of this paper.
Open Research
Data Availability Statement
Data concerning this paper can be available from the first or corresponding author once request.




