Skeletal Deformities in Farmed Rainbow Trout, Oncorhynchus mykiss, at an Early Stage of Development: A Case Study of Indian Himalayan States
Abstract
The rainbow trout, Oncorhynchus mykiss, is the most extensively cultured coldwater fish species in India. However, skeletal deformities remain a significant concern and are frequently reported in rainbow trout farming operations. The incidence of skeletal deformities can serve as an indicator of the quality of rearing conditions and environmental factors. The present study was conducted to assess the skeletal deformities of rainbow trout, from selected farms in Himachal Pradesh, and Uttarakhand, India. During the study, physicochemical parameters such as water temperature, pH, dissolved oxygen (DO), nitrogen and phosphorus compounds, and chlorides were recorded. The brain, gill, and muscle tissues were examined under optical microscope to inspect myxozoan parasite infections. Nevertheless, myxozoan parasites were not detected in the microscopic examination. For histopathological analysis, tissue samples from the internal organs were processed. The degree of acinar cell necrosis, fibrosis, and macrophage aggregation were determined through histopathological examination. The skeletal deformities, whirling behavior, and mortality were directly associated with the major pancreatic anomalies but no signs of parasite presence.
1. Introduction
In the Indian subcontinent, two trout species, viz., the brown trout Salmo trutta fario and rainbow trout Oncorhynchus mykiss were introduced from Europe, primarily to develop sport fishing [1]. Among these, rainbow trout have remained confined to the raceway culture system in India. Rainbow trout farming is the most promising industry in the Indian–Himalayan region for adopting the traditional flow-through system. The first rainbow trout stock imported to India was from England, whereas several other strains were imported from other European countries during the eighties to replenish the existing old stock. Trout farming has progressed steadily in India, and in the last 15 years, the coldwater aquaculture industry has developed tremendously in Himalayan states. Over time, this coldwater fish became popular in the hill states of India, including Himachal Pradesh, Jammu Kashmir, Uttarakhand, Sikkim, and Arunachal Pradesh [2].
With an annual production of 598 metric tonnes of rainbow trout, the union territory of Jammu and Kashmir leads the list of coldwater regions, followed by Himachal Pradesh with 456 metric tonnes (official website of the Jammu and Kashmir Fisheries Department; https://jkfisheries.in/). Compared to other Indian states, Himachal Pradesh has a higher number of private trout growers (572 no.) [3]. About 16 million tons of eyed ova are produced overall from 32 no. of rainbow trout hatcheries in India [4]. In the various agroclimatic zones of India’s Himalayan states, rainbow trout have varying breeding periods [5]. In Himachal Pradesh and Uttarakhand, the first rainbow trout spawning begins the first week of December and January, respectively [6]. In the expansion of rainbow trout production in India, the major challenges faced are climate change, reduced water flow, and poor water quality sources due to the construction of river valley projects [4, 7, 8].
The incidence of disease and deformities is high in cultured fishes, which are most susceptible to changing environmental conditions, as their growth and well-being depend mainly on the quality of water [9] and health status [10]. In farmed rainbow trout, fluctuations in temperature and parasite infections can affect physiology and lead to the development of skeletal deformities [11, 12]. Skeletal anomalies lead to economic and animal welfare issues in farmed fishes [13]. The incidence of skeletal deformities is dependent on the species type, developmental stage, environmental condition, nutrition, and presence of microbial agents such as Myxobolus cerebralis or pancreatic disease. Many farmed fishes such as rainbow trout, O. mykiss [14, 15]; gilt seabream, Sparus aurata [16]; Atlantic salmon, Salmo salar [17, 18]; Atlantic halibut, Hippoglossus hippoglosus, L. [19]; Senegalese sole, Solea senegalensis [20]; Japanese flounder, Paralichthys olivaceus [21] are found to have deformities in their vertebral column. Reduced growth, welfare, swimming ability, inappetence, lethargy, and farmed product quality are the unintended negative effects of vertebral deformities [22, 23]. Also, skeletal anomalies are also associated with the mortality of farmed fish mostly in juveniles that can range from 10% to 50% [24, 25].
Rainbow trout farmers of Himachal Pradesh and Uttarakhand, India, frequently report vertebral deformities along with whirling movements of fish and mortality as a significant problem in the farms and hatcheries. Consumers often hesitate to buy deformed fish from farms because they sell them either at a lower price or discard. Indeed, there are a lot of publications about the deformities of the vertebrae in farmed rainbow trout [14, 15, 26, 27]. Most of the literature in rainbow trout however focused on the type and location of anomalies and did not report about whirling movement of fish. Hence, the study was envisaged to determine the prevalence, possible cause of skeletal anomalies, whirling movement and mortality in rainbow trout farms using microscopic and histopathological tools.
2. Materials and Methods
2.1. Fish and Sampling
Rainbow trout specimens show similar symptoms of whirling movements, blackening of the body, a curved tail, the presence of a cyst before the anal fin on the lateral line, hemorrhage on the gills, and skeletal deformities (Figure 1) were taken from three farms of Himachal Pradesh and one farm of Uttarakhand, India (Figure 2). The sampling period spanned three years, from 2017 to 2019. The percentage prevalence of deformity was calculated from each farm on the basis of the total number of skeletal deformed fish observed to the total sample collected from each population and expressed as a percentage [28]. During these three years, approximately 424 randomly collected fish samples (Table 1) showing signs of deformity and black caudal peduncle were brought to the fish parasitology and mycology laboratory of the ICAR-Directorate of Coldwater Fisheries Research (DCFR), Bhimtal, for postmortem analysis. Furthermore, the possible causes and impacts of skeletal anomalies were investigated via various approaches.




| Farm ID | 2017 | 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample size | No. of deformed fish observed (n) | % deformed fish | Sample size | No. of deformed fish observed (n) | % deformed fish | Sample size | No. of deformed fish observed (n) | % deformed fish | |
| UK1 | 290 | 40 | 13.79 | — | — | — | — | — | — |
| HP1 | 300 | 40 | 13.33 | 310 | 44 | 14.19 | 262 | 35 | 13.35 |
| HP2 | 255 | 35 | 13.72 | 482 | 65 | 13.48 | 465 | 70 | 15.05 |
| HP3 | 200 | 30 | 15 | 250 | 35 | 14 | 230 | 30 | 13.04 |
- Total deformed fish observed n = 424.
2.2. Physiological Parameters of the Water
The physicochemical parameters of water from rainbow trout raceways, such as temperature, pH, dissolved oxygen (DO), phosphate (PO4), nitrogen–ammonia (NH3N), nitrate-nitrogen(NO3N), and chlorides, were recorded during this period. The temperature (°C) and pH were measured via a digital meter (Hanna, USA). The DO concentration (mg L−1) was measured via a multiparameter probe (Oakton DO 6+ Dissolved Oxygen Meters, Coleparmer, USA). PO4, NH3N, NO3N, and chloride contents were estimated via commercially available kits (Himedia, India) following the manufacturer’s protocol.
2.3. Histopathology
The moribund trouts were anesthetized with clove oil (40 μL/L) until they remained motionless and were fixed in Bouin’s fluid. For analysis of the cellular pathology of various tissues in deformed fishes, tissue samples (caudal fin, skin and muscle, visceral mass, spleen, kidney, gill, head, and brain) were processed for histopathological analysis. The protocol for histology followed that of Sharma et al. [29]. Briefly, samples were processed in a series of alcohols (70%, 85%, 95%, and 100%), xylene, and finally embedded in paraffin blocks. The skin and muscle tissues were cross-sectioned at a thickness of 4 μm and stained with hematoxylin and eosin (Sigma). Histopathological observations were made with an upright microscope (Nikon Eclipse Ci) with a digitally attached camera (Nikon Ds Fi2) to record pathological changes through microphotography.
2.4. Parasite Investigation
Skin scrapings and squash smear preparations of mucus from the fins, gills, brain, head kidney, liver, and intestine of moribund fishes were mounted on glass slides with a drop of saline solution for parasite observation under a microscope as suggested by Karlsbakk et al. [30].
2.5. Whole-Mount Study
The moribund trouts were anesthetized with clove oil (40 μL/L) until they remained motionless and were fixed in 10% formalin. Whole-mount staining of bone and cartilage was performed with a modified protocol of the alcian blue technique by Dingerkus and Uhler [31] for skeletal anomalies. The fish were first fixed in 10% formalin and washed with distilled water for 48 h. The staining technique was started by placing the fish in 10 mg alcian blue, 80 mL ethyl alcohol, and 20 mL glacial acetic acid for up to 48 h. Subsequently, the alcian blue stain was removed, and further processing was carried out with 96%, 75%, 40%, and 15% graded ethyl alcohol for 3 h and an enzyme solution of 30 mL saturated aqueous sodium borate, 7 mL distilled water, and 1 gm trypsin for 72 h. The process was again continued with 0.5% aqueous potassium hydroxide (KOH) with alizarin red S for 24 h and 0.5% KOH–glycerine series (3 : 1, 1 : 1, 1 : 3) to pure glycerin. A few drops of thymol were added to the glycerin and hydrogen peroxide (H2O2) solutions for the final stage.
3. Results and Discussion
3.1. Percentage Prevalence of Deformity and Clinical Signs
During the three years of sampling period, mortalities were observed in rainbow trout fry aged 90–110 days, with an average weight of 2 ± 1.2 g. Approximately 8–15 trout fry die daily in outdoor raceways, and an approximately 13%–15% prevalence of skeletal anomalies is reported during the sampling years (Table 1). Notably, the symptoms manifested in the initial week of June and persisted until the final week of September. Fish were lethargic, and there was low acceptance to feed. At present, only a limited percentage of farms can consistently produce 100% of nondeformed fish. Earlier records on vertebra deformity rates in wild fish have shown a range of 3%–43% in salmonids [32, 33].
The affected fish displayed a black tail, which was the first sign of spinal instability. The appearance of curvy caudal region, frenzied tail-chasing motion was evident from the samples. According to Chong [34], abnormal whirling behavior and blackening probably happen due to damage to the sympathetic nerves of the spinal cord, which are located caudal to the site of spinal cartilage damage and regulate the melanin pigmentation.
3.2. Parasitological Investigations
Skeletal anomalies may arise due to parasite infection (Myxobolus sp.), elevated incubation temperatures [18, 35–37], or other possible reasons. Since, whirling movement and mortality were associated with the deformity, it was first suspected to be myxozoan infection. However, the myxozoan parasites were absent in all the tested samples in microscopy examination. The absence of Myxobolus spores in various tissues led to the hypothesis that abiotic, nutritional factors could be possible reasons for these skeletal anomalies.
The farmed rainbow trout in both states were fed a balanced diet (50% fish meal, 15% soybean meal, 15% wheat flour, 10% linseed oil, 8% yeast extract, and 2% vitamin and mineral mixture) and an appropriate ration size; hence, suspecting/conspiring the feed or nutrients as a factor responsible for triggering the deformities was unsubstantiated.
3.3. Physicochemical Parameters
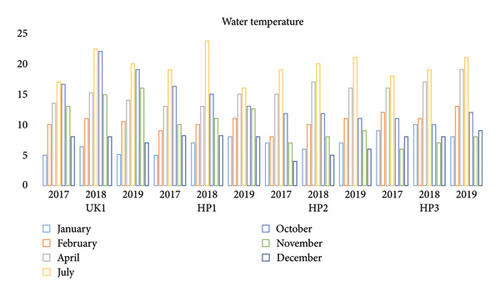
The physicochemical parameters of the trout raceways were as follows: water temperature, 3.3°C–23.70°C (Figure 3); pH, 8.0–9.5; DO, 5.8–10.7 ppm; PO4, 0.08–0.95 ppm; NH3N, 0.136–0.72 ppm; NO3N, 0.02–0.747 ppm; and chlorides, 1.63–7.44 ppm. Rainbow trout spawn from autumn to winter months (November to January) in both states of India [2]. The optimum incubation period for rainbow trout is 370° days. The temperature recorded from all the farms and hatcheries ranged from 4°C to 8°C from December to January, and the water temperature of the sampled rainbow trout farms ranged from 17°C to 23.7°C from June–July. The ideal temperature range for incubating rainbow trout eggs is 7°C–12°C; lower incubation temperatures, such as 4°C-5°C, and more than 12°C–14°C have a detrimental impact on the early stages of development [38].
3.4. Histopathological and Whole-Mount Investigations
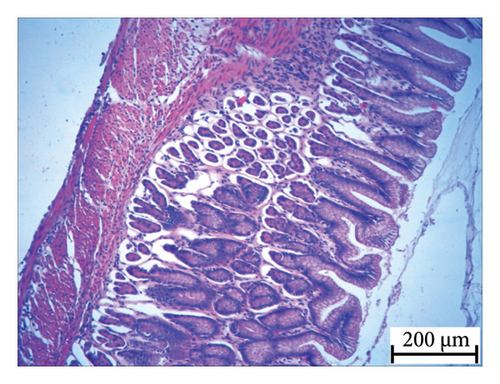
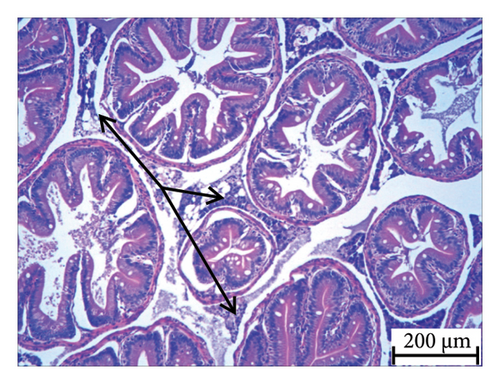
Histopathology and whole-mount and molecular biology are essential laboratory tools for assessing vertebral anomalies in fish [20, 36]. Histology was performed for different tissues, such as the stomach, liver, pyloric caeca, and pancreas (Figure 4), of which the pancreas was the most infected organ where degeneration of acinar cells was observed in addition to vacuolation. The stomach, pyloric caeca, and intestine were found to be normal, with intact epithelium, glands, and muscle layers (both inner circular and outer longitudinal). The ultrastructure of the spleen was normal; however, some smears of pigmented macrophages were observed. Yellowish to brown pigmented macrophage aggregates were found in the pancreatic tissue, and the space of degenerated acinar cells was replaced by fibrosis. Macrophage aggregates in internal organs have been linked to a range of phenomena, including infectious diseases and parasite infestations [39], and heat stress [40].
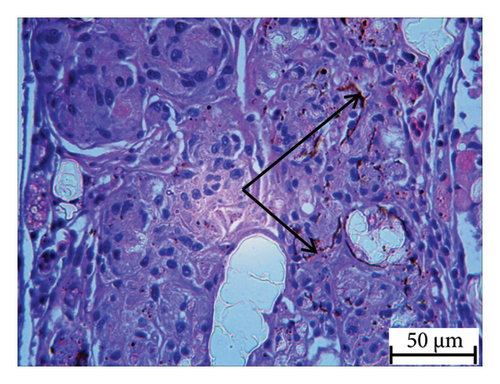
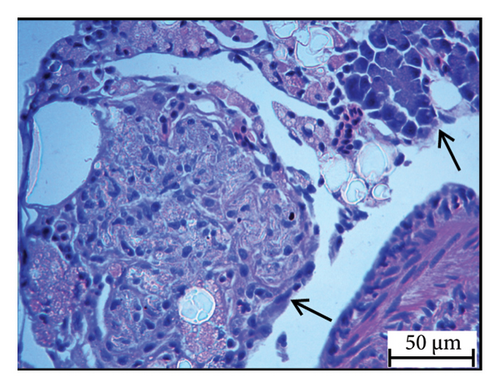
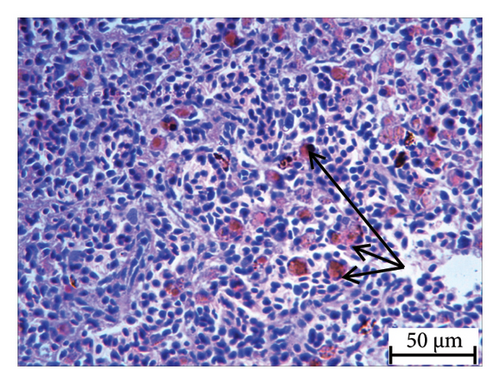
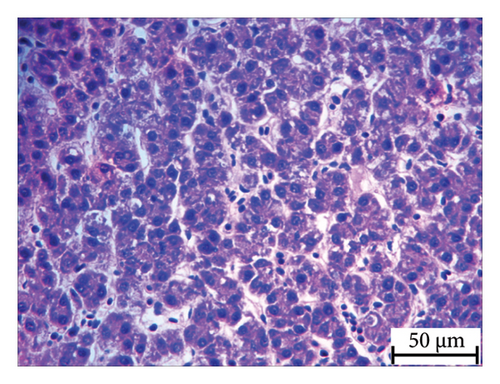
Furthermore, inflammatory leukocytes infiltrated the liver, and hepatocytes exhibited some degree of lipid infiltration. Lipid accumulation in the livers of captive fish is likely a consequence of the fluctuating water temperature. Indeed, some studies reported that temperature was an important factor that modulated the liver lipid accumulation in Atlantic salmon and steel head trout [41–43].
Rainbow trout stained with alcian blue and alizarin red presented anomalies in caudal regions (Supporting File). A similar result was observed in cultured Sole, Solea sengalenis [20], rainbow trout, O. mykiss [44], and Salmo gairdneri [45]; Atlantic halibut, Hippoglossus hippoglosus [19]; and European seabass, Dicentrarchus labrax [46], at the early stages of development. In salmonid fishes, high temperatures during both incubation and rearing are the principal factors that negatively impact skeletal development [47]. During an experimental larval rearing of rainbow trout under three temperatures, the range of deformities was between 2% and 8% when the temperature was below 13°C, and substantial larval losses were reported, with 13%–20% deformed stock when the temperature was above 13°C–14.5°C [48]. The phenomenon, how favorable environmental conditions change the skeletal phenotype, including the induction of skeletal deformities, was portrayed by Martini et al. in 2020. Moreover, in both geographic locations, the diurnal water temperature fluctuates rapidly, which could lead to deformities in rainbow trout fry and fingerlings.
In nutshell, the skeletal deformities, whirling behavior and mortality were associated with the major pancreatic anomalies, abnormal build up of lipid in liver with no signs of parasites presence. An observation was made by authors in [49, 50] during an outbreak of pancreatic disease in Atlantic salmon from Ireland, observing whirling movement and mortality due to skeletal myopathy associated with pancreatic acinar cell degeneration and liver cell necrosis. McLoughlin et al. made similar observations in 1995, 1996, and 2002 during an outbreak of pancreatic disease in Atlantic salmon from Ireland, observing whirling movement and mortality due to skeletal myopathy associated with pancreatic acinar cell degeneration and liver cell necrosis.
4. Conclusion
The current study found that rainbow trouts farmed in India’s Western and Central Himalayan regions were suffering from skeletal deformities, which could be attributed to pancreatic disease and temperature fluctuations. Pancreatic disease is often linked with the virus such as IPN, Salmonid alphavirus (SAV) in salmonids. Therefore more research is needed to substantiate the present finding and to prevent the skeletal anomalies, which could make a significant contribution to rainbow trout farming in India.
Ethics Statement
The institutional ethical committee approved this study (ICAR-DCFR/Ref. No. IAEC/4-4(127)/2008/DC/3842-48) and the protocols for sampling, maintenance, handling during experiments, and sacrificing of the fishes.
Consent
All the authors listed have consented to the submission of the case report to the journal.
The research content of the manuscript is original and has not been published elsewhere. All the authors agree to publish the paper in the Journal of Ichthyology.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ritesh Shantilal Tandel: original draft preparation, conceptualization, and experimental trial;
Pragyan Dash: data curation, and review and editing.
Raja Aadil Hussain Bhat: visualization, methodology, and review and editing.
Kavya Kalingapuram: investigation,
Prakash Sharma: histology and review and editing.
Funding
This research was funded by the National Fisheries Development Board (Grant number: G/Nat. Surveillance/2014) and the Indian Council of Agricultural Research (Grant number: ICAR DCFR) for providing the necessary financial support and facilities to conduct this research work successfully.
Acknowledgments
The authors would like to express their sincere thanks to the director, ICAR DCFR, farmer, and officers of the Department of Fisheries, Himachal Pradesh and Uttarakhand, India, for allowing them to perform periodic sampling. The kind help of Mr. Ravindra Posti, YP, in the preparation of the GIS map is acknowledged.
Supporting Information
Figure S1: Whole mount of rainbow trout show anomalies. Figure S1 shows anomalies, absent hemal spine(AHS), bifurcated hemal spine(BHS), and fusion of last hemal vertebra (FLV), also deformed condition, anomalies at prehemal regions, and anomalies at hemal regions of the rainbow trout.
Open Research
Data Availability Statement
Data sharing is not applicable as no datasets were generated or analyzed during the current study.




