Environmental Mold Management in Negative-Pressure Isolation Wards During the COVID-19 Pandemic: A Potential Indicator of COVID-19-Associated Pulmonary Aspergillosis
Abstract
High concentration of mold spores in inhaled air is an important risk factor for invasive mold infections. COVID-19 (coronavirus disease 2019)-associated pulmonary aspergillosis (CAPA) is a serious complication of severe COVID-19. To investigate the mold distribution in negative-pressure isolation wards and its potential association with CAPA incidence, we conducted microbiological air sampling and retrospectively analyzed CAPA cases in a tertiary care hospital in Korea during the COVID-19 pandemic. Air sampling was conducted in January 2022 at multiple sites in four negative-pressure isolation wards designated for managing severe COVID-19 patients. A portable microbial air sampler (MAS-100 NT) was used for air sampling, and Tryptic Soy Agar plates were incubated to identify mold isolates at the genus level. CAPA cases (January 2021–June 2023) were defined by antifungal treatment, mycological evidence (serum galactomannan index > 0.5), and radiological findings. Immunomodulator use, including dexamethasone and tocilizumab, was analyzed to identify clinical risk factors influencing CAPA incidence. Among the isolated molds, Aspergillus (86.7%) was the most prevalent, followed by Penicillium (53.3%), Mucorales (20%), and Paecilomyces (13.3%). Mold concentrations were highest in areas adjacent to a construction site. Intervention procedures, including installing air purifiers and reinforcing seals between the adjoining construction site and isolation ward, effectively reduced mold concentrations and paralleled a decline in CAPA incidence. While increased foot traffic after the relaxation of COVID-19 restriction measures led to a rise in spore concentration, the incidence of CAPA did not increase, likely due to the decreased use of immunomodulators such as corticosteroids and tocilizumab. Construction-related mold spore increases can be mitigated with appropriate interventions, and pedestrian traffic near isolation wards may need regulation. Monitoring and mitigating environmental mold contamination are crucial to preventing opportunistic respiratory mold infections in negative-pressure isolation wards and should be interpreted in conjunction with clinical risk factors.
1. Introduction
The unprecedented coronavirus disease 2019 (COVID-19) pandemic has highlighted the significance of opportunistic mold infections [1, 2]. The pathophysiology of severe COVID-19 involves a hyperinflammatory immune response triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), necessitating immunomodulating treatments such as dexamethasone and tocilizumab [3–8]. In addition, respiratory viruses cause direct damage to the airway epithelium, facilitating fungal spore invasion and thereby increasing the susceptibility to COVID-19-associated pulmonary aspergillosis (CAPA) [9]. CAPA is a serious complication associated with increased mortality in patients with severe COVID-19, with an overall incidence ranging from 2.5% to 35.0% [10, 11]. The concentration of Aspergillus spores in hospital air is a major extrinsic risk factor for nosocomial invasive aspergillosis, highlighting the importance of indoor air quality in controlling CAPA incidence [12–15]. As COVID-19 patients are treated in negative-pressure isolation rooms to prevent viral transmission, ensuring air quality is crucial for reducing CAPA risk. The COVID-19-associated mucormycosis (CAM) outbreak was concentrated in India, indicating that regional variations in environmental mold distribution may be important [2]. Nevertheless, there is insufficient regional data on air culture in negative-pressure isolation wards. Herein, we report for the first time the mold distribution in negative-pressure isolation wards identified by air sampling, interventions to improve air quality, and trends in CAPA occurrence at a single center during the COVID-19 pandemic.
2. Methods
We investigated the environmental mold distribution in air and CAPA incidence trends in the negative-pressure isolation wards of a tertiary care hospital in Korea. Our center was designated for the management of severe COVID-19 patients requiring high-flow oxygen support, including high-flow nasal cannula, noninvasive mechanical ventilation, or invasive mechanical ventilation. During the COVID-19 pandemic, we operated isolation rooms in four wards (A, B, C, and D). Wards A and B had negative-pressure isolation facilities that were completed before the COVID-19 pandemic. The negative-pressure system of Ward C was established during the pandemic, while construction on the ward opposite the elevator hall occurred from the fourth quarter of 2021 (2021 Q4) to 2022 Q2. Ward D, previously a general ward, was temporarily converted into a negative-pressure facility to reinforce the isolation beds during 2021 Q4–2022 Q2. There were no significant issues with water leakage, dampness, ventilation, or air-conditioning reported during the study period, including in the negative-pressure isolation wards. Environmental surface cleaning and disinfection were carried out according to standard protocols outlined in the Centers for Disease Control and Prevention and institutional guidelines, using a 1000 ppm sodium hypochlorite solution, a chlorine-containing disinfectant [13].
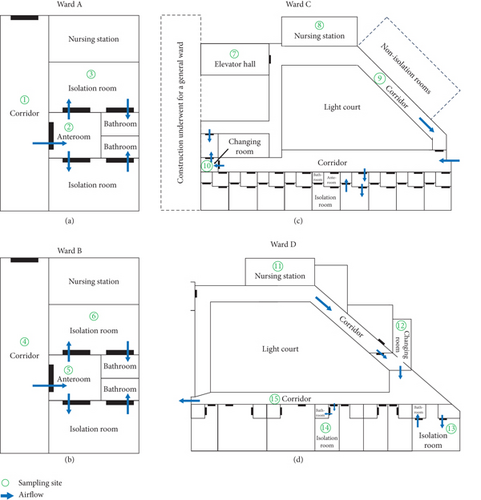
Air sampling was performed at multiple sites in each negative-pressure isolation ward in January 2022. All air samples were collected on the same day during daytime hours, at times when patient and healthcare worker activity was minimal. The sampling site numbers for each ward are presented in Figure 1. Microbiological air sampling was conducted using a portable microbial air sampler (MAS-100 NT; Merck Millipore, Merck KGaA, Darmstadt, Germany) which utilizes the solid surface impaction method, recognized as suitable for sampling fungal spores in healthcare environments [13]. A Tryptic Soy Agar (TSA) plate was placed in the sampler. The air sampler passed air at a rate of 100 L/min for 5 min, and the total volume collected from each sample was 500 L. The TSA plates were incubated at 30°C for 72 h, followed by incubation at room temperature for an additional 18 days. Growth was monitored visually on a daily basis. When mold colonies were observed, the plate was stained with lactophenol cotton blue for microscopic examination. Mold isolates were identified at the genus level based on key macroscopic and microscopic characteristics described by Lars et al. [16]. The colony-forming units (CFU) were calculated and reported as CFU per cubic meter.
We conducted a retrospective investigation into the incidence and clinical aspects of CAPA in patients with severe COVID-19 who were admitted to our center between January 2021 and June 2023. CAPA was defined as COVID-19 cases treated with antifungal agents in conjunction with both mycological evidence (serum galactomannan index > 0.5) and radiological evidence (new lung infiltrates or nodules or both on chest x-rays) [10]. The number of CAPA cases and their incidence were investigated quarterly. To investigate clinical factors that may affect the decreasing CAPA incidence, we explored the use of immunomodulators administered to severe COVID-19 patients. The doses of dexamethasone and tocilizumab were evaluated based on the day of treatment (DOT). We calculated DOT per admission rather than DOT per patient day because mandatory isolation periods changed during the COVID-19 pandemic. The study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No. SMC 2023-12-132). Informed consent was waived due to the retrospective nature of the study. All data were anonymized, with no access to identifiable information, ensuring ethical compliance.
3. Results
The results of air sampling, including mold distribution, species identification, and colony counts, are summarized in Table 1. A total of 15 samples were collected at various locations in each isolation ward. The numbering of sampling sites in Table 1 corresponds to those shown in Figure 1. Among the isolated molds, Aspergillus was the most prevalent (87%, 13/15), followed by Penicillium (53%, 8/15), Mucorales (20%, 3/15), and Paecilomyces (13%, 2/15). The average colony count was 4.5 CFU/m3, ranging from 1 to 10 CFU/m3. Because the elevator hall of Ward C, adjacent to the construction site (Sampling Site Number 7), had the highest mold concentration, several interventions were implemented to reduce the mold concentration. Specifically, two air purifiers were installed in the elevator hall. The exhaust systems, comprising two air exhaust fans and two dust collectors, were installed around the closed entrance to the construction site. Additionally, Ward C maintained a positive pressure of +0.8 Pa compared with the elevator hall. Reinforcement was performed to maintain the well-sealed status between the construction site and the negative-pressure isolation ward. Follow-up air sampling was conducted in the elevator hall of Ward C in February 2022, and the colony count decreased to 3 CFU/m3.
| Ward | No. | Sampling site | Mold species | CFU/m3 |
|---|---|---|---|---|
| A | 1 | Corridor | Aspergillus | 7 |
| 2 | Anteroom | Aspergillus, Penicillium | 7 | |
| 3 | Isolation room | Penicillium | 2 | |
| B | 4 | Corridor | Aspergillus, Penicillium | 6 |
| 5 | Anteroom | Aspergillus, Penicillium | 6 | |
| 6 | Isolation room | Aspergillus, Penicillium | 6 | |
| C | 7 | Elevator hall | Paecilomyces | 10 |
| 8 | Nursing station | Aspergillus | 4 | |
| 9 | Corridor | Aspergillus, Penicillium | 4 | |
| 10 | Changing room | Aspergillus | 6 | |
| D | 11 | Nursing station | Aspergillus, Mucorales | 2 |
| 12 | Changing room | Aspergillus, Mucorales | 1 | |
| 13 | Isolation Room 2 | Aspergillus, Mucorales | 1 | |
| 14 | Isolation Room 3 | Aspergillus, Penicillium | 4 | |
| 15 | Corridor | Aspergillus, Penicillium, Paecilomyces | 1 | |
- Note: The sampling site numbers in the table match those shown in Figure 1.
- Abbreviations: CFU, colony-forming unit; No., number.
After the fifth domestic outbreak wave led by the Omicron variant, Ward C was used primarily for the management of COVID-19 patients. At that time, the pedestrian corridor traffic in Ward C increased because construction opposite Ward C was completed and used as a general ward, with several nonisolation rooms in Ward C also being used (Figure 1c). Additionally, as the pandemic transitioned into the endemic phase, the Korea Disease Control and Prevention Agency downgraded COVID-19 from a Class 2 to a Class 4 infectious disease and relaxed associated restrictions in June 2023. To assess the potential impact of these changes, air sampling was conducted in the nursing station and elevator hall of Ward C in July 2023. Aspergillus was detected at both sites, with colony counts of 22 CFU/m3 in the nursing station and 44 CFU/m3 in the elevator hall. This represented a considerable increase compared to January 2022, when colony counts were 4 and 10 CFU/m3, respectively.
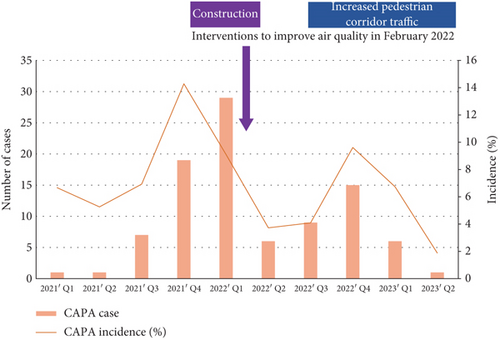
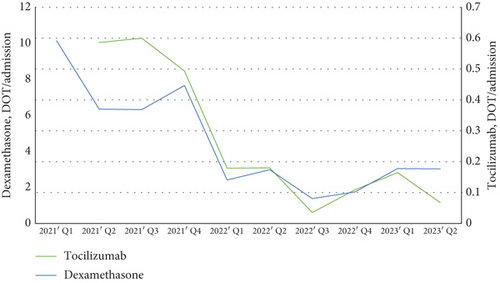
Next, we investigated the incidence and clinical aspects of CAPA. From January 2021 to June 2023, a total of 1266 patients with severe COVID-19 were admitted to our center. The number and incidence of CAPA cases are shown in Figure 2a. During the Delta variant–dominated outbreak period from 2021 Q3 to 2022 Q1, the number of CAPA cases increased from 7 to 29 per quarter. After the interventions to improve air quality in February 2022, there was a downward trend in CAPA incidence in 2022 Q2. However, CAPA incidence temporarily increased in 2022 Q4, possibly due to increased pedestrian corridor traffic around Ward C. Nonetheless, CAPA incidence steadily decreased again after 2022 Q4. Doses of immunomodulators evaluated based on the DOT are shown in Figure 2b. The use of both dexamethasone and tocilizumab decreased remarkably since 2022 Q1 when the Delta variant–dominated outbreak passed. We speculated that a decrease in the number of critically ill patients requiring dexamethasone and tocilizumab, as well as a reduction in the dosage of immunomodulators, prevented a resurgence of CAPA incidence despite increasing pedestrian traffic.
4. Discussion
This study highlights that elevated environmental mold concentrations around negative-pressure isolation wards may increase the risk of CAPA. Several risk factors for CAPA have been reported, including old age, invasive respiratory support, chronic lung disease, and high-dose corticosteroids, but these are nonmodifiable factors [1, 8]. Our findings suggest that improving air quality through environmental control is a potentially modifiable factor in reducing opportunistic respiratory infections, including CAPA. Several interventions, such as installing air purifiers and sealing construction sites, effectively reduced airborne mold concentrations, which correlated with a decline in CAPA incidence. Interestingly, while fungal spore concentrations increased with pedestrian traffic, the incidence of CAPA did not rise, likely due to an overall reduction in clinical risk factors. These findings reflect the multifactorial nature of CAPA, which should be considered in conjunction with the clinical risk of opportunistic infections.
Previous studies have reported that high concentrations of airborne Aspergillus spores increase the risk of pulmonary aspergillosis; however, little is known about mold distribution in negative-pressure isolation wards [13, 14]. Aspergillus and Penicillium were the most frequently isolated molds around the negative-pressure isolation wards. Mucorales was also identified from some sites but at a low frequency and concentration. These findings align with a previous study investigating environmental mold profiles in another Korean tertiary hospital in 2017 [17]. The outbreaks of CAM in India were preceded by an increase in Mucorales spore concentrations, which correlated with increased temperature and relatively lower humidity. While many Mucorales spores were identified in air culture in India, few were identified in indoor and outdoor air in Korea [2, 18]. Korea has a temperate climate characterized by distinct seasons, including a cold winter and a humid summer, which may limit the continuous proliferation of Mucorales. These findings may explain why Korea has fewer CAM cases than India. However, given that the concentration of Mucorales spores in the atmosphere may be affected by climate change, regular monitoring will be necessary to reflect potential environmental changes.
This study has several limitations. First, it was conducted at a single center with a limited number of air culture samples, primarily due to a significant shortage of personnel during the COVID-19 pandemic. However, air sampling was conducted at various locations in all negative-pressure isolation wards, and the findings were interpreted in conjunction with clinical factors. The outdoor air quality and seasonal variations were not assessed in this study. However, it is important to note that patients in negative-pressure isolation wards are inherently susceptible to respiratory opportunistic infections, regardless of external air conditions or seasonal fluctuations. Initial sampling was conducted, followed by prompt interventions, with a follow-up sampling performed approximately 1 month later. Given the short time interval and minimal climatic variations during this period, seasonal differences are unlikely to significantly affect the results. Second, due to the multifactorial nature of COVID-19 management, providing statistical evidence to demonstrate a direct correlation between the interventions for air quality improvement and the reduction of CAPA was challenging. Nonetheless, the concentration of airborne Aspergillus spores is well known to be a major risk factor for nosocomial invasive aspergillosis [13–15]. Third, while microbiological air sampling in healthcare facilities aids in evaluating indoor air quality and dust control measures, it remains controversial due to the lack of standard testing protocols [13]. Although the COVID-19 pandemic is over, respiratory infections caused by other pathogens may threaten the world again. Further studies are needed to establish standard protocols for quality control of the air environment in negative-pressure isolation wards.
5. Conclusions
We described the airborne mold distribution in negative-pressure isolation wards during the COVID-19 pandemic, along with CAPA incidence and clinical factors. This study highlights the importance of environmental mold management in negative-pressure isolation wards to reduce the risk of opportunistic respiratory infections, including CAPA. Construction-related increases in airborne fungal spores were effectively mitigated through appropriate interventions. Despite rising fungal spore concentrations linked to increased pedestrian traffic, CAPA incidence remained stable, likely due to a reduction in clinical risk factors. These findings emphasize the necessity of integrated strategies combining environmental and clinical risk management. Regular air quality monitoring and the establishment of standardized protocols are essential to prevent nosocomial mold infections. This study contributes to improving infection control practices in negative-pressure isolation wards.
Ethics Statement
The study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB No. SMC 2023-12-132). Informed consent was waived due to the retrospective nature of the study. All data were anonymized, with no access to identifiable information, ensuring ethical compliance.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
J.Y.H. and J.-H.K. are co-first authors. J.Y.H. and J.-H.K. contributed equally to this article. Conceptualization: J.Y.H., J.-H.K., S.Y.C., and D.R.C. Data curation: J.Y.H., J.-H.K., and S.Y.C. Formal analysis: J.Y., K.H., C.-I.K., and K.R.P. Investigation: J.Y., K.H., C.-I.K., and K.R.P. Methodology: T.Y.K., H.J.H., and N.Y.L. Software: J.Y.H. and J.-H.K. Supervision: J.Y.H., J.-H.K., and D.R.C. Validation: J.Y.H. and J.-H.K. Visualization: J.Y.H. and J.-H.K. Writing–original draft: J.Y.H. and J.-H.K. Writing–review and editing: J.Y.H., J.-H.K., and D.R.C.
Funding
This study was supported by the Future Medicine 2030 Project of Samsung Medical Center (Grant Number SMX1250021).
Acknowledgments
We would like to thank Ju-hyung Kim and Junki Lee for conducting the microbial air sampling and culture procedures. The first draft of this manuscript was edited for language by eWorldEditing.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




