Association Between House Dust Endotoxins and Increased All-Cause Mortality in Adults
Abstract
Background: Exposure to house dust endotoxins is known to cause diseases across various organ systems; however, their effect on mortality remains unclear.
Objective: This study is aimed at investigating the association between house dust endotoxins and mortality in US adults.
Methods: National Health and Nutrition Examination Survey data from 2005 to 2006 were used in this study. Participants were linked to mortality data from the date of the survey through December 31, 2019. A multivariate Cox regression analysis was used to determine the association between house dust endotoxins and mortality. All analyses were performed in the overall population and across different sensitization statuses.
Results: This cohort study included 3171 adults aged 20 years or older (weighted median age (P25–P75): 45 (32–58) years; 49.2% male). Among them, 1287 participants were sensitized to inhalant allergens. During a median follow-up of 13.8 years, 672 deaths occurred. In participants sensitized to inhalant allergens, house dust endotoxins were significantly associated with all-cause mortality. The hazard ratio (HR) comparing the highest and lowest tertiles of house dust endotoxin levels was 1.98 (95% confidence interval (CI): 1.32–2.97) for all-cause mortality. An association was observed between endotoxin concentration and cardiovascular disease mortality when analyzed as a continuous variable (HR: 1.29, 95% CI: 1.01–1.56). In nonsensitized participants, no significant association was found between house dust endotoxins and mortality.
Conclusion: Exposure to house dust endotoxins was associated with all-cause mortality in adults sensitized to inhalant allergens.
1. Introduction
Endotoxin is a glycolipid released from the cell wall of gram-negative bacteria. Low-dose endotoxins are widely present in the environment, and previous studies have shown that they can be commonly detected in household dust [1]. Compared to occupational exposure levels, endotoxins in everyday environments are typically present at lower concentrations but have higher detection rates [2]. However, the concentration range of endotoxins in dust varies considerably across reports. Studies from different countries [3–6] have shown detectable concentrations of endotoxins in indoor dust samples. Therefore, it is essential to assess the health risks associated with environmental exposure to low-dose endotoxins.
Exposure to high-dose endotoxins can lead to airway inflammation and changes in various inflammatory factors [7]. This has been demonstrated in numerous animal studies and research involving occupationally exposed humans [8–11]. Studies have further revealed that low-dose endotoxin exposure is associated with various respiratory diseases, with the strongest evidence linking dust endotoxins with asthma, although findings are inconsistent [12–14]. In addition, dust endotoxins are associated with emphysema, chronic bronchitis [15], and rheumatoid arthritis [16]. Exposure to endotoxins present in indoor environmental dust can also alter white blood cell levels, suggesting that even low-dose exposure may exert systemic effects on humans [17].
Only a few prospective cohort studies have examined the association between dust endotoxin exposure and long-term health outcomes. Although exposure to endotoxins in dust has been associated with diseases such as asthma and chronic bronchitis [15], its impact on mortality remains unexplored. Long-term mortality serves as a key indicator for assessing the impact of prolonged exposure to dust endotoxins, offering insight into adverse effects that may influence public health policy. Mendy et al. [15] conducted a cross-sectional analysis using National Health and Nutrition Examination Survey (NHANES) data (2005–2006) and reported increased risks of chronic bronchitis and emphysema exclusively among sensitized individuals. Building on this foundation, our study extends their findings by employing a prospective design to evaluate the long-term mortality risks associated with indoor dust endotoxin exposure. We used indoor dust endotoxins data from the 2005–2006 NHANES, combined with 2019 mortality data from the National Center for Health Statistics (NCHS), to analyze the effect of indoor dust exposure on long-term mortality among sensitized and nonsensitized adults in the United States.
2. Method
2.1. Study Population
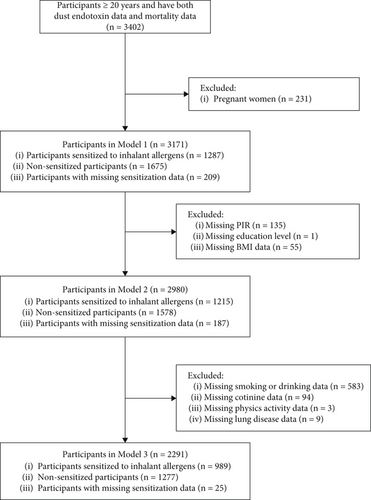
The NHANES is a cross-sectional study conducted in 1999 by the NCHS of the Centers for Disease Control and Prevention (CDC). It used a complex method to select participants to ensure representative sampling. The participants provided data on their demographic characteristics, socioeconomic status, nutritional intake, environmental exposure, medical conditions, and other information. In this study, because only the NHANES 2005–2006 cycle contains data on house dust, we examined only the corresponding population. Among participants older than 20 years, 3403 had information on house dust endotoxins. Among them, 3402 were followed up for long-term survival. After excluding pregnant women, 3171 participants were enrolled in our study. Among them, 1287 participants were sensitized to inhalant allergens and 1675 were not. Participants without serum data on inhalant allergens were included in the overall analyses but not the stratified analysis based on sensitization status. The CDC and the Agency for Toxic Substances and Disease Registry determined that our research did not meet the criteria for human research under federal regulations and therefore did not require review.
2.2. Indoor Dust Detection
Household dust sample collection procedures are detailed on the NHANES webpage (https://www.cdc.gov/Nchs/Nhanes/2005-2006/ALDUST_D.htm). Briefly, dust samples were collected from each respondent’s bed and bedroom floor using a Sanitaire Model 3683 vacuum cleaner and a Mitest dust collector (Indoor Biotechnologies Inc., Charlottesville, Virginia). “Bedroom” was defined as the room or area where the participant slept regularly and where their main bed was located. We defined a “bed” as the furniture on which the participant usually slept. For each sample collected, we marked a 1-square yard area on the bedside and the adjacent bedroom floor. Each site was individually processed for 2 min (total vacuum time: 4 min per sample collection). For sampling from the bed, the technician was required to vacuum the surface of the sheet whenever possible. For sampling from the floor, for rooms with both carpeted and uncarpeted floor areas, technicians were instructed to place the sample template on the carpet rather than a smooth floor surface. When two participants slept in the same bed, only one sample of bed/floor dust was collected, and the results were applied to both participants. Endotoxin concentrations are reported in endotoxin units (EUs) per mass of sieved dust (milligrams) or per square inch of vacuumed surface (milligrams per square inch, calculated by multiplying EU per milligram of dust by the total mass of dust divided by the collection area). The lower limit of detection was 0.0005 EU/mg. Based on the weighted proportions of endotoxin in the dust samples, three groups were assigned: Tertile 1 (0%–33.3%), Tertile 2 (33.3%–66.6%), and Tertile 3 (66.6%–100%).
2.3. Mortality Detection
Mortality was assessed using public-use linked mortality files (LMFs) for 2019, published by the NCHS in May 2022. An introduction to public-use LMFs and instructions for use can be found on the CDC website (https://www.cdc.gov/nchs/data/datalinkage/public-use-linked-mortality-file-description.pdf). The public-use versions of the NCHS LMFs were subjected to data perturbation techniques to reduce the risk of participant reidentification. We converted the originally downloaded files into formats compatible with statistical software, using the software programs provided by the NCHS. The NCHS classified deaths related to cardiovascular disease (CVD) as being due to heart disease (Codes I00–I09, I11, I13, and I20–I51) or cerebrovascular disease (Codes I60–I69) and cancer mortality as being due to malignant neoplasms (Codes C00–C97). Lower respiratory disease mortality was similarly classified as being due to respiratory system–related ailments (Codes J40–J47) [18]. We calculated the follow-up time for each person as the difference between the NHANES examination date and the last known date the individual was reported alive or removed from the LMF.
2.4. Covariates
- •
“Have you ever been told by a doctor that you have asthma?”
- •
“Have you ever been told by a doctor that you have chronic bronchitis?”
- •
“Have you ever been told by a doctor that you have emphysema?”
We divided physical activity into four categories based on the answers to the question, “how much physical activity do you do in a day?” We included age and BMI in the model as continuous variables. Our selection of these covariates was based on previous studies [15, 18]. We built three models using different covariates to improve data stability. The first model contained no covariates, and the second model included sex, age, BMI, ethnicity, education level, and PIR. For the third model, we added serum cotinine, smoking status, drinking status, and lung disease status. Because samples with any missing covariate data were excluded from the analyses, serum cotinine was used as a marker of smoking (including active smoking and exposure to secondhand smoke) in our main analysis instead of using the secondhand smoke questionnaire. In the sensitivity analysis, we used a secondhand smoke questionnaire in place of serum cotinine values. In this analysis, secondhand smoke exposure was defined as having a smoker at home or reporting the smell of cigarettes in the workplace.
As a major stratification variable, we defined sensitization status as having serum values of any one of 15 inhalant allergens greater than 0.35 Ku/L (Alternaria alternata, Aspergillus fumigatus, Bermuda grass, birch, cat, cockroach, dog, dust mites, mouse, oak, ragweed, rat, Russian thistle, or ryegrass).
2.5. Statistical Analyses
The NHANES program employs a complex, multistage, probability sampling design to represent the national, civilian, and noninstitutionalized population of the United States. Therefore, data analyses require applying sample weights according to NHANES documentation (https://www.cdc.gov/nchs/nhanes/tutorials/Module3.aspx). As the study used only one cycle, the dust allergen subsample 2-year weight from the dust file was used directly as the weight variable without additional processing. All analyses were performed using survey design methods. Baseline data were expressed as the weighted mean ± standard error, interquartile range, and rate. The three categories of endotoxins in dust were segmented according to a weighted cut-off, ensuring that each tertile contained 33% of cases after weighting. A weighted chi-square test was employed to compare mortality between the three groups corresponding to the endotoxin concentration tertiles. A weighted Cox regression was used to compare the effect of dust endotoxins on participants’ mortality. The outcomes of the Cox regression are presented as hazard ratio (HR) values, confidence intervals (CIs) of the HRs, and p values. The p trend was calculated by assigning numeric values to tertiles. Simultaneously, survival curves were drawn, incorporating all covariates with the weighted data. Generalized additive models (GAMs) were applied in R using the mgcv package to examine nonlinear relationships between mortality and house dust endotoxin exposures, incorporating smooth terms for house dust endotoxin concentration. Nonlinear associations between mortality and house dust endotoxin concentration were visualized using partial effect plots.
Further, stratified and interaction analyses were conducted to determine whether the association differed by sensitization status, age, sex, PIR, smoking status, and drinking status based on the interaction p value. A p value less than 0.05 was considered statistically significant, and p values less than 0.001 were denoted as < 0.001. Two sensitivity analyses were used to demonstrate the stability of the study. First, as stated in the Covariates section, different secondhand smoke assessment methods (serum cotinine and secondhand smoke questionnaire) were selected as input variables. Second, the E value was also used to assess the impact of unknown confounders on the study [19, 20]. All analyses were conducted using R (Version 4.0.5). The packages employed included survey, survival, and survminer.
3. Results
3.1. Baseline Data
Based on data available for the different covariates, Models 1, 2, and 3 included 3171, 2980, and 2291 participants, respectively (Figure 1). The baseline characteristics were reasonably similar across all three models. Therefore, only the baseline data from Model 1 are presented. Among participants included in Model 1, the median age was 45 (32, 58) years, and 49.2% were male. Most participants were non-Hispanic White. The median follow-up time was 166 months (13.8 years), and these data are presented in Table 1. Of the 3171 participants, 672 had died at the time of follow-up, with 214 deaths attributed to heart-related illnesses, 136 to tumors, and 39 to chronic lower respiratory diseases. The weighted endotoxin concentrations for each tertile and the weighted proportion for each tertile are presented in Table S1.
| All participants (N = 3171) | Sensitized (N = 1287) | Nonsensitized (N = 1675) | |
|---|---|---|---|
| Age (years), median [IQR] | 45 [32–58] | 42 [31–55] | 49 [36–62] |
| Ratio of family income to poverty | |||
| ≤ 1.3 | 18.8 | 18.4 | 18.9 |
| 1.31–3.50 | 40.9 | 41.4 | 40.6 |
| > 3.50 | 40.4 | 40.2 | 40.5 |
| BMI (kg/m2), median [IQR] | 27.55 [24.05–32.32] | 27.41 [24.06–32.00] | 27.64 [24.13–32.77] |
| Sex | |||
| Male (%) | 49.2 | 54.3 | 45.3 |
| Female (%) | 50.8 | 45.7 | 54.7 |
| Race | |||
| Hispanic (%) | 10.9 | 11.5 | 10.7 |
| Non-Hispanic White (%) | 71.7 | 68.0 | 75.3 |
| Non-Hispanic Black (%) | 11.4 | 13.6 | 9.1 |
| Other race (%) | 5.9 | 7.0 | 4.8 |
| Education level | |||
| Less than high school (%) | 18.8 | 15.6 | 20.9 |
| High school graduate/GED or equivalent | 26.4 | 27.2 | 25.4 |
| Some college or AA degree | 31.1 | 30.7 | 31.8 |
| College graduate or above | 23.8 | 26.4 | 21.9 |
| Smoking | |||
| Current smokers (%) | 26.0 | 24.1 | 28.3 |
| Never smokers (%) | 48.8 | 52.3 | 45.1 |
| Former smokers (%) | 25.2 | 23.6 | 26.6 |
| Drinking | |||
| No alcohol | 20.7 | 16.1 | 24.8 |
| ≤ 4 drinks per week (%) | 69.1 | 73.6 | 64.4 |
| > 4 drinks per week (%) | 10.2 | 10.3 | 10.8 |
| Ever told by doctor had asthma, emphysema, or chronic bronchitis | |||
| No (%) | 81.1 | 76.0 | 84.8 |
| Yes (%) | 18.9 | 24.0 | 15.2 |
| Follow-up time (month), median [IQR] | 166 [160–173] | 166 [161–173] | 166 [158–173] |
| Endotoxin in dust (EU/mg), median [IQR] | 14.60 [7.01–29.75] | 14.52 [7.90–29.44] | 14.60 [6.63–29.65] |
| Tertile proportions of endotoxin in dust (%) | |||
| Tertile 1 | 33.3 | 33.9 | 32.2 |
| Tertile 2 | 33.3 | 32.7 | 34.5 |
| Tertile 3 | 33.3 | 33.3 | 33.3 |
3.2. Effect of Different Levels of Endotoxins on Mortality
The effect of different endotoxin tertiles on mortality was first assessed using a weighted chi-square test. In the general population, although all-cause mortality increased progressively across endotoxin tertiles, no statistically significant differences were observed. Among participants sensitized to inhalant allergens, the all-cause (p = 0.008) and chronic lower respiratory-related mortality (p = 0.022) significantly increased in higher endotoxin groups. Among nonsensitized participants, all-cause, CVD, and cancer mortality did not differ significantly across endotoxin tertiles (Table 2). Because mortality appeared to differ significantly by sensitization statuses, a multivariate Cox regression model was constructed to analyze the relationship between sensitization status and mortality after adjusting for covariates. The results showed that sensitization status was not significantly associated with all-cause, CVD, or tumor mortality after adjustment for covariates (data not shown).
| All participants | Sensitized | Nonsensitized | |
|---|---|---|---|
| All-cause mortality | |||
| Tertile 1 | 182/999 (14.02%) | 45/389 (7.58%) | 123/542 (18.47%) |
| Tertile 2 | 200/989 (15.41%) | 56/415 (10.28%) | 131/511 (19.65%) |
| Tertile 3 | 290/1183 (17.47%) | 101/483 (14.65%) | 166/622 (19.46%) |
| p | 0.102 | 0.008 | 0.879 |
| CVD mortality | |||
| Tertile 1 | 57/999 (4.62%) | 16/389 (3.46%) | 38/542 (5.55%) |
| Tertile 2 | 61/989 (3.89%) | 19/415 (2.94%) | 38/511 (4.71%) |
| Tertile 3 | 96/1183 (5.56%) | 36/483 (5.22%) | 54/622 (5.88%) |
| p | 0.154 | 0.197 | 0.531 |
| Cancer mortality | |||
| Tertile 1 | 46/999 (3.30%) | 13/389 (1.75%) | 29/542 (4.43%) |
| Tertile 2 | 44/989 (3.77%) | 11/415 (2.34%) | 28/511 (4.78%) |
| Tertile 3 | 46/1183 (2.60%) | 15/483 (1.80%) | 27/622 (3.08%) |
| p | 0.281 | 0.676 | 0.311 |
| Chronic lower respiratory mortality | |||
| Tertile 1 | 10/999 (1.03%) | 1/389 (0.18%) | 8/542 (1.70%) |
| Tertile 2 | 8/989 (0.77%) | 2/415 (0.28%) | 5/511 (0.95%) |
| Tertile 3 | 21/1183 (1.50%) | 7/483 (1.28%) | 14/622 (1.84%) |
| p | 0.337 | 0.022 | 0.482 |
- Note: Number of cases were counted using unweighted data; proportions and p values were calculated with weighted data.
- Abbreviation: CVD, cardiovascular disease. Bold values mean p < 0.05.
3.3. Results of the Cox Regression
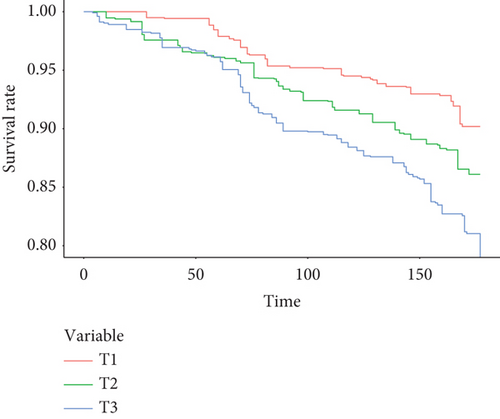
The Cox regression results were consistent with those of the chi-square test, even after adjusting for all covariates. In participants sensitized to inhalant allergens, higher concentrations of dust endotoxins were associated with increased all-cause mortality, regardless of whether endotoxin levels were modeled as tertiles (T1 vs. T3, HR: 1.98 (1.32–2.97), p trend: < 0.001) or as natural log-transformed continuous variables (HR: 1.37 (1.2–1.56)). Among nonsensitized participants, dust endotoxin levels were not significantly associated with all-cause mortality (Table 3). For CVD mortality, only the fully adjusted model in the sensitized population demonstrated a significant association with log-transformed endotoxin concentrations (HR: 1.09 (1.01–1.18)) (Table 4). No association was observed between endotoxin concentrations and cancer mortality in either nonsensitized or sensitized participants (Table 5). After excluding participants who died from CVD or chronic lung disease, the effect of dust endotoxins on mortality was re-evaluated. The results indicated that exposure to dust endotoxins remained significantly associated with increased mortality in participants sensitized to inhalant allergens (Table S2). The survival curves adjusted for all covariates are presented in Figure 2 and Figure S1. GAM plots indicate that natural logarithmic transformation dust endotoxin level (ln(EU/mg)) did not show a significant nonlinear association with mortality (Figure S2).
Model 1a HR (95% CI) |
Model 2b HR (95% CI) |
Model 3c HR (95% CI) |
|
|---|---|---|---|
| All | N = 3171 | N = 2980 | N = 2291 |
| Tertile 1 | Reference | Reference | Reference |
| Tertile 2 | 1.11 (0.86–1.44) | 1.18 (0.93–1.49) | 1.2 (0.98–1.47) |
| Tertile 3 | 1.28 (1.06–1.55) | 1.18 (0.97–1.43) | 1.16 (0.95–1.42) |
| ln(endotoxin)d | 1.12 (1.03–1.21) | 1.06 (0.97–1.16) | 1.09 (1.01–1.18) |
| p trend | 0.007 | 0.094 | 0.15 |
| Sensitized | N = 1287 | N = 1215 | N = 989 |
| Tertile 1 | Reference | Reference | Reference |
| Tertile 2 | 1.42 (0.88–2.28) | 1.38 (0.7–2.75) | 1.7 (0.93–3.1) |
| Tertile 3 | 2.05 (1.36–3.07) | 1.83 (1.03–3.25) | 1.98 (1.32–2.97) |
| ln(endotoxin)d | 1.30 (1.14–1.49) | 1.25 (1.02–1.52) | 1.37 (1.2–1.56) |
| p trend | < 0.001 | 0.019 | < 0.001 |
| Nonsensitized | N = 1675 | N = 1578 | N = 1277 |
| Tertile 1 | Reference | Reference | Reference |
| Tertile 2 | 1.07 (0.76–1.51) | 1.1 (0.89–1.34) | 1.07 (0.87–1.32) |
| Tertile 3 | 1.07 (0.83–1.38) | 0.97 (0.8–1.17) | 0.96 (0.74–1.26) |
| ln(endotoxin)d | 1.05 (0.96–1.15) | 0.99 (0.9–1.08) | 1.01 (0.92–1.11) |
| p trend | 0.608 | 0.761 | 0.805 |
- Note: Bold values mean p < 0.05.
- aModel 1: unadjusted model.
- bModel 2: adjusted for age, sex, BMI, PIR, education level, and race.
- cModel 3: adjusted for covariates in Model 2 plus smoking status, drinking status, serum cotinine, secondhand smoke exposure, history of lung disease, and physical activity.
- dEstimated by modeling the natural logarithm of endotoxin levels (ln(EU/mg)) as a continuous variable. HRs reflect the mortality risk associated with a 1-unit increase in ln(endotoxin) (ln(EU/mg)).
Model 1a HR (95% CI) |
Model 2b HR (95% CI) |
Model 3c HR (95% CI) |
|
|---|---|---|---|
| All | N = 3171 | N = 2980 | N = 2291 |
| Tertile 1 | Reference | Reference | Reference |
| Tertile 2 | 0.85 (0.56–1.31) | 0.96 (0.58–1.58) | 0.93 (0.54–1.60) |
| Tertile 3 | 1.24 (0.87–1.76) | 1.17 (0.76–1.81) | 1.03 (0.61–1.74) |
| ln(endotoxin)d | 1.06 (0.89–1.26) | 1.01 (0.80–1.27) | 1.02 (0.81–1.29) |
| p trend | 0.219 | 0.468 | 0.905 |
| Sensitized | N = 1287 | N = 1215 | N = 989 |
| Tertile 1 | Reference | Reference | Reference |
| Tertile 2 | 0.87 (0.42–1.79) | 0.97 (0.43–2.19) | 1.52 (0.71–3.27) |
| Tertile 3 | 1.63 (0.83–3.21) | 1.46 (0.61–3.51) | 1.57 (0.65–3.81) |
| ln(endotoxin)d | 1.25 (0.95–1.63) | 1.11 (0.74–1.65) | 1.29 (1.01–1.66) |
| p trend | 0.15 | 0.38 | 0.337 |
| Nonsensitized | N = 1675 | N = 1578 | N = 1277 |
| Tertile 1 | Reference | Reference | Reference |
| Tertile 2 | 0.85 (0.50–1.44) | 0.87 (0.56–1.35) | 0.77 (0.44–1.35) |
| Tertile 3 | 1.07 (0.66–1.74) | 1.04 (0.69–1.57) | 0.87 (0.51–1.48) |
| ln(endotoxin)d | 0.99 (0.82–1.20) | 0.95 (0.77–1.18) | 0.92 (0.7–1.21) |
| p trend | 0.781 | 0.88 | 0.573 |
- Note: Bold values mean p < 0.05.
- aModel 1: unadjusted model.
- bModel 2: adjusted for age, sex, BMI, PIR, education level, and race.
- cModel 3: adjusted for covariates in Model 2 plus smoking status, drinking status, serum cotinine, secondhand smoke exposure, history of lung disease, and physical activity.
- dEstimated by modeling the natural logarithm of endotoxin levels (ln(EU/mg)) as a continuous variable. HRs reflect the mortality risk associated with a 1-unit increase in ln(endotoxin) (ln(EU/mg)).
Model 1a HR (95% CI) |
Model 2b HR (95% CI) |
Model 3c HR (95% CI) |
|
|---|---|---|---|
| All | N = 3171 | N = 2980 | N = 2291 |
| Tertile 1 | Reference | Reference | Reference |
| Tertile 2 | 1.15 (0.71–1.86) | 1.23 (0.82–1.84) | 1.24 (0.87–1.76) |
| Tertile 3 | 0.81 (0.58–1.13) | 0.81 (0.56–1.17) | 0.86 (0.53–1.40) |
| ln(endotoxin)d | 0.96 (0.84–1.10) | 0.93 (0.81–1.08) | 0.96 (0.83–1.11) |
| p trend | 0.218 | 0.256 | 0.546 |
| Sensitized | N = 1287 | N = 1215 | N = 989 |
| Tertile 1 | Reference | Reference | Reference |
| Tertile 2 | 1.34 (0.59–3.03) | 1.20 (0.48–3.02) | 1.90 (0.74–4.90) |
| Tertile 3 | 1.08 (0.57–2.04) | 0.90 (0.37–2.20) | 1.26 (0.57–2.78) |
| ln(endotoxin)d | 0.99 (0.78–1.27) | 0.90 (0.66–1.23) | 1.08 (0.87–1.34) |
| p trend | 0.806 | 0.81 | 0.618 |
| Nonsensitized | N = 1675 | N = 1578 | N = 1277 |
| Tertile 1 | Reference | Reference | Reference |
| Tertile 2 | 1.08 (0.6–1.95) | 1.13 (0.71–1.80) | 1.05 (0.77–1.44) |
| Tertile 3 | 0.70 (0.47–1.06) | 0.72 (0.44–1.17) | 0.78 (0.44–1.40) |
| ln(endotoxin)d | 0.94 (0.80–1.10) | 0.92 (0.77–1.10) | 0.93 (0.78–1.11) |
| p trend | 0.09 | 0.185 | 0.397 |
- aModel 1: unadjusted model.
- bModel 2: adjusted for age, sex, BMI, PIR, education level, and race.
- cModel 3: adjusted for covariates in Model 2 plus smoking status, drinking status, serum cotinine, secondhand smoke exposure, history of lung disease, and physical activity.
- dEstimated by modeling the natural logarithm of endotoxin levels (ln(EU/mg)) as a continuous variable. HRs reflect the mortality risk associated with a 1-unit increase in ln(endotoxin) (ln(EU/mg)).
Similar results were obtained in the sensitivity analysis when the secondhand smoke–related questionnaire was used instead of serum cotinine concentrations as the covariate representing secondhand smoke exposure (Table S3). In sensitized participants, the E value for all-cause mortality was 2.08 for the point estimate and 1.69 for the lower confidence bound when dust endotoxin was analyzed as a natural log-transformed continuous variable. The E value was 3.37 for the point estimate and 1.97 for the lower confidence bound when comparing dust endotoxin concentrations between Tertiles 3 and 1. None of the HR point estimates or lower confidence bounds associated with all-cause mortality exceeded the E value for any covariate included in the study. These findings demonstrate the robustness of the results. Similar results were obtained when endotoxin per square inch of vacuumed surface was used as the exposure variable (Tables S4–S6).
3.4. Subgroup Analysis Results
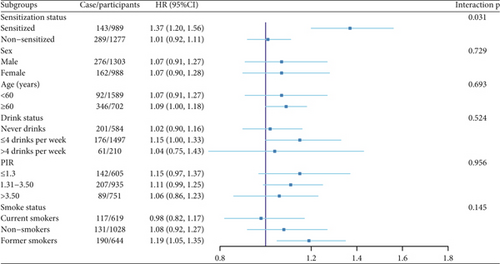
A subgroup analysis was conducted using data adjusted for all covariates. A significant interaction between subgroup factors and endotoxin levels in dust was observed when comparing different sensitization statuses; no significant interactions were found in other subgroups (Figure 3).
4. Discussion
Our study found that exposure to endotoxins present in indoor dust can increase all-cause mortality in adults sensitized to inhalant allergens but has less effect on the nonsensitized population. We found an association between endotoxin exposure and CVD mortality when analyzing endotoxin concentration as a continuous variable in a fully adjusted model. Although no significant differences were found when analyzing endotoxin concentration as tertiles (despite all RR point estimates being greater than 1.5), these results still suggest that the long-term effects of endotoxin on the cardiovascular system cannot be ignored. We also found a possible association between endotoxin exposure and chronic lower respiratory disease mortality in sensitized populations; however, due to the low incidence of mortality, this association warrants further validation. Regardless of sensitization status, endotoxin exposure was not significantly related to mortality due to cancer.
To our knowledge, this is the first article to evaluate the effect of dust endotoxins on all-cause mortality. The observed health effects of exposure to dust endotoxins appear contradictory, as such exposures have been linked to both beneficial and harmful health outcomes. Some research has suggested that exposure to low-dose endotoxins in dust is linked to the development of conditions, such as asthma, chronic obstructive pulmonary disease, and emphysema [4, 14, 15]. Additionally, exposure has been found to induce alterations in certain systemic conditions, such as leukopenia [17]. Conversely, other studies have proposed that early-life exposure to microbial agents may prevent the development of atopy and asthma [21, 22]. Several investigations have reported a reduced risk of atopy, hay fever, and asthma in children and adolescents from farming backgrounds who are believed to have high levels of exposure to bacterial endotoxins [23, 24]. In our study, we identified that exposure to endotoxins in dust appears to be primarily associated with increased mortality among participants sensitized to inhalant allergens. This suggests the potential for long-term effects stemming from low-dose endotoxin exposure in specific populations. In addition, we found that endotoxin had a greater impact on mortality in the sensitized population. People who are sensitized are more vulnerable to endotoxins. The effects of exposure to dust-containing endotoxins on emphysema and chronic bronchitis are more pronounced in sensitized populations [15]. The synergistic association of exposure to endotoxins and particulate matter 2.5 (PM2.5) has also been observed only in sensitized populations [25]. Our findings align with these studies, suggesting that the interaction between sensitization status and endotoxin exposure remains valid.
Exposure to dust endotoxins mainly affects the respiratory system, which acts as the primary site for absorption. We found that exposure to environmental dust endotoxins tended to increase deaths attributable to chronic lung disease and CVD. Due to the close connection between the respiratory and cardiovascular systems, pollutants inhaled by the respiratory system, such as PM2.5 [26–28] and nitrogen dioxide [29, 30], usually also impact the cardiovascular system. However, thus far, research on dust endotoxins has mostly focused on the respiratory system. Our findings highlight the need for further research to examine the impact of dust endotoxins on the cardiovascular system, especially in sensitized populations. Conversely, exposure to dust-containing endotoxins was associated with mortality even when individuals who died from chronic respiratory disease and CVD were excluded. This suggests that respiratory diseases and CVD might not be the only reasons for the increased mortality from exposure to endotoxins in house dust. A previous study also found that endotoxins in house dust can alter a person’s white blood cell count, which may lead to changes in multiple systems throughout the body [17]. Hence, follow-up studies are needed to explore the possible effects of endotoxins in indoor dust on systems besides the respiratory system. Alternatively, could low doses of endotoxin at indoor environmental levels, in some uncertain way, directly impact mortality without necessarily involving disease? Additional investigation is warranted, as there is uncertainty surrounding this hypothesis. Furthermore, although studies on occupational endotoxin exposure suggest that occupational-level endotoxins may be associated with a high risk of cancer, our study did not find an association between low-dose endotoxin exposure in house dust and tumor-related mortality [31, 32].
The increased mortality attributed to endotoxin appears to stem from its induction of an inflammatory response. Prolonged inflammation can exacerbate long-term mortality rates [33]. Endotoxin can trigger the innate immune system, initiating the release of proinflammatory cytokines [7]. When exposed to high doses, this systemic cytokine release can precipitate systemic inflammatory response syndrome (SIRS), leading to multiple organ dysfunction [34]. Conversely, low-dose exposure can incite chronic inflammation within the human body. A study correlating dust endotoxin exposure with human leukocyte levels also demonstrated the impact of dust endotoxin on inflammation [17]. Chronic low-grade inflammation plays a pivotal role in various CVDs [35]. This could elucidate the connection between dust endotoxin and cardiac-related mortality. Furthermore, the respiratory effect of indoor endotoxin exposure may contribute to diminished cardiac function, subsequently elevating the risk of cardiac-related mortality [36]. In our investigation, house dust endotoxins predominantly affect sensitized populations. Previous studies have demonstrated that house dust endotoxins influence sensitive populations differently than the general population in the respiratory system [15], though the mechanisms remain unclear. One review posits that the immune response to endotoxin exhibits greater complexity in asthmatic populations and that asthma may alter endotoxin tolerance mechanisms in the general population, potentially accounting for the heightened impact of endotoxins on individuals sensitive to inhalants [2]. However, experimental evidence remains insufficient to fully understand the molecular mechanisms linking dust endotoxins to mortality, particularly in susceptible populations. Future research should prioritize elucidating its signaling pathways and population-specific effects.
This study has several strengths. First, to our knowledge, this is the first study to identify an association between house dust–related exposure to endotoxins and mortality. Since the effects of house dust can be mitigated in simple ways, such as effective cleaning and the use of certain types of carpet [37], these findings may have implications for public health policy. Second, we utilized NHANES data, which covers a broad, representative section of the US population. In addition, the NHANES study on endotoxins in dust appears to be the largest nationwide study on endotoxins conducted to date. These advantages allowed for a better representation of the target population, which enabled us to maximize the size of our study. The adjustment for potential confounding factors ensured that our study had good reliability.
This study had some limitations. First, although we attempted to include as many participants as possible, the sample size was still relatively small because NHANES only collected house dust data in one cycle. A small sample size can make it difficult to conduct complex stratified studies, and at the same time, it is hard to derive clear conclusions for certain causes of mortality (such as deaths from respiratory diseases). However, our study still obtained credible results on the effect of house dust endotoxin on all-cause mortality, indicating the long-term risk of dust endotoxin exposure. Second, we cannot rule out the possibility of misclassifying causes of death. However, the NHANES LMF identifies causes of death by linking to the National Death Index, which is based on death certificates and is considered a reliable source. Additionally, our primary findings were based on all-cause mortality and were less affected by misclassification. Finally, dust endotoxin levels were measured only once and, hence, may not reflect the participants’ exposure throughout the follow-up period [38]. Long-term measurements may yield different results from a single measurement, and subsequent research should continue measuring endotoxin levels multiple times over a specific period to further clarify the relationship between endotoxin and mortality. However, previous studies have found that endotoxin concentrations are associated with lifestyle habits (e.g., using carpets and having pets) that are relatively stable in adulthood [5]. Therefore, the endotoxin concentrations used in our study may be representative of dust endotoxin exposure over a considerably long period.
In conclusion, we found that increased exposure to endotoxins in dust was associated with a high all-cause mortality in individuals sensitized to inhalant allergens. Mortality due to CVD and chronic respiratory disease showed a higher trend in those with greater endotoxin exposure levels among sensitized adults. Dust endotoxins did not demonstrate an association with cancer mortality, and there was no significant association between dust endotoxins and mortality in nonsensitized populations.
Ethics Statement
The CDC/ATSDR has determined that our research did not meet the criteria for human research as per federal regulation and therefore did not require review. Details of the IRB approval are available here: http://www.cdc.gov/nchs/nhanes/irba98.htm.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC/ATSDR.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Project administration: Yu Sun. Resources: Jia Yan. Software: Ren Zhou. Supervision: Hong Jiang. Visualization: Lei Zhang. Roles/writing—original draft: Ren Zhou and Zongzong Quan. All authors have read and approved the final manuscript. Ren Zhou and Zongzong Quan contributed equally to this work.
Funding
This work was supported by the Clinical Research Plan of SHDC (Grant Number SHDC2020CR3043B) and the Science and Technology Commission of Shanghai Municipality (STCSM) (23Y11908100 and 23YF1422700).
Acknowledgments
This study utilized data from the National Health and Nutrition Examination Survey (NHANES); the authors would like to thank all contributors and participants of NHANES.
Open Research
Data Availability Statement
This study utilized data from the National Health and Nutrition Examination Survey (NHANES). The raw data can be found on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). The data that support the findings of this study are available from the corresponding authors upon reasonable request.




