Characterizing Risk Through Particle Size Analysis of Airborne Bacteria and Fungi in a Wholesale Traditional Fish Market
Abstract
Wholesale traditional markets (WTMs) have established the most comprehensive and advanced auction systems for fresh seafood, meat, and fruits. Understanding the levels of bioaerosols of different sizes in WTMs can help to develop strategies to reduce the spread of infectious diseases. This study was aimed at analyzing and comparing the size distributions of culturable airborne bacteria (AB) and airborne fungi (AF) in a typical wholesale traditional fish market (WTFM). The AB and AF concentrations in the WTFM were relatively both high during and after the operation. The average AB concentration significantly increased from 4.73 × 103 during operation to 8.58 × 103 CFU/m3 after operation. The highest concentration of AB was observed at the fourth stage (2.1–3.3 μm), accounting for 26.22% and 28.15% of the total AB measured during and after operation, respectively. The average AF concentration remained steady from 2.77 × 103 during operation to 2.53 × 103 CFU/m3 after operation. The fourth stage also showed the highest AF concentration postoperation, comprising 35.47% of the total AF measured. Particles in this size range can be easily inhaled and deposited in the bronchial tubes, posing significant health risks. This study identified four and two types of possible pathogenicity in dominant AB and dominant AF, respectively. Commonly pathogenic Flavobacterium spp. frequently found in seafood and the highly pathogenic AF species Aspergillus tamarii and Aspergillus ochraceus were also detected. These pathogens and ultrafine biological aerosols (< 1 μm) can induce respiratory conditions such as aspergillosis. Based on these findings, the WTFM management should implement targeted interventions to reduce the concentration of harmful particles.
1. Introduction
Taiwan, located in a subtropical region, experiences high average annual temperature and relative humidity, typically ranging between 70% and 80% (with an average of 74.8% from 1991 to 2020 at the Taipei Astronomy and Weather Station, Taiwan) [1]. These conditions facilitate the production of indoor biological pollutants, primarily in the form of bioaerosols containing bacteria and fungi. Exposure to these bioaerosols can lead to respiratory issues, such as asthma, bronchitis, rhinitis, and sinusitis, and may also impair lung function. In addition, bioaerosols can trigger allergic reactions, including hay fever and other atopic conditions. In recent years, emerging infectious diseases, such as COVID-19, have been identified as airborne infections, often transmitted within public buildings with high-density populations [2–4]. Consequently, bioaerosol research has regained international attention since 2020, focusing on indoor air quality (IAQ), bioaerosol transmission, and health risks. The size of pathogenic aerosol particles significantly affects their airborne residence time and increases the risk of human exposure [5]. Bioaerosols are a significant concern for the IAQ in Taiwan. Poor IAQ owing to high bioaerosol concentrations can lead to various health issues. Long-term exposure to bioaerosols can have serious health implications, particularly for vulnerable populations, such as children and older adults. These health effects underscore the importance of monitoring and controlling bioaerosol exposure, particularly in occupational and indoor environments [6].
Indoor bioaerosols, including bacteria, fungi, viruses, and their byproducts (e.g., endotoxins and spores), can vary significantly in size and can range from approximately 0.05 to 100 μm. These particles can remain airborne for extended periods and have a significant impact on human health. Understanding the size distribution of bioaerosols is crucial for assessing their effects on human health and for developing effective mitigation strategies. These small particles can be inhaled and deposited in the respiratory tract, leading to various health effects owing to their ability to penetrate into the respiratory system [7]. The size distribution of indoor bioaerosols is influenced by various biotic and abiotic factors. Biotic factors include human activities, microbial sources, and biological decay. The abiotic factors include temperature, humidity, ventilation systems, air exchange rates, and seasonal variations. These factors collectively determine the concentration and size distribution of bioaerosols in indoor environments and can have significant implications for IAQ and human health.
Taiwan’s wholesale traditional markets (WTMs) have developed the most comprehensive and advanced auction system for fresh seafood, meat, and fruits, providing producers, suppliers, sellers, and consumers with top-notch professional services. WTMs also attract customers who operate restaurants, retail shops, and street carts seeking fresh food and household supplies. The health risks to indoor WTM staff and customers exposed to high concentrations of bioaerosols require careful evaluation as these individuals spend long periods in the market for stall preparation, product selling, and cleaning. However, this information on the concentration of bioaerosols in WTMs is limited. A previous study showed airborne bacterial (AB) contamination in a typical wet market with a live poultry trade [8, 9]. Corynebacterium minutissimum and other pathogenic bacteria accounted for 0.81%–8.02% of the microbial community. Bioaerosols containing AB and airborne fungi (AF) can be generated, often as a result of poor sanitation and interactions with staff and customers. Moreover, food-processing areas in hot and humid climate environments must consider the airborne biological hazards associated with seafood. These hazards may arise from seafood spoilage and transmission of foodborne diseases [10].
The distribution of AB and AF in wholesale traditional fish markets (WTFMs) depends on the goods sold. For example, Serratia liquefaciens, Pseudomonas spp., Kocuria rhizophila, and Flavobacterium spp. are often found in the air in fish processing plants. In particular, the bleeding areas in both factories exhibited medium to high bacterial counts over several days [11]. Seafood processing workers are at health risk due to exposure to bioaerosols [11, 12]. Cleaning of seafood and the environment by workers after their activities increases endotoxin levels in the environment, increasing the exposure risk [11, 13]. In WTFMs, such as seafood markets in the coastal regions of Mexico, bioaerosols forming the indoor microbial community can vary depending on biotic or abiotic factors, such as the products sold. Each WTFM may have unique characteristics influenced by the local culture, types of seafood available, and regional practices. High counts of aerobic mesophiles, molds, and yeasts have been quantified in the air of popular fish processing and marketing. This indicates that the air in seafood processing zones is laden with biological hazards, potentially leading to food contamination and health risks for workers [10].
This study is aimed at analyzing and comparing the size distributions of culturable airborne AB and AF in a typical WTFM during and after market operations. It also identifies representative AB and AF species frequently found in WTFMs and evaluates bioaerosol concentrations of different particle sizes to staff and customer activities. Several key innovations are researched in this study. First, it systematically examines the size distributions of culturable AB and AF under varying operational conditions, offering insights into how human activity influences bioaerosol concentrations. Second, it identifies bacterial and fungal species, enhancing our understanding of microbial exposure risks in WTFMs. Third, by correlating bioaerosol levels with market activities, including vendor transactions and cleaning processes, this study provides dynamics on fluctuations of bioaerosols. Lastly, its findings contribute to assessing occupational and public health risks associated with bioaerosol size variations in WTFMs, supporting air quality management strategies to control the spread of infectious diseases.
2. Methods
2.1. Basic Information on the Case Study of the WTFM
The indoor WTFM selected in this study was located on the edges of urban areas in northern Taiwan, serving as the greatest hub for fishery producers, processors, and consumers. The WTFM, built from reinforced concrete in 1975, had a 12,924 m2 indoor area. No mechanical ventilation was used in the building during the experiment. Mechanical ventilation systems usually reduce the concentration of bioaerosols [14, 15]. Advanced mechanical ventilation systems, particularly those with increased fresh air changes per hour and HEPA filters, are highly effective in reducing indoor bioaerosol concentrations [16]. Currently, the WTFM relies solely on natural ventilation, such as open windows; however, the implementation of a mechanical ventilation system is being considered for future improvements.
The WTFM operates daily from 2:00 AM to 12:00 PM, Tuesday through Sunday. The WTFM has more than 300 stalls, which offer a wide variety of seafood, ranging from freshly caught local fish to premium imported items. Moreover, there are many delicatessens for market staff in the resting area. A statistical report in 2021 shows the WTFM traded approximately 20,000 tons of fishery products daily. During auction operating hours, hundreds of people, including staff and customers, stayed in the WTFM simultaneously.
2.2. Sampling Design
According to an analysis of climate change trends in Taiwan [1], the temperature–humidity index in northern Taiwan has increased annually since the baseline period (1995–2014). This indicates that the environmental conditions of WTFMs in northern Taiwan will continue to worsen and that the health risks posed by AB and AF in indoor WTFMs will only intensify for workers. The indoor temperature of the market was an average of 23.3°C, the humidity was an average of 86%, and the wind speed ranged from 0 to 1.0 m/s in studied area. To understand the differences in bioaerosol distribution between indoor and outdoor environments, nine sampling points were designed. An additional outdoor sampling location, serving as a control, was marked in the legend “※” in the legend. Table 1 lists the vendors around each sampling location and the coordinates of the WTFM. The sampling in the WTFM was conducted at 09:35–12:00 (during the operation) and 12:30–16:20 (after the operation) on June 2, 2010. This study specifically focused on the impact of bioaerosol particle size distribution within the WTFM on immediate exposure risks to receptors. Therefore, sampling locations were selected near vendor stalls. To avoid disrupting market operations, only a single day’s sampling data could be collected. Until now, the WTFM’s operational model and environmental conditions remained unchanged. The aerobic plate counts of AB and AF present in the indoor WTFM were analyzed using culture-based standard methods. A distance meter (Laser HD 50, Trimble Inc., CO, United States) was used to measure the distance between the sampling points, and a configuration diagram was created, as shown in Figure 1.
| Sampling location no. | Position coordinates (x, y)a |
|---|---|
| a | (0, 0.5) |
| b | (0, 8.5) |
| c | (0, 16.5) |
| d | (−8, 0.5) |
| e | (−8, 8.5) |
| f | (−8, 16.5) |
| g | (−14, 0.5) |
| h | (−14, 8.5) |
| i | (−14, 16.5) |
| Control | (−17, 1) |
- aUnit: millimeters.
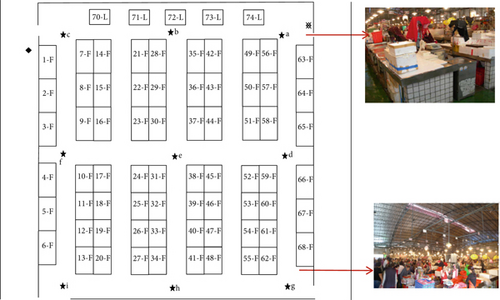
The method of bioaerosol collection in the WTFM followed previous studies [9, 17], based on the Sampling Indoor Bacteria Method (NIEA E301.15C) and the Sampling Indoor Fungi Method (NIEA E401.15C) of Taiwan’s Ministry of Environment (https://www.moenv.gov.tw/nera). This study used an Andersen six-stage viable Anderson Cascade Impactor (TE-10-800, Tisch Environmental Inc., OH, United States), which was wiped and sterilized using 70% ethanol before and after each sampling period. The sampling flow rate was 28.3 L/min, which was adjusted using a dry-flow calibration equipment (Defender L520, MesaLabs, CO, United States). Bioaerosol sampling was performed in triplicate at each sampling location on June 2, 2010. The sampled Petri dishes of AB and AF were incubated at 30°C ± 1°C for 48 ± 2 h and at 25°C ± 1°C for 4–5 days, respectively. The number of AB/AF colonies in each plate was determined. A positive hole correction factor was applied to adjust the colony-forming units (CFUs) according to Anderson’s conversion table [18]. Two-dimensional contour plots of the AB and AF concentration distributions within the WTFM were analyzed using contour lines with Surfer Version 12.0.
2.3. Principal Component Analysis (PCA)
PCA is a powerful tool for processing environmental monitoring data, such as bioaerosols and water parameters. This study was aimed at clarifying the relationship between various sized particles of bioaerosols and the WTFM, following our previous study [17]. In this study, R software (X64, Version 4.2.1) was used to analyze and visualize the relationships between the various characteristics of the different sizes of AB/AF at various sampling points in the WTFM using PCA. The raw data analysis was performed by the built-in PCA command of the R language, and the visualization of analyzed data used the package “factoextra.” The correlation plot was analyzed and illustrated by the package “corrplot” in R.
2.4. Strain Identification
The single colonies of representative bacterial and fungal strains that appeared at a high frequency on the Petri dishes were isolated and purified completely using the streaking method using growth on tryptone soy agar at 30°C ± 1°C for 48 h and on malt extract agar (MEA) at 25°C ± 1°C for 4–5 days, respectively. The bacterial colonies were isolated and identified with all sequencing regions (V1–V9) in the 16S rRNA whole genes by Mission Biotech (Taipei, Taiwan). Fungal colonies were identified with the extraction of genomic DNA, PCR amplification of the ITS1/ITS2 18S rRNA region, and sequencing by Mission Biotech. The results were used to search the NCBI database for the closest taxonomic relatives of the selected strains.
3. Result and Discussions
3.1. Distribution of Cultural AB/AF in the WTFM
Figure 2a shows that the average AB concentration is 4.73 × 103 CFU/m3 with a range of 2.76 × 103 (Sampling Point i) to 7.74 × 103 CFU/m3 (Sampling Point e) during WTFM operations (auction hours). The AB concentration at the outdoor sampling location (the control) is 2.36 × 103 CFU/m3. The AB concentrations at all nine sampling points exceeded the Taiwan IAQ standard of 1500 CFU/m3. The AB size distribution was similar in the indoor traditional wet markets (TWMs), with the highest proportion of smaller particle bioaerosols. These particles are likely deposited in the bronchial tubes of the respiratory tract after inhalation. Moreover, the higher concentrations of AB sampling points (hotspots) in the WTFM, specifically Points d, e, and f, ranged from 5.34 × 103 to 7.74 × 103 CFU/m3. This aisle is at the center of the market, where many vendors are clustered, and customer density is high. There is a wide range of AB concentrations in different markets owing to the types of products sold. Typical non-air-conditioned markets selling fresh seafood, vegetables, meat, and poultry in Hong Kong showed low concentrations of ABs, ranging from 4.01 × 103 to 7.43 × 103 CFU/m3 [19]. The highest concentration of culturable airborne microorganisms (9.62 × 104 CFU/m3) in these markets was found along the indoor footpath in Hong Kong. After the market operation, the average AB concentration was 8.58 × 103 CFU/m3, as shown in Figure 2b. The concentration ranged from 8.49 × 102 (Sampling Point a) to 2.78 × 105 CFU/m3 (Sampling Point b). The AB concentration at the outdoor sampling location (the control) is 2.01 × 103 CFU/m3. The cessation of operation in the WTFM did not reduce the concentration; instead, it increased by 81%, with each stage contributing to the increase, as shown in Figure 3. The higher concentrations of AB at Sampling Points b, c, f, and g (hotspots) ranged from 1.05 × 105 to 2.78 × 105 CFU/m3. This increase was attributed to the sampling locations being at entrances and locations where the market staff continued cleaning activities, restocking, and preparation for the next day. This finding is consistent with international research [11]. Additionally, many food vendors at Sampling Locations b and c remained open and served customers after market operation. These activities can stir dust and bioaerosols, thus increasing the AB concentration in the air.
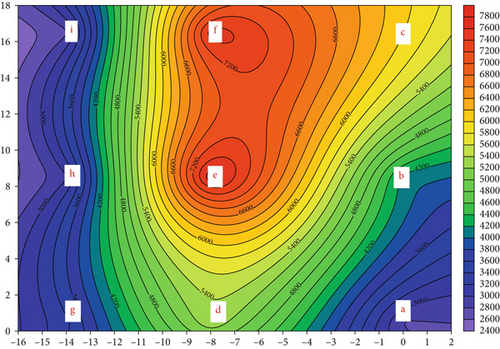


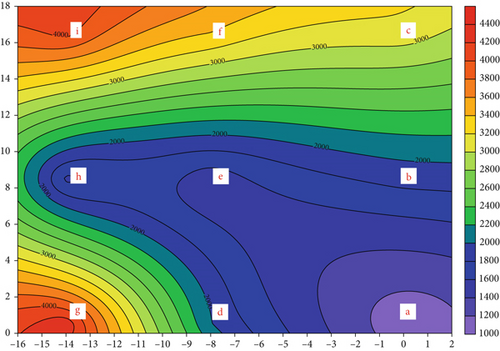
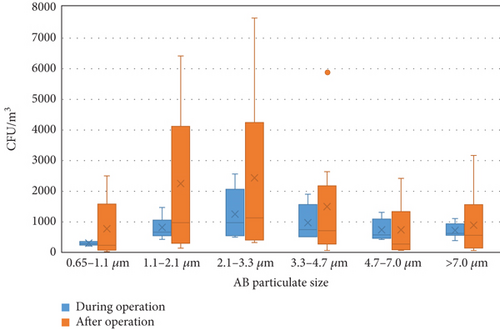
Figure 2c shows that the average concentration of AF at the nine sampling points is 2.77 × 103 CFU/m3 during operation, with a range of 1.73 × 103 (Sampling Point b) to 4.28 × 103 CFU/m3 (Sampling Point e). The AF concentration at the outdoor sampling location (the control) is 1.66 × 103 CFU/m3. A similar AF level was reported in our previous study [9]. After market operations had finished, the bioaerosol levels in the livestock and poultry fresh raw meat districts of the TWM were 1.06 × 103 and 6.60 × 102 CFU/m3, respectively. Another study reported that AF concentrations in the WTFM ranged from 1.13 × 105 to 1.04 × 106 CFU/m3 in the vegetable market due to the presence of fungal spores [20]. Figure 2d shows that the average AF concentration decreases to 2.53 × 103 CFU/m3 after the operation. The AF concentration at the outdoor sampling location (the control) is 1.48 × 103 CFU/m3. The AF concentration decreased by only 9% in the WTFM compared to that during operation. Figure S4 shows the detailed size distribution of the AF concentration after WTFM operation, with the highest portion (31.7%–41.0%) in the fourth stage at eight sampling points (a, c, d, e, f, g, h, and i) and the lowest portion (0.0%–5.7%) in the sixth stage at all sampling points.
3.2. A Comparison of Size Distribution AB Bioaerosols in the WTFM During and After the Operation
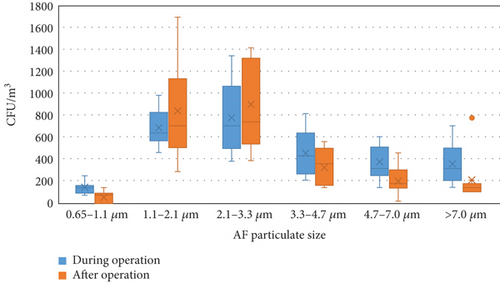
Figure 3 shows the distribution of the AB concentrations of different sizes in the WTFM. Figure S1 illustrates the size distribution of AB concentrations during operation, with the highest portion (24.22%–33.11%) on the fourth stage (2.1–3.3 μm) at five sampling points (b, d, e, f, and g) and the lowest portion (3.78%–8.96%) on the sixth stage (0.65–1.1 μm) at all sampling points. The concentration at the fourth stage (2.1–3.3 μm) was the highest, with an average concentration of 1.24 × 103 ± 7.83 × 102 CFU/m3, accounting for 26.22% of the total AB measured, followed Stage 3 (3.3–4.7 μm) with an average concentration of 9.85 × 102 ± 5.85 × 102 CFU/m3, accounting for 20.83%. The sixth stage (0.65–1.1 μm) had the smallest concentration, with an average concentration of 7.93 × 102 ± 3.27 × 102 CFU/m3, accounting for 6.06% of the total AB measured. The size distribution of AB concentrations at the outdoor sampling location (the control) is different with the highest portion (22.38%) on the first stage (> 7.0 μm), the second portion (20.90%) on the third stage (3.3–4.7 μm) and the fourth stage (2.1–3.3 μm), and the lowest portion (4.48%) on the sixth stage (0.65–1.1 μm). Conversely, Figure S2 shows the size distribution of AB concentrations after operation, with the highest portion (27.3%–37.5%) on the fourth stage (2.1–3.3 μm) at six sampling points (a, b, d, g, h, and i) and the lowest portion (5.05%–10.07%) on the second stage (4.7–7.0 μm) at six sampling points (a, b, c, e, f, and g). The concentration at the fourth stage (2.1–3.3 μm) remained the highest, with an average concentration of 2.41 × 103 ± 2.58 × 103 CFU/m3, accounting for 28.15% of the total AB measured, followed by the fifth stage (1.1–2.1 μm) with an average concentration of 2.23 × 103 ± 2.27 × 103 CFU/m3, accounting for 26.09%. The second stage (4.7–7.0 μm) had the smallest concentration, with an average concentration of 7.42 × 102 ± 8.26 × 102 CFU/m3, accounting for 9.33% of the total AB measured. The size distribution of AB concentrations at the outdoor sampling location (the control) is different with the highest portion (28.05%) on the fourth stage (2.1–3.3 μm), the second portion (19.31%) on the second (4.7–7.0 μm) stage, and the lowest portion (3.53%) on the sixth stage (0.65–1.1 μm).
The size distribution of bioaerosols critically governs their deposition within the human respiratory tract, with implications for occupational health in high-risk environments such as WTFMs. The aerodynamic diameter of bioaerosols determines their interaction with airway structures and transport mechanisms, primarily through inertial impaction, sedimentation, diffusion, interception, and electrostatic forces [21]. Large bioaerosols (> 5 μm), including pollen, fungal spores, and certain bacteria, predominantly deposit in the upper respiratory tract (nasopharynx and oropharynx) via inertial impaction and sedimentation due to their mass and momentum [22]. Such depositions of bioaerosols may precipitate localized infections (e.g., sinusitis) or hypersensitivity reactions. Intermediate-sized bioaerosols (1–5 μm), such as single bacterial cells, primarily undergo sedimentation, with some inertial impaction, enabling penetration into the lower respiratory tract, including bronchi and bronchioles [23]. This size range is critical for airborne transmission of diseases like tuberculosis and influenza, as reduced airflow velocity in smaller airways facilitates particle settling onto epithelial surfaces. Specifically, bioaerosols within the 2.1–3.3 μm range are prevalent and pose significant health risks due to their ability to penetrate the lower respiratory tract [24]. Particles in the 1–2.5 μm range can reach the alveoli via diffusion, while those in the 2.5–5 μm range may deposit in the trachea, potentially causing infections, allergies, or toxic reactions [25]. Notable pathogens include Pseudomonas aeruginosa (1.5–3 μm), an antibiotic-resistant bacterium prevalent in market meat, which poses infection risks due to its environmental adaptability and virulence [26]. Similarly, Mycobacterium tuberculosis (2–4 μm) is associated with respiratory infections [27], while fungal spores like Aspergillus fumigatus (2–3 μm) can induce conditions such as aspergillosis, particularly in immunocompromised individuals, who face elevated risks of invasive, potentially fatal infections [28]. Smaller bioaerosols (< 1 μm), including viruses and ultrafine biological aerosols, are governed by diffusion (Brownian motion), allowing prolonged suspension in the air for at least 12 h and deep penetration into the pulmonary alveoli [5, 29]. Additionally, bioaerosols in the WTFM could carry antibiotic resistance genes (ARGs), which, upon inhalation, may transfer to human pathogens via horizontal gene transfer, exacerbating the risk of recalcitrant infections [30]. Vulnerable populations, including children, the elderly, and individuals with chronic respiratory or cardiovascular conditions [31], face heightened risks from prolonged exposure in the WTFM. These findings underscore the need for targeted mitigation strategies to reduce occupational health risks associated with bioaerosol exposure in such environments.
PCA was used to explore the correlation between the particle size distribution of the AB in the WTFM during and after the operation in this study. Figure 4 shows the PCA profile of AB, which explains 97.7% of the variance and is divided into Groups I and II. Figure S5 shows a scree plot of the PCA eigenvalues for the AB concentration of the WTFM during and after the operation. Table S1 shows the comprehensive evaluation values for AB in the indoor WTFM during and after operation. Group I included all nine sampling locations in the WTFM during the operation, five sampling locations (2a, 2d, 2e, 2h, and 2i) in the WTFM after the operation, and two outdoor sampling locations (1NC and 2NC). Group II includes four sampling locations (2b, 2c, 2g, and 2f) in the WTFM after operation. It was inferred that the characteristics of the AB size distribution at the five sampling locations (a, d, e, h, and i) were similar in the WTFM during and after the operation. In this study, the distribution of bioaerosols in TWMs was significantly influenced by activities and products sold, including fresh seafood. Seafood is a primary source of bioaerosols in the WTFM. Vendors in these sampling locations primarily sold fresh seafood on ice or in open containers, such as fish and other marine products (shrimp, clams, crabs, and mussels). These products can carry a variety of microorganisms, which become airborne during selling, handling, gutting, filleting, or washing during and after the operation. Bioaerosols contained protein, parvalbumin (a fish allergen), and endotoxins, directly linked to the fish being processed [13, 32]. These bioaerosols contain various components such as aerosolized seafood particulates and gram-negative bacteria, which can lead to adverse health effects. On the other hand, seafood processing bioaerosols present significant health risks, particularly respiratory issues, to workers in the industry [12, 33]. Workers exposed to higher allergen levels during seafood processing are at a higher risk of developing respiratory symptoms, asthma, and impaired lung function. Exposure to gram-negative bacteria is a concern in salmon processing plants, which can be linked to increased respiratory symptoms among workers in the presence of endotoxins [34]. Additionally, biological residues such as blood, guts, and contaminated storage ice are generated from fish processing and selling in the WTFM. The WTFM staff used powerful water jets to clean the ground, raising dust and dirt in the WTFM after the operation. This process can aerosolize microorganisms in the water used for cleaning and contribute to higher AB concentrations during and after the operation. Using water jets can inadvertently raise dust and dirt in the air, particularly if the pressure is not optimized [35]. This can lead to temporary air quality issues. For example, a cleaning process using agitated water spouts decreased the concentration of bacterial bioaerosols in the market by an average of 64% [9]. Conversely, the same cleaning process increased the AF concentration by approximately 2.4 times. The use of high-pressure water during cleaning can release bioaerosols in the salmon processing, where higher exposure levels were noted in workers using water hoses [32].
3.3. A Comparison of Size Distribution AF Bioaerosols in the WTFM During and After the Operation
Figure 5 shows the AF concentration distributions for different particle sizes in the WTFM. During the operation, the average concentration in the fourth stage (2.1–3.3 μm) in the WTFM was the highest (average concentration of 7.73 × 102 ± 3.24 × 102 CFU/m3), accounting for 27.91% of the total AF measured, followed by that in the fifth stage (1.1–2.1 μm) with an average concentration of 6.82 × 102 ± 1.69 × 102 CFU/m3, accounting for 24.64%. The particle concentration in the sixth stage (0.65–1.1 μm) was the smallest (average concentration of 1.33 × 102 ± 5.5 × 101 CFU/m3), accounting for 4.82% of the total AF measured. The size distribution of AF concentrations at the outdoor sampling location (the control) is different with the highest portion (36.16%) on the fourth stage (2.1–3.3 μm), the second portion (23.41%) on the fifth (1.1–2.1 μm) stage, and the lowest portion (4.27%) on the sixth stage (0.65–1.1 μm). Figure S3 shows the size distribution of the AF concentration during operation, with the high portion (24.51%–33.01%) in the fourth stage (2.1–3.3 μm) in eight sampling points (a, b, c, d, e, f, g, and i) and the low portion (2.01%–9.20%) in the sixth stage (0.65–1.1 μm) in all nine samplings points. After the operation, the concentration in the fourth stage (2.1–3.3 μm) remained the highest (average concentration of 8.99 × 102 ± 4.10 × 102 CFU/m3), accounting for 34.57% of the total AF measured, followed by the fifth stage (1.1–2.1 μm) (average concentration of 8.40 × 102 ± 4.44 × 102 CFU/m3), accounting for 33.14%. The sixth stage (0.6–1.1 μm) had the smallest concentration (average concentration 5.6 × 101 ± 5.0 × 101 CFU/m3), accounting for 2.22% of the total AF measured. Figure S4 shows the size distribution of the AF concentration after the operation, with the highest portion (30.17%–41.02%) in the fourth stage (2.1–3.3 μm) in all sampling points and the lowest portion (0.00%–5.66%) in the sixth stage (4.70–7.0 μm) in all sampling points. The size distribution of AF concentrations at the outdoor sampling location (the control) is different with the highest portion (33.36%) on the fourth stage (2.1–3.3 μm), the second portion (19.07%) on the fifth stage (1.1–2.1 μm), and the lowest portion (7.14%) on the sixth stage (0.65–1.1 μm).
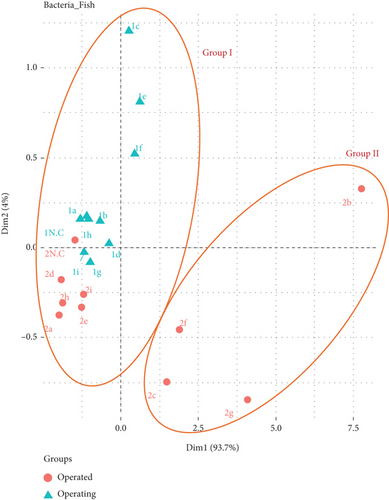
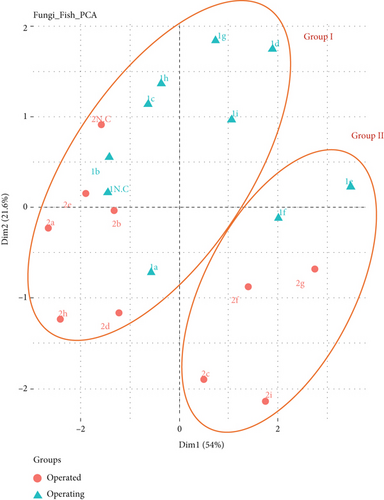
PCA was used to explore the correlation between the particle size distribution of AF in WTFM during and after operation. Figure 6 shows the PCA profile of AF in the WTFM, which explains 75.6% of the variance and is divided into Groups I and II. Figure S6 shows a scree plot of the PCA eigenvalues for the AF concentration of the WTFM during and after the operation. Table S2 shows the comprehensive evaluation values for AF in the indoor WTFM during and after the operation. Group I included seven sampling points (1a, 1b, 1c, 1d, 1g, 1h, and 1i) in the WTFM during the operation, five sampling points (2a, 2b, 2d, 2e, and 2h) in the WTFM after the operation, and two outdoor sampling locations (1NC and 2NC). Group II included two sampling points (1e and 1f) in the WTFM during the operation and four sampling points (2c, 2f, 2g, and 2i) in the WTFM after the operation. Similar characteristics of the AF size distribution were observed at four sampling locations (a, b, d, and h) in Group I and one sampling location (f) in Group II during and after operation. The distribution ratio of the various AF particle sizes is related to customer activity and the different products sold in the WTFM. For Group I, vendors around the sampling locations (a, b, d, and h) observed the ongoing refrigeration and storage process of fresh fish during and after operation. Fresh fish products are highly perishable, requiring proper refrigeration and storage to maintain quality and ensure safety. However, refrigeration units of fresh fish products in the WTFM may not be consistently maintained due to high usage and poor maintenance. These high risks are primarily due to the growth of fungi and the production of mycotoxins. Psychrotrophic fungi such as Penicillium and Aspergillus are capable of surviving and growing in refrigerated environments, making them significant concerns for food storage and safety [36]. These fungi can thrive at low temperatures and are commonly found in the air of food storage refrigerators. Common AF species associated with fishes include Aspergillus, Penicillium, and Fusarium. These fungi are prevalent in fresh and dried fish, often because of poor handling and storage conditions [37–39]. The workplace in the WTFM also has high moisture from melting ice and cleaning and inadequate storage conditions, which create ideal conditions for fungal growth. AF species, such as Aspergillus flavus and Aspergillus parasiticus, are increasing in humid environments, leading to aflatoxin production [39, 40]. Fungal populations such as yeasts and molds can increase significantly under refrigeration within a few days [41]. Moreover, mycotoxins such as aflatoxin B1, ochratoxin A (OTA), and T-2 are common contaminants in fish products. These toxins pose significant health risks to consumers and are present in WTFMs under poor storage conditions [37, 42]. For Group II, a few vendors at the sampling point f processed fresh seafood and prepared to send it to other markets. AF in seafood processing environments could pose significant health risks owing to the presence of pathogenic fungi and mycotoxins in the WTFM. Vendors at the sampling point for fresh fish (seafood) processing may introduce biological contamination during handling and packaging before transportation to other markets. Potential sources of cross-contamination include exposure to unclean surfaces, inadequately sanitized equipment, contaminated water utilized in the processing stages, or infected personnel. Studies have identified a high diversity of fungal species in dried fish products obtained from the Zhanjiang market in China [37]. The AF produces mycotoxins that are hazardous to human health. Moreover, AF can lead to various health issues, including hypersensitivity pneumonitis and immunotoxic diseases. Exposure to other bioaerosols can exacerbate these conditions, potentially decreasing normal respiratory conditions or interacting with diseases such as COVID-19 [43].
3.4. Dominant AB Identification and Their Possible Pathogenicity in the WTFM
There were 15 different AB colonies in the WTFM identified by culture, comprising eight gram-negative pure strains and seven gram-positive pure strains. Table 2 shows the three bacterial genera frequently observed in the WTFMs. Domain species have been identified in markets that sell products from different countries. For example, gram-positive Bacillus and Penicillium spp. in traditional bazaars in Iran have the highest bacterial and fungal populations, respectively [44]. The most frequently detected bacteria were Bacillus, Corynebacterium, Diplococcus, Micrococcus, Acinetobacter, Alcaligenes, Enterobacter, and spore-forming rods. Another study reported that AB species were frequently (> 50%) detected in an arcade-type traditional market in Anseong, South Korea, including three pathogenic species, Bacillus subtilis, Bacillus licheniformis, Staphylococcus xylosus, and one nonpathogenic species Bacillus megaterium [45]. This study reveals the characteristics of AB that pose potential health risks to humans. Flavobacterium spp. are gram-negative, nonmotile, motile, rod-shaped bacteria comprising > 130 recognized species. Most species are aerobic, whereas others are microaerobic or anaerobic. They are found in the soil and freshwater in various environments. Flavobacterial diseases cause devastating losses to farmed and wild fish populations worldwide. Infections are the second leading cause of mortality in pond-raised catfish in the southeastern United States [46]. Three species, including all the species identified in this study, are known to cause diseases in freshwater fish. It has been detected in raw meat, dairy, and other foods as well as in clinical specimens from hospital settings and humans. Flavobacterium psychrophilum causes bacterial cold-water diseases in salmonids and rainbow trout fry. Infection can occur horizontally, between fish via waterborne and contact exposure, and vertically, because of its association with the early life stages of fish [47]. Flavobacterium columnare causes cotton-wool disease in freshwater fish, one of the oldest known diseases among warm-water fish, and manifests as an infection commonly known as columnar [48]. Flavobacterium branchiophilum causes bacterial gill disease in trout, which is characterized by the presence of numerous bacteria on the surface of the gill epithelium that severely affects the respiratory function of infected fish [49].
| Strain IDa | Species (NCBI identify, %) and gram stain’ resultc | Microscopic picture | Appearance of frequency (%) |
|---|---|---|---|
| I-B-1 | Flavobacteriaceae sp. (98%) G− |  |
55% (5/9)b |
| I-B-2 | Kocuria marina (98%) G+ |  |
100% (9/9)b |
| I-B-3 | Staphylococcus sp. (98%) G+ |  |
88% (8/9)b |
| I-B-5 | Staphylococcus sciuri (99%) G+ |  |
77% (7/9)b |
| I-F-2 | Aspergillus tamarii (99%) |  |
66% (6/9)b |
| I-F-3 | Aspergillus ochraceus (99%) |  |
77% (7/9)b |
- aB, bacteria; F, fungi.
- bNumber of sampling points that airborne bacteria or fungi were found per total number of sampling points.
- cG+ is gram-positive bacteria; G− is gram-negative bacteria.
Kocuria sp. is a gram-positive, nonencapsulated, nonhalophilic, nonendospore-forming bacterial species. The most common types of infections are bacteremia, skin and soft tissue infections, endophthalmitis, infective endocarditis, and peritonitis, most commonly peritoneal–dialysis-associated [50]. Kocuria marina is typically considered nonpathogenic to humans. It is commonly found in marine environments and has not been reported to cause infections or illnesses in healthy individuals. However, in rare cases, it may cause opportunistic infections in immunocompromised patients. The effect of K. marina on health can vary depending on an individual’s overall health status and immune system function. A case of spontaneous bacterial peritonitis due to K. marina has been reported in a 2-year-old child with no underlying risk factors [51]. Moreover, Kocuria carniphila is a type of bacteria that is commonly found in the soil environment and is of low pathogenicity to humans. In general, K. carniphila isolated from meat or food [52] is not known to cause infections or illnesses in humans; however, it may cause infections in immunocompromised individuals in rare cases.
Staphylococcus is a genus of gram-positive, oxidase-positive, coagulase-negative bacteria that appear spherical (cocci) and form grape-like clusters under a microscope. Staphylococcus species are facultative anaerobic organisms. Although many Staphylococcus species are harmless, some can cause infections when they enter the body through breaks in the skin or mucous membranes. The effects of Staphylococcus on health can range from minor skin infections to life-threatening conditions, particularly in individuals with weakened immune systems or underlying health issues. It can rapidly transition from methicillin-sensitive to methicillin-resistant states and possesses a set of highly efficient virulence factors [53]. Moreover, specific Staphylococcus sciuri, identified in the WTMs, is considered part of the normal skin flora of humans and animals, and it can also act as an opportunistic pathogen, causing infections in certain circumstances [54]. It is an emerging bacterial pathogen that has rarely been isolated from humans. S. sciuri infections can lead to skin infections, wound infections, bacteremia (bloodstream infections), and other more severe conditions, such as infective endocarditis, urinary tract infection, and pelvic inflammatory disease [55].
3.5. Dominant AF Identification and Their Possible Pathogenicity in the WTFM
Different AF colonies were identified by culture comprising 18 strains in the WTFM. Table 2 shows two mold genera, Aspergillus, that are frequently observed in WTMs. Aspergillus spp. can produce citrinin and toxin OTA, one of the most abundant food-contaminating mycotoxins [56]. The filamentous fungus contains biseriate conidiophores. Airborne spores are potential causes of asthma in children and lung diseases in humans, such as allergic bronchopulmonary aspergillosis [57]. Aspergillus spp. are present in various ecological niches, including agricultural commodities, farmed animals, and marine species. Pig and chicken populations on farms are mostly affected by this fungus and its mycotoxins [58]. Airborne fungal genera, such as Acremonium, Aspergillus, Cladosporium, Penicillium, and Sporotrichum, are the most frequently investigated genera in Thailand [59]. A previous study reported that AF species detected frequently (> 50%) in a traditional market in South Korea were Aspergillus niger, Fusarium sporotrichioides, Penicillium variabile, and Rhizomucor pusillus [45]. The detailed characteristics of Aspergillus spp. isolated are described below. The prevalence of Aspergillus tamarii in the WTFM was 66%. This filamentous fungus has characteristic biseriate conidiophores and produces the dihydroisocoumarin mellein. The consumption of this fungus by humans and animals can lead to chronic neurotoxic, immunosuppressive, genotoxic, carcinogenic, and teratogenic effects [60]. In contrast, the appearance of Aspergillus ochraceus was 77% in the WTFM. OTA, a mycotoxin produced by A. ochraceus, contaminates food and initiates apoptosis in plant cells, thereby affecting humans [61]. Consumption of OTA has neurotoxic, immunosuppressive, genotoxic, carcinogenic, and teratogenic effects on humans. OTA has strong carcinogenic effects on the human liver and kidneys. Renal failure has been reported in humans following OTA inhalation. Allergic bronchopulmonary aspergillosis is caused by the antigenic effects of A. ochraceus [62]. A. ochraceus is associated with the development of asthma in children. In addition to lung diseases, instances of A. ochraceus causing paranasal sinusitis have been reported. Occupational environmental hazards have also been documented owing to the presence of this fungus in the organic dust of the poultry industry [63]. Workers on poultry farms exposed to contaminated organic dust suffer from lung inflammation and decreased pulmonary function.
4. Conclusions
High concentrations of indoor bioaerosols at a typical WTFM during and after operation were reported in this study. The average AB and AF concentrations during operation reached 4.73 × 103 and 2.77 × 103 CFU/m3, respectively. Moreover, the average AB concentration reached 8.58 × 103 CFU/m3 after operation, whereas the average AF concentration decreased to 2.53 × 103 CFU/m. The presence of AB and AF in TWMs poses biological hazards to market staff and customers. The highest frequency of these bioaerosols was observed within the size range of 2.1–3.3 μm during and after market operations, which is significant because these particles can deposit in the bronchial tubes when inhaled. The PCA revealed significant differences based on the impact of vendors selling products and staff performing cleaning activities at the WTFM. The dominant pathogenic species of AB and AF, such as Flavobacterium sp., A. tamarii, and A. ochraceus, were identified in the WTFM during and after the operation. To control the dispersion of indoor bioaerosols, environmental sanitation should be enhanced through proper waste collection and disposal, and market staff should consider implementing more effective cleaning methods and upgrading ventilation systems to ensure continuous air exchange in WTFM, thereby reducing the accumulation of AB and AF. In addition, a comprehensive long-term risk assessment framework for WTFMs should be developed in the future. The experimental data is applied to determine how exposure to specific AB/AF concentrations correlates with health outcomes. The exposure data is combined with dose–response models to evaluate health risks. This approach must incorporate detailed environmental monitoring data, including variables such as temperature, humidity, and wind speed, during and after operational hours. The scope of the assessment should also be expanded to evaluate a broader range of bioaerosols, such as viruses and endotoxins, to better address public health risks.
5. Study Limitations
This study presents a case study with certain limitations that should be considered when interpreting the findings. First, the experiment was conducted with a single-day limit on the number of samples collected owing to the operating WTFM, allowing only a restricted time for the study to minimize the inconvenience to customers and market staff. Sampling the AB/AF ratio of this WTFM during regular opening hours on multiple days was not possible. A single-day dataset may not represent dynamic conditions, including variations in weather or customer flow. Furthermore, the analysis of bioaerosol distribution characteristics in WTFM was based solely on samples collected in 2010. The absence of time-series data prevented this study from analyzing how the AB/AF distribution might vary over time, such as hourly, daily, or seasonally. This limitation reduces the study’s ability to generalize its findings to other WTFMs or to understand long-term trends. While this constraint limits deeper insights into temporal influences on AB/AF distribution, it is reasonable to infer that the observed patterns stem from similar biological sources and customer activities, given the consistent operational model of the WTFM in the study area to date. Moreover, this study focused solely on this specific WTFM and compared the AB/AF concentrations during and after operation to assess the receptor’s immediate exposure risk associated with different bioaerosol particle size, rather than cumulative risk over time.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization: Yi-Tang Chang. Methodology: Cheng-Che Chiang and Yi-Tang Chang. Investigation: Cheng-Che Chiang. Writing: Cheng-Che Chiang, I-Chun Chen, and Yi-Tang Chang. Original draft preparation: Wen-Te Liu, I-Chun Chen, Yi-Tang Chang, and Sai Hung Lau. Writing, reviewing, and editing: Wen-Te Liu, Yi-Tang Chang, and I-Chun Chen. Supervision: Yi-Tang Chang. Project administration: Yi-Tang Chang. Funding acquisition: Yi-Tang Chang. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Taiwan National Science and Technology Council (Grant Number MOST106-2221-E-031-001-MY2).
Acknowledgments
The authors thank Dr. Hsi-Ling Chou for assisting with airborne bioaerosol sampling in this study and Dr. Da-Jiun Wei for his help in visualizing the figures in this paper.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




