Fabrication of Cinnamaldehyde-Loaded Polycaprolactone (PCL) Electrospun Nanofibers as a Potential Antibiofilm Preventative Approach Against Escherichia coli
Abstract
The bacterial biofilm formation plays an important role in the spread of antibiotic resistance. Therefore, there is an urgent need to find an alternative approach to antibiotics to inhibit biofilm-related infections. Electrospinning of nanofibers provides a high level of drug loading capacity and encapsulation efficiency. This study is aimed at inhibiting and dispersing the biofilm formation of Escherichia coli by using cinnamaldehyde-loaded polycaprolactone (PCL) electrospun nanofibers. Two percent cinnamaldehyde-loaded 12% PCL nanofibers were fabricated using the electrospinning technique. The morphology of fabricated nanofibers was examined using scanning electron microscopy (SEM). The biofilm formation inhibition and dispersion activity of cinnamaldehyde-loaded PCL nanofibers were tested using a crystal violet assay. Cytotoxicity assessment of cinnamaldehyde against HFF-1 cell line was conducted in vitro using the MTS assay. Cinnamaldehyde at a sub-MIC level of 70 and 150 μg/mL was able to inhibit the biofilm formation by 65% and 100%, respectively. For the biofilm dispersal, it was able to remove the formed biofilm by 87% and 100%, respectively. The cinnamaldehyde-loaded PCL nanofibers were fabricated using the electrospinning technique with an EE of 83.58% and DL of 119.35 μg/mg. Similarly, no growth of bacterial cells was observed at 6 mg/mL disk of 2% of cinnamaldehyde-loaded 12% PCL nanofibers, with a complete (100%) removal of biofilm. However, all cinnamaldehyde concentrations (5000–30 μg/mL) exhibited inhibitory effects on HFF-1 cells. Due to its toxicity, the current study suggests using cinnamaldehyde on nonliving surfaces as a powerful antibacterial agent. This study demonstrated the effectiveness of cinnamaldehyde-loaded PCL nanofibers in inhibiting and dispersing E. coli biofilm formation. This approach may be considered a possible strategy to inhibit hospital-acquired infections by covering surfaces and biomedical devices with cinnamaldehyde-loaded PCL nanofibers.
1. Introduction
Biofilm-related infections are increasing in healthcare, both in devices and nondevice-related infections [1]. Most biofilms are tolerant to antibiotics and immune responses. Therefore, there is an urgent need to find an alternative approach to antibiotics for inhibiting biofilm-related infections [1]. Biofilm can be overcome by inhibiting its formation or by dispersal/removal of the already formed biofilm [2]. In general, antibiofilm agents include natural compounds, peptides, and extracellular polymeric substances (EPSs) degrading enzymes that target biofilm formation [2]. Cinnamaldehyde, also known as trans-cinnamaldehyde, cinnamal, or cinnamic aldehyde, is the main component of cinnamon essential oil, naturally occurring in parts of cinnamon trees of Cinnamomum species [3, 4]. Among essential oils, the antimicrobial potential was obtained from Cinnamomum cassia (C. cassia) and Cinnamomum zeylanicum (C. zeylanicum) [3]. C. cassia, traditionally known as “Chinese cinnamon,” is a permanent tree native to the southern China [5]. Generally, C. cassia is used in Western medicine to treat illnesses such as diarrhea, cramps, lack of appetite, flatulent, gastric ulcers, cough, bronchitis, renal weakness, spasms, palpitation, arthritic angina, and fungal/bacterial infections of the skin [3, 6, 7]. C. zeylanicum is a native tree in some areas of India and Ceylon, which is known as “cinnamon-of-ceylon” [8]. C. zeylanicum is used in folk medicine due to its various medicinal properties, including antiseptic, astringent, aperitif, aphrodisiac, stimulant and vasodilator, aromatic, sedative, carminative, digestive, antidiabetic, antinociceptive, and diuretic [3, 9]. Many studies proved the antimicrobial activities of cinnamaldehyde [3, 4, 10]. It was reported that cinnamaldehyde has antibiofilm activities against Gram-negative and Gram-positive bacteria and yeasts [3, 4, 10–13].
Additionally, it was reported that cinnamaldehyde can disrupt biofilm formation by reducing the expression of intracellular levels of c-di-GMP [14]. Generally, high levels of c-di-GMP stimulate the expression of several adhesins and biofilm-associated exopolysaccharides and decrease the expression or activity of flagella [15]. Cinnamaldehyde also inhibits the quorum sensing of Pseudomonas aeruginosa (P. aeruginosa) by repressing the expression of rhlA, lasB, and pqsA [16]. The mode of action of cinnamaldehyde on the bacterial cell may be mediated via membrane depolarization [14], where its hydrophobicity property facilitates the bacterial cell entry and disturbs the lipid bilayer of the cell membrane, causing an increase in the membrane permeability. The leaky cell membrane could release ions and vital molecules outside the bacterial cells, resulting in bacterial death [11]. Cinnamaldehyde may also interact with the cell membrane and cause rapid inhibition of energy metabolism [17].
Design and synthesis of efficient drug delivery systems are necessary for medicinal and pharmaceutical applications [12]. Material development using nanotechnology has synergistically stimulated the progress of drug delivery. Many polymers have been studied in designing nanodrug delivery systems [12]. Electrospinning is a simple and versatile technique that applies electrical forces to fabricate fibers with diameters ranging from 2 nm to several micrometers using natural and synthetic polymers [13, 18]. Electrospun nanofibers have numerous biomedical applications, such as filtration, drug and gene delivery, wound dressings, enzyme immobilization, sensors, and catalysts [18, 19]. Nanofibers were utilized extensively in the delivery of various drugs, including antibiotics, anticancer drugs, cardiovascular drugs, nonsteroidal anti-inflammatory drugs, antihistamines, gastrointestinal drugs, and contraceptives [20]. Compared with other nanotechnological techniques, electrospinning of nanofibers provides a high level of drug loading (DL) capacity and encapsulation efficiency (EE), with a minimal loss of the nanodrug delivery system [21].
The used materials and their rate of degradation are two crucial factors in the fabrication of electrospun nanofibers [22]. In addition, various pharmacists and researchers consider nanofibers necessary due to their advantages, such as easy functionalization, small diameters (nano/microsized fiber), high surface-to-volume ratio, high porosity, flexible control, and relatively inexpensive costs [22].
One of the most commonly used synthetic polymers in medical and industrial fields is polycaprolactone (PCL) due to its attractive properties such as biocompatibility, biodegradability, structural stability, mechanical strength, cost efficiency, and the approval of the US Food and Drug Administration (USFDA) for its use in humans [22]. However, its hydrophobic nature can lead to a very slow rate of degradation ranging from 2 to 4 years, which has limited its application in the biomedical fields [22]. On the other hand, this may also be an important feature of the PCL to maintain long-term drug retention. Therefore, this study is aimed at exhibiting the inhibition of biofilm formation of a widely infectious bacterium Escherichia coli (E. coli) by using the naturally available flavonoid cinnamaldehyde-loaded PCL nanofibers as a potential means of application in the biomedical field.
2. Materials and Methods
2.1. Materials
Cinnamaldehyde (100% purity), PCL with an average molecular weight of 80,000 g/mol, dimethyl sulfoxide (DMSO), chloroform, dimethylformamide (DMF), brain–heart infusion (BHI), Mueller Hinton (MH), and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Sigma-Aldrich (St. Louis, MO, United States). E. coli (ATCC 25922) was purchased from ATCC as a reference bacteria and was used as a biofilm producer and model organism for antimicrobial susceptibility testing.
2.2. Minimum Inhibitory Concentration (MIC) of Cinnamaldehyde
The MIC of cinnamaldehyde against E. coli ATCC 25922 was performed using the broth microdilution method using the reference protocol of the Clinical and Laboratory Standards Institute (CLSI) [23]. Cinnamaldehyde at a concentration of 10,000 μg/mL was prepared as follows: 100 μL of cinnamaldehyde was diluted in 10 mL of 0.5% DMSO. A serial dilution of cinnamaldehyde ranging from 10,000 to 70 μg/mL was added into the 96-well flat bottom microtiter plates containing MH broth at a final volume of 100 μL in each well. The volume of 10 μL (106 CFU/mL) of bacterial inoculum was added to each 96-well flat bottom microtiter plate (Corning No. 3474, United States). The plate was incubated for 24 h at 37°C with a continuous shaking speed of 180 rpm. The experiment was performed in triplicates. The bacterial growth inhibition was measured using a PowerWave XS2 plate reader (bioMérieux, Marcy L’Etoile, France) at a UV absorbance of 600 nm. The wells containing only E. coli ATCC 25922 and MH broth media were used as positive and negative controls, respectively. MIC was defined as the lowest concentration of cinnamaldehyde that inhibits the growth of the bacterium, which can be detected visually.
2.3. Minimum Bactericidal Concentration (MBC) of Cinnamaldehyde
To determine the MBC, 70 μL of the suspension of MIC wells was streaked on Mueller–Hinton agar (MHA) plates and incubated at 37°C for 24 h. The MBC was defined as the lowest concentration of drug that killed the bacteria.
2.4. Minimum Biofilm Inhibition Concentration (MBIC) of Cinnamaldehyde
To determine the antibiofilm activity of cinnamaldehyde against E. coli ATCC 25922, cinnamaldehyde was serially diluted in 100 μL of BHI culture medium with a final concentration ranging from 1000 to 70 μg/mL. A volume of 10 μL (106 CFU/mL) of bacterial inoculum was added to the wells of the 96-well flat bottom microtiter plate and incubated under static conditions at 37°C for 24 h. E. coli ATCC 25922 was used as a positive control, and culture medium BHI broth only was used as a negative control. After incubation, biofilm quantification was performed using a crystal violet (CV) assay [24]. Briefly, the media and planktonic cells were washed out using a BioTek ELx50 Microplate Strip Washer (BioTek, United States). Adhered cells in the plates were stained with 110 μl of 1% CV solution and incubated for 7 min at room temperature, then washed out using a BioTek ELx50 Microplate Strip Washer (BioTek, United States). The CV bound to the biofilm of each well was resolubilized with 110 μl of 30% acetic acid and incubated for 10 min at room temperature. The biofilm formation was measured using a PowerWave XS2 plate reader (bioMérieux, Marcy L’Etoile, France) at a UV absorbance of 560 nm. The experiment was performed in triplicates. The MBIC was defined as the lowest concentration of drug that inhibited bacterial biofilm formation.
2.5. Biofilm Dispersal of Cinnamaldehyde
E. coli ATCC 25922 biofilm was initially formed as described above. Subsequently, the 96-well flat bottom microtiter plate was washed out to remove the planktonic cells using BioTek ELx50 Microplate Strip Washer (BioTek, United States). The adhered cells of the biofilm were treated with cinnamaldehyde concentrations ranging from 10,000 to 70 μg/mL in a fresh BHI medium. The microtiter plate was then incubated at 37°C for 24 h. After incubation, biofilm quantification was performed using a CV assay [24]. The experiment was performed in triplicates. The biofilm formation was measured using a PowerWave XS2 plate reader (bioMérieux, Marcy L’Etoile, France) at a UV absorbance of 560 nm.
2.6. Fabrication of Cinnamaldehyde-Loaded PCL Nanofibers
Fabrication of cinnamaldehyde-loaded PCL nanofibers was conducted according to Almousained et al. [25] with minor modifications as follows: The PCL polymer solution was prepared by dissolving 12% (w/v) of PCL powder in 70% (v/v) chloroform and 30% (v/v) DMF. The solution was kept under stirring at 500 rpm, at room temperature (15–25°C), for 24 h. Cinnamaldehyde at a concentration of 2% (w/v) was added to the PCL solution and stirred at 500 rpm for 4 h at ambient temperature to reach a homogeneous mixture. The polymer–drug solution was taken into a 5-mL sterile disposable syringe. The electrospinning instrument (Spraybase, Dublin, Ireland) was used to prepare the nanofibers at the following parameters: a flow rate of 1 mL/h, a needle diameter of 0.55 mm, a needle tip-to-collector distance of 20 cm, and a voltage of 8–9 kV. Blank nanofibers (i.e., drug-free nanofibers) were prepared under the same conditions. The electrospinning was performed at an ambient temperature and relative humidity of 30% to 35%, and the metal collector was covered with aluminum foil.
2.7. Morphology and Diameter Assessment of Cinnamaldehyde-Loaded Nanofibers
Scanning electron microscopy (SEM; JSM-IT500HR SEM, JEOL Inc., Peabody, MA, United States) was used to assess the morphology of the fabricated nanofibers and to measure their diameter. A small piece of the nanofiber depositing aluminum foil was observed under the SEM at an accelerating voltage of 5 kV, and the average diameter of fibers was measured using the SEM software (SEM Operation, 3.010, Akishima, Tokyo, Japan).
2.8. Cinnamaldehyde Quantification Using High-Performance Liquid Chromatography (HPLC) Calibration Curve
A calibration curve was obtained by preparing a serial dilution of cinnamaldehyde in acetonitrile at a concentration range between 490 and 49 μg/mL using a previously modified HPLC method [26]. A reversed C18 column was used at ambient temperature, while the mobile phase of methanol:acetonitrile:water at a ratio of 35:20:45 was used throughout the system, with a flow rate of 1 cm3/min. The cinnamaldehyde samples were injected at 10 μL, and the detection wavelength was set up at 221 nm. The standard calibration curve was plotted using OriginPro 2020 software (OriginLab Corporation, Northampton, MA, United States).
2.9. EE % and DL of Cinnamaldehyde-Loaded Nanofibers
The results were presented as average ± standard deviation (SD) of triplicates.
2.10. Antibiofilm Activity of Cinnamaldehyde-Loaded Nanofibers
The antibiofilm of cinnamaldehyde-loaded PCL nanofibers against E. coli ATCC 25922 was performed using 96-well microtiter plates. The nanofibers were cut into circles with an average weight of 6 mg and were sterilized under the UV for 10 min. The nanofibers were added to the wells containing 200 μL of BHI culture medium. A 106 CFU/mL bacterial suspension was added to the plate and incubated under static conditions at 37°C for 24 h. After incubation, the biofilm quantification was performed using the CV assay [24, 27]. BHI broth and blank PCL nanofibers were used as experimental controls. The experiment was conducted in triplicates.
2.11. Biofilm Dispersal of Cinnamaldehyde-Loaded Nanofibers
The biofilm was formed for 24 h as described above. After incubation, the plate was washed out to remove the planktonic cells using the BioTek ELx50 Microplate Strip Washer (BioTek, United States). The 6 mg of cinnamaldehyde-loaded nanofibers were added to the wells of the adhered biofilm cells containing fresh BHI medium and incubated at 37°C for 24 h. After incubation, biofilm quantification was performed using the CV assay, as described above. The experiment was performed in triplicates.
2.12. In Vitro Cytotoxicity Assessment of Cinnamaldehyde
2.13. Statistical Analysis
The results were represented as mean ± SD. The results of the antibiofilm activity of cinnamaldehyde with and without nanofibers against E. coli ATCC 25922 were analyzed by paired Student’s t-test, in which the p values between p < 0.05 and p < 0.001 were considered statistically significant.
3. Results
3.1. Determination of MIC and MBC Values of Cinnamaldehyde Against E. coli
In vitro inhibition of bacterial growth was conducted to investigate the antimicrobial action of cinnamaldehyde on E. coli ATCC 25922 using the broth microdilution method. After 24 h of incubation, turbidity was noticed. Cinnamaldehyde was able to completely inhibit bacterial growth at a concentration of 300 μg/mL. In contrast, the MBC of cinnamaldehyde was observed at 600 μg/mL, which was considered to have a bactericidal effect.
3.2. Antibiofilm Activity of Cinnamaldehyde Against E. coli
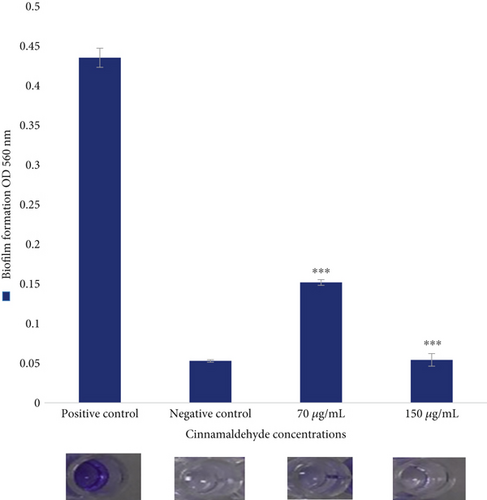
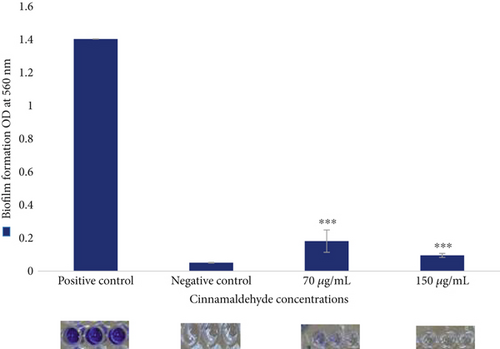
The antibiofilm activity of the sub-MIC levels of cinnamaldehyde against E. coli ATCC 25922 was determined using the CV assay. For biofilm inhibition, cinnamaldehyde at a sub-MIC level of 70 μg/mL was able to reduce 65.14% of the biofilm, p value = 0.001. However, no biofilm was observed at the sub-MIC level of 150 μg/mL (i.e., 100% inhibition). As a result, the MBIC of cinnamaldehyde was at a sub-MIC level of 150 μg/mL (Figure 1). For biofilm dispersal, cinnamaldehyde at the sub-MIC level of 70 μg/mL removed 87.08% of the biofilm, p value = 0.001. In contrast, no biofilm was observed at the sub-MIC level of 150 μg/mL, which indicated that cinnamaldehyde at a concentration of 150 μg/mL could remove the biofilm completely (Figure 2).
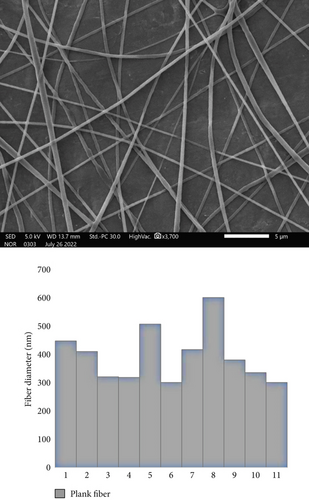
3.3. Morphological Characterization of Cinnamaldehyde-Loaded PCL Nanofibers
The cinnamaldehyde-loaded PCL nanofibers were successfully fabricated using the electrospinning technique, and their morphology was evaluated using the SEM. Uniform, smooth, and unbeaded nanofibers were obtained for both blank and drug-loaded nanofibers, with average fiber diameters of 300 ± 500 and 400 ± 980 nm, respectively (Figures 3 and 4).
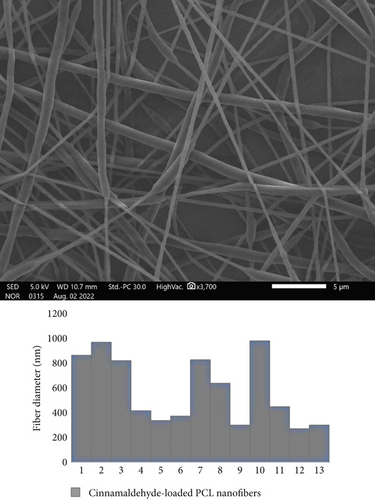
3.4. Cinnamaldehyde Determination Using HPLC
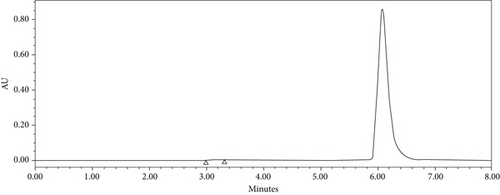
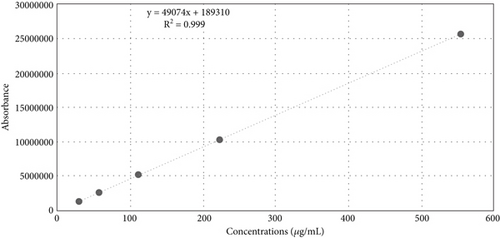
The developed HPLC method for cinnamaldehyde showed a good drug separation at 6.1 min (i.e., retention time Rt) (Figure 5), an excellent linearity, with a regression coefficient of determination (R2 = 0.999), and a regression equation of Y = 49,074X + 189,310, as shown in (Figure 6).
3.5. DL and EE
The percentage of EE plays a crucial role in the therapeutic efficiency of the drug. The EE percentage of cinnamaldehyde in PCL nanofiber was significantly high (83.58%). It was observed that 100% of the drug was released from the PCL nanofibers (95% acetonitrile and 5% chloroform) within 15 min. The average DL of cinnamaldehyde in 1 mg of PCL nanofiber was 119.35 μg/mg. Accordingly, 6 mg of PCL nanofiber was ≈600 ± 700 μg/mg of cinnamaldehyde.
3.6. Antibacterial and Antibiofilm Activities of Cinnamaldehyde-Loaded PCL Nanofibers Against E. coli ATCC 25922
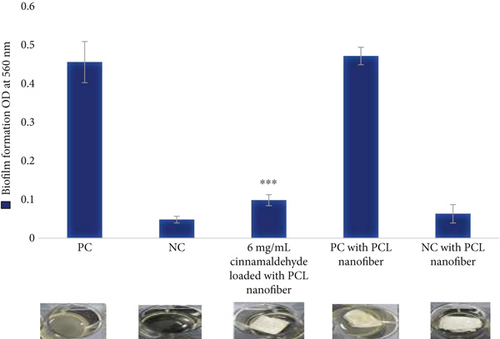
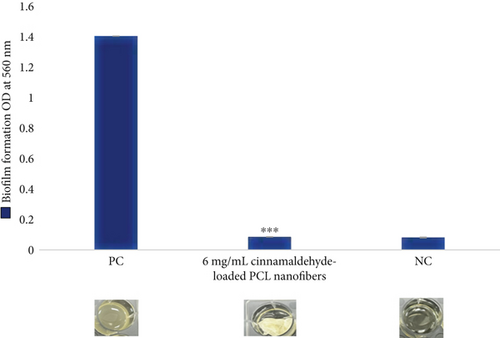
For the biofilm formation inhibition, no growth of bacterial cells was observed at a 6 mg disk of the cinnamaldehyde-loaded nanofibers, as shown in Figure 7 and Table 1. For biofilm dispersal, the cinnamaldehyde-loaded PCL nanofibers were able to remove the biofilm completely 100%, as shown in Figure 8 and Table 1, which confirmed the role of PCL nanofibers in increasing the efficiency of cinnamaldehyde in the inhibition and dispersal of biofilm.
| Strains | OD 560 | |
|---|---|---|
| Biofilm inhibition | 6 mg/mL of cinnamaldehyde-loaded PCL nanofiber |
|
| Positive control | ||
| Biofilm dispersion | 6 mg/mL of cinnamaldehyde-loaded PCL nanofiber |
|
| Positive control | ||
| Control | Positive control with PCL nanofibers | 0.455667 |
| Negative control with PCL nanofibers | 0.063 |
- Note: Percentage of biofilm reduction = 100 − (OD 560 of treated cell/OD 560 of control cell × 100). Paired t-test student, p value = 0.001.
3.7. Cytotoxicity Evaluation of Cinnamaldehyde Against Human Dermal Fibroblasts
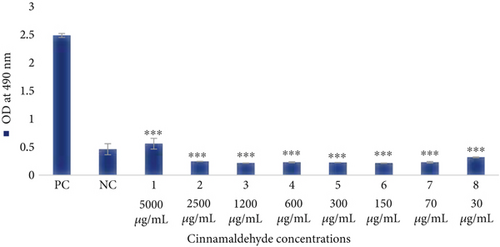
The effect of different concentrations of the cinnamaldehyde on the cellular metabolic activity of HFF-1 cells was conducted using the MTS assay. All cinnamaldehyde concentrations (5000–30 μg/mL) exhibited inhibitory effects on HFF-1 cells, which indicates the high toxicity of cinnamaldehyde on human cells, as shown in Table 2 and Figure 9.
| Cinnamaldehyde concentrations | OD at 490 nm |
|---|---|
| Cells treated with 5000 μg/mL | 0.55925 |
| Cells treated with 2500 μg/mL | 0.24325 |
| Cells treated with 1200 μg/mL | 0.21425 |
| Cells treated with 600 μg/mL | 0.22875 |
| Cells treated with 300 μg/mL | 0.224 |
| Cells treated with 150 μg/mL | 0.21325 |
| Cells treated with 70 μg/mL | 0.2285 |
| Cells treated with 30 μg/mL | 0.317 |
| Positive control (HFF-1 cell) | 2.485 |
| Negative control | 0.46075 |
| Paired t-test student | p value = 0.001 |
4. Discussion
Cinnamaldehyde is one of the most active components derived from cinnamon, which has long been utilized as a natural flavoring agent. Recent research has evaluated its positive effects as an antibacterial, antioxidant, anticancer, and anti-inflammatory [29, 30].
The current findings showed that cinnamaldehyde has potent bacteriostatic and bactericidal effects on E. coli ATCC 25922. The MIC value for cinnamaldehyde was 300 μg/mL, which is similar to earlier studies where the MIC values of cinnamaldehyde ranged from 250 to 400 μg/mL [28, 29]. Additionally, a prior study found that E. coli membranes were lysed by cinnamaldehyde at a concentration of 300 μg/mL. At the molecular level, the fimH, fimA, focA, papG, sfaA, and sfaS genes, which are essential for E. coli adhesion and host cell invasion, were downregulated by cinnamaldehyde [26]. Cinnamaldehyde is a powerful antibiofilm agent since it has been shown to lower the expression of the curli genes, restrict the growth of E. coli biofilms [10, 31], and reduce the adherence of E. coli to human epithelial Type 2 (HEp-2) cells [28]. Overall, the results showed that cinnamaldehyde was effective in eliminating both E. coli growth and biofilm formation, whether it was loaded with or without PCL nanofibers. However, PCL nanofibers assisted in increasing the effectiveness of cinnamaldehyde in eliminating biofilm inhibition.
According to previous studies, cinnamaldehyde has been found to possess several biological activities, including antimicrobial, antioxidant, anti-inflammatory, and anticancerous effects. However, most of these studies lacked normal cell lines as the control [32, 33]. Also, despite extensive reports that cinnamaldehyde is considered safe because it is well tolerated by both humans and animals [32, 33], the findings showed that all tested cinnamaldehyde concentrations have inhibitory effects on the proliferation of HFF-1 cells. Because they are easy to collect and culture, fibroblast cells are the most common cells used in cell culture trials. Fibroblast cells are present in all body tissues; therefore, if fibroblast cells are adversely affected by cinnamaldehyde, the whole body cells would be affected if cinnamaldehyde was used to treat cancer.
Moreover, it was reported that the cytotoxic and genotoxic effects of cinnamaldehyde were observed on adult human pulmonary fibroblasts (hPFs) and human embryonic stem cells (hESCs) at concentrations ranging from 2.2 to 140,000 μg/mL [34]. The previous study also found that low cinnamaldehyde concentrations resulted in altered cell morphology and motility; decreased cell growth, spreading, and attachment; increased DNA strand breaks; and increased cell death [35]. This result is consistent with the current study observation that even low cinnamaldehyde concentrations (30–70 μg/mL) were toxic to HFF-1 cells. Cell separation and noticeably enhanced cell death were seen on both healthy skin fibroblasts and malignant HeLa cells at 100 μg/mL of cinnamaldehyde [35]. Because cinnamaldehyde lacks a unique targeting pathway, the previous study theorized that it possesses similar cytotoxic mechanisms that similarly affect both malignant and noncancerous cells [35]. Due to its toxicity, the current study suggests using cinnamaldehyde on nonliving surfaces as a powerful antibacterial agent.
Similarly, because of their hydrophobicity, PCL nanofibers loaded with cinnamaldehyde can be applied to surfaces and materials in the healthcare industry to maintain sterility over an extended period. A surface’s physical and chemical interactions with bacteria are changed to provide antibacterial ability [36]. Antimicrobial surfaces are divided into hydrophobic surfaces and hydrophilic surfaces. For a large group of microorganisms, including bacteria, viruses, and fungi, hydrophobic surfaces are efficient. By preventing or limiting microbe attachment to surfaces, the hydrophobicity of the surface confers antibacterial properties [36]. A preprint has previously been published [37]. In order to effectively prevent bacterial infections linked to hospital surfaces and materials, cinnamaldehyde-loaded PCL nanofibers may be used to cover the surfaces of healthcare facilities.
5. Conclusion
The study demonstrated the strong bactericidal activity of cinnamaldehyde against E. coli. This study also supported the importance of PCL nanofibers in boosting cinnamaldehyde’s effectiveness in inhibiting and dispersing E. coli biofilm formation. Cinnamaldehyde-loaded PCL nanofibers may be a potent option to manage infections linked to hospital surfaces and medical materials because of its strong bactericidal activity and hydrophobicity.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study is supported by the King Abdulaziz City for Science and Technology (10.13039/501100004919, 1-18-01-001-0007), King Saud University (KSU), and Inaya Medical Colleges, Saudi Arabia.
Acknowledgments
The authors would like to thank King Abdulaziz City for Science and Technology (KACST) and King Saud University (KSU) for their full support of this project. The authors also acknowledge the support offered by Inaya Medical Colleges, Saudi Arabia.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding authors. The data are not publicly available due to privacy or ethical restrictions.




