Evaluating the Impact of Seed-Borne Mycoflora on Seed Quality and Health of Various Oryza sativa Varieties
Abstract
Seed mycoflora poses significant challenges to rice production, leading to substantial economic losses. Fourteen samples of stored and freshly harvested seeds of five rice varieties were collected from the Agricultural farm of Orissa University of Agriculture and Technology, Bhubaneswar, Odisha, to study the morphology of the mycoflora associated with rice seeds and their impact on seed health and quality. Eight fungal species, including Fusarium pallidoroseum, Fusarium fujikuroi, Curvularia lunata, Helminthosporium oryzae, Trichoconis padwickii, Aspergillus flavus, Rhizopus sp., and Penicillium sp., were isolated, identified, and morphologically characterized based on colony growth, mycelial features, conidial structures, and spore measurements. The rice varieties, viz., Hema, Jyotirmayee, Swarna, Lalat, and Annapurna, were artificially inoculated with F. pallidoroseum, F. fujikuroi, T. padwickii, C. lunata, and H. oryzae. Seeds showed that the fungi had a variable effect on seed quality parameters like germination, seedling vigor index I and seedling vigor index II, speed of germination, and seed health. A maximum of 16.33% reduction of germination was observed by T. padwickii, followed by F. fujikuroi (14.80% in Hema). Seedling vigor (1754.81) incited by T. padwickii inoculation was recorded to be the lowest. Seed rot up to 15.8% was observed in the case of F. fujikuroi. The percentage of root discoloration reached 40.0% by F. pallidoroseum and T. padwickii. In Jyotirmayee, C. lunata caused an 18.88% germination reduction and the lowest seedling vigor (1866.03) by T. padwickii. T padwickii also caused the highest reduction of seedling dry weight (36.12%) in this variety. The result reveals the diversity and prevalence of fungal pathogens in rice seeds. Hence, this study underscores the importance of seed sterilization practices in reducing fungal contamination and the requirement of thorough seed examination to reduce the effects of seed mycoflora associated with rice seeds, which could affect rice production.
1. Introduction
Rice, scientifically known as Oryza sativa L., is a self-pollinating cereal plant under the Poaceae family [1]. It is widely cultivated as a primary food source in tropical and subtropical regions, covering an extensive land area of 165 million hectares. Global rice production has reached 744.4 million tons, with 496.4 million tons milled [2]. Rice consumption and production are prevalent in Asia [3], with India being a prominent producer. Key rice-growing regions in India include West Bengal, Odisha, Uttar Pradesh, Madhya Pradesh, Punjab, and Bihar. Rice cultivation is practiced across most Indian states, encompassing around 44 million hectares and yielding over 100 million tons annually. With more than half of the population depending on rice as a staple crop, it holds significant dietary importance and supports the livelihood of approximately 70% of the population [4]. Rice, a staple food source for half of the world’s population, is seriously threatened by diseases, specifically those caused by seed-borne pathogens [5, 6]. Approximately 50 biotic entities, including fungi, bacteria, viruses, nematodes, and insects, pose a significant threat to yield. Among these, fungal pathogens have emerged as a major obstacle to sustaining rice production [7]. Seeds are crucial for agriculture, ensuring healthy and uniform crop growth. The world’s food security depends on producing sufficient small seed cereals, which are the cheapest source and provide 70% of absorbable energy [8]. High-quality seeds with genetic and physical purity, high germination rates, and freedom from seed-borne diseases and insects are essential [9]. The significance of having disease-free and viable seeds in rice cultivation cannot be overstated. Infected seeds often display discoloration, indicating poor grain quality [10]. Seed is crucial, encompassing the presence of disease-causing organisms, including fungi, nematodes, bacteria, viruses, and insects associated with the seed [11]. Farmers frequently neglect the importance of evaluating the quality of rice seeds despite the potential for seeds to carry pathogens within the seed [12]. Both external and internal seed-borne pathogens pose a risk to seed health, potentially leading to reduced vigor, poor germination rates, and negative impacts on appearance and chemical composition [13, 14]. The presence of pathogens in stored seeds can significantly compromise their overall quality. This may lead to reduced crop yields and accelerate the deterioration of seeds [15]. Seed transmission plays a crucial role in the spread of plant diseases, with fungi being the most common group of pathogens affecting seeds’ quality and market value [13, 16]. Over a hundred species of fungi are reported to be associated with rice seeds, and their impact varies based on location, sampling time, and rice variety [17–19]. Different seed-borne diseases of rice caused by economically important seed-borne fungi are Fusarium moniliforme causing bakanae disease of rice, F. pallidoroseum and T. padwickii (Alternaria Padwickii) causing stackburn disease, H. oryzae causing brown spots, Curvularia sp. causing black kernel, Pyricularia oryzae causing blast disease, Sarocladium oryzae causing sheath blight, and Microdochium oryzae causing leaf scald [20–22]. Gopalakrishnan et al. [19] identified eight genera and 12 species of fungi on contaminated rice seed. Among these, the severity of Bipolaris oryzae and A. padwickii was found to be the highest, at 59% and 53%, respectively. Ahmed et al. [23] found nine different species of fungi in rice seeds, including Fusarium oxysporum, F. moniliforme, B. oryzae, A. padwickii, C. lunata, A. flavus, A. niger, Penicillium sp., and Nigrospora oryzae.
High-quality seeds are crucial in successful rice cultivation and achieving high yields. Unfortunately, rice seeds are often imported and sold without undergoing health testing. Addressing this issue is vital to minimize substantial losses. Implementing effective seed treatment measures can enhance seed quality and boost overall yield. The present investigation seeks to assess the influence of diverse seed-borne mycoflora on seed quality and health across different rice cultivars in this context.
2. Material and Methods
2.1. Planting Material
Fourteen working samples representing five rice varieties (Annapurna, Hema, Lalat, Jyotirmayee, and Swarna) were collected from storage and fields from the Central Agricultural Research Farm. Following the International Seed Testing Association (ISTA) [24] rules, one sample per variety (each weighing about 0.5 kg) was randomly selected from rice experiment trials. These samples were labeled correctly, sealed in polythene bags, and refrigerated at 5°C ± 1°C in the Seed Pathology Laboratory for subsequent analysis.
2.2. Detection and Isolation of Rice Seed Mycoflora
ISTA techniques [24–26] were used to detect and isolate seed-borne mycoflora of rice. The methods used for seed health testing were as follows: inspection of dry seeds or visual examination; incubation method, rolled paper towel method, standard blotter method, and agar plate method; and NaOH soaking test, test tube agar test, and pathogenicity test.
2.3. Blotter Method
To assess the presence of seed-borne mycoflora on newly harvested and stored rice seeds, the blotter method recommended by ISTA [24] was utilized. To set up the procedure, filter papers were first soaked in distilled water and sterilized for 15 min at 15 p.s.i. Representative samples from each seed lot were placed on a blotter with 25 seeds plated in a Petri dish, arranged in three concentric rings, 15 seeds in the outer ring first, nine in the middle ring, and one in the center. Two hundred seeds were sterilized with a 2% sodium hypochlorite solution and tested for each variety, with eight replications maintained. The Petri dishes were then incubated at 22°C ± 2°C under 12-h cycles of near-ultraviolet (NUV) light and darkness as per ISTA [25] for seven days. Finally, the incidence of different seed-borne fungi was recorded by examining the seeds under a microscope.
2.4. Rolled Paper Towel Method
2.5. Agar Plate Method
The agar plate method was used to detect seed-borne mycoflora. Surface-sterilized seeds were plated on potato dextrose agar (PDA) medium and incubated at 22°C–25°C under alternating cycles of light and darkness. Fungi growing from the seeds were identified based on colony characters and morphology of sporulation structures under a microscope [28, 29]. More than one type of fungal colony was produced in some cases, and identification was made based on the most frequently occurring colony in all the Petri dishes. Results were presented as a percentage of incidence.
2.6. Water Agar Test Tube Method
The water agar test tube method was used by Khare et al. [30] to study the symptoms of seedlings raised from infected seeds. Treated seeds were sown in test tubes, one seed per tube, on water agar and then incubated at 20°C ± 2°C under artificial daylight tubes, 12/12 h cycle. Observations were taken after 9 days and separated into three groups: healthy-looking seedlings showing symptoms and seeds that did not germinate.
The symptoms were recorded and examined under a compound microscope for confirmation.
2.7. Pot Culture Method
2.8. Morphology Studies of the Isolated Pathogens
Pure cultures of fungi were obtained, and identification was made by studying their morphological features and using reference manuals. The frequency of fungal isolation was also recorded. Some fungal isolates were also sent to the Indian Type Culture Collection Centre (ITCC), New Delhi, for identification. A pure culture of a fungal colony smear was grown for 7 days and then stained and mounted on a slide for microscopic examination. This was done to evaluate various morphological and taxonomic characteristics. In order to compare, spores of individual fungi from a 10-day-old culture were measured under a microscope using an ocular micrometer. The measurements of spores and conidia were recorded. The fungi were identified based on mycelial growth colony characters such as color, texture, growth habit, size, shape, color, and septations. The conidia were examined using a microscope to help with the identification.
2.9. Effect of Seed Inoculation on Seed Quality
The procedure for obtaining healthy seeds from seed lots was carried out by separating the seeds without any visible disease symptoms. These seeds were then subjected to surface sterilization using a 2% sodium hypochlorite solution. The sterilized seeds were thoroughly washed multiple times in distilled sterile water. Subsequently, spore suspensions with a concentration of @107–108 per mL were prepared from actively sporulating cultures of eight isolated fungi using distilled sterile water. Equal volumes of spore suspension of each fungal culture were poured on the surface of sterilized seeds and soaked for 4 h. One sheet of germination paper was moistened with sterilized distilled water. Twenty-five seeds were placed on a moistened sheet evenly, another germination paper was kept on the first sheet, and the seeds were wetted carefully. The seeds were then rolled on a sterilized blotter of 8.5 cm diameter and left overnight in Petri dishes for drying. Both sheets were rolled and incubated in the seed germinator at 25°C for seven days. At the end of the incubation period, rolled towel papers were carefully opened, and the seeds were used to test seed health and seed quality.
2.10. Statistical Analysis
In this study, no statistical analysis was conducted due to the nature of the research design/methodology/data.
3. Result
3.1. Identification of Seed Mycoflora Associated With Rice Varieties
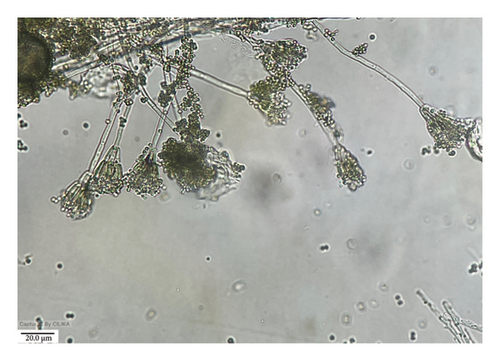
Investigating the association of seed mycoflora with rice varieties, we identified eight fungal species, viz., F. pallidoroseum, F. fujikuroi, C. lunata, H. oryzae, T. padwickii, A. flavus, Rhizopus sp., and Penicillium sp. (Figures 1(a), 1(b), 1(c), and 1(d)). The results of mycoflora associated with different varieties of rice seed, both unsterilized and surface-sterilized, from stored and freshly harvested samples provided significant insights into the diversity and frequency of different fungi.

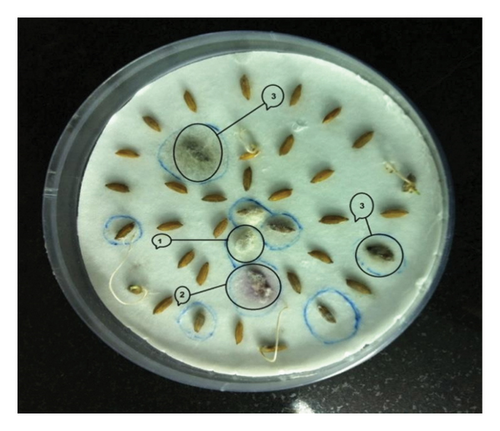


3.2. Mycoflora Association in Store-Collected Rice Seeds
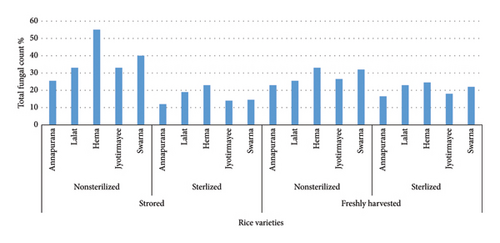
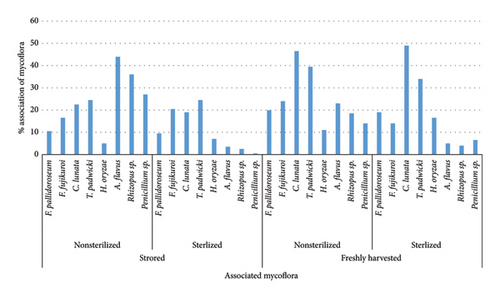
When examining unsterilized rice seeds collected from the store (Figure 2), it was observed that the Hema variety exhibited the highest association of seed mycoflora at 55.0%, followed by Swarna at 40.0%. T. padwickii was found to be the most predominant fungi across all varieties, both in the case of unsterilized and sterilized conditions, with Hema having the highest association at 11.5%, followed by 10.5% in the case of Swarna variety (Figure 3). Fusarium sp., C. lunata, and T. padwickii populations remained stable regardless of surface sterilization. However, after surface sterilization, significant reductions were seen in A. flavus, Rhizopus sp., and Penicillium sp. populations. A. flavus decreased to 3.5%, Rhizopus sp. to 2.5%, and Penicillium sp. nearly disappeared in sterilized seeds compared to nonsterilized ones. For instance, Annapurna recorded a decrease in total fungal count from 25.5% to 12.0% after surface sterilization (Table S1a and S1b).
3.3. Mycoflora Association in Freshly Harvested Rice Seeds
In freshly harvested rice seeds, both unsterilized and surface-sterilized, seed mycoflora were consistently present across all varieties, but their level varied (Figure 2). The Hema variety showed the highest mycoflora association among unsterilized seeds at 33.0%, followed by Swarna at 32%. C. lunata was found to be the most predominant fungi across all varieties, both in the case of unsterilized and sterilized conditions, with Swarna having the highest association at 8.5%, followed by 7.5% in the case of the Hema variety (Figure 3). Lalat exhibited the highest association among surface-sterilized seeds at 23.0%. Surface sterilization led to a notable reduction in fungal populations, particularly in fungi like Fusarium sp., A. flavus, Rhizopus sp., and Penicillium sp., indicating its efficacy in reducing seed-borne fungal contamination in freshly harvested rice seeds. For example, Hema showed a decrease in total fungal count from 33.0% to 24.5% after surface sterilization. In comparison, Lalat exhibited a reduction from 25.5% to 19.0%, highlighting variability in the response to sterilization treatment among different rice varieties (Table S2a and S2b).
3.4. Morphological Studies of the Identified Mycoflora
Detailed information regarding the morphology of different fungi has been recorded with great attention, highlighting their unique features and development patterns (Table S3).
3.4.1. Fusarium pallidoroseum
The growth of F. pallidoroseum on PDA was observed to be dull white to slightly dark, covering the entire plate within 7 days. The mycelium exhibited an orange-to-pink color on the back side of the PDA plate and appeared hyaline septate. Conidiophores were single and literal, while conidia were hyaline, tapering toward both ends, primary macroconidia with wedge-shaped foot cells, with 0–4 septa, measuring 6.5–23 × 2–4 μm. Secondary macroconidia with typical heeled foot cells, 2–6 septa, 15–31 × 2.5–5 μm, formed from phialides usually grouped in sporodochia. The culture was identified as F. pallidoroseum by the ITCC in New Delhi (ID No. 10188.16) (Figure 4).

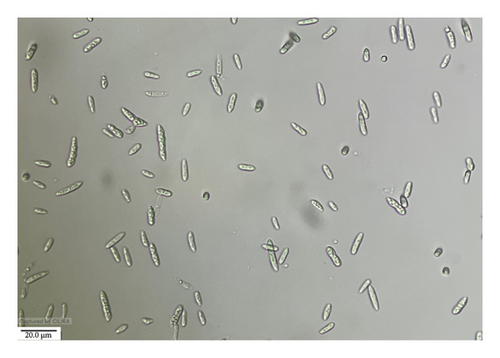
3.4.2. Fusarium fujikuroi
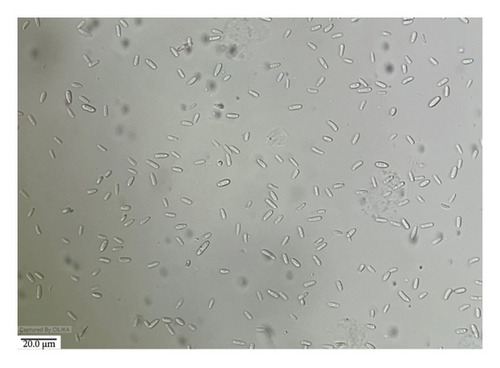
Growth on PDA was moderately fast and covered the entire plate in 7 days. It was floccus to slightly felted, white tinged with pink at the center. The color at the reverse side of the PDA plate was slightly zoned and white with a purplish center. Conidia were hyaline, fusiform, ovate, or clavate, and flattened somewhat at both ends; one- or two-celled microconidia are measured 3.5–11 × 1.5–3 μm, occasionally with one septum. Macroconidia were boat-shaped, hooked foot cells 2–6 septate, measuring 11–36 × 1.5–2.5 μm. The culture was sent to ITCC, New Delhi, for identification and identified as F. fujikuroi (ID No. 10189.16) (Figure 5).

3.4.3. Curvularia lunata
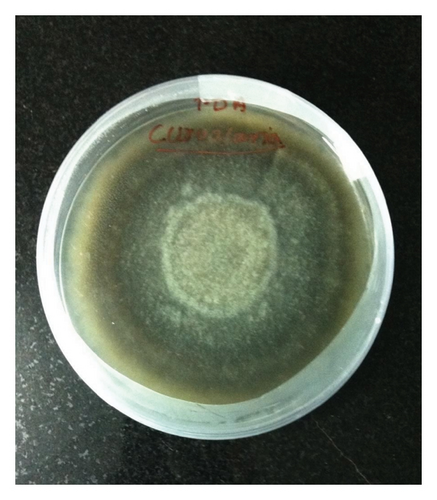
C. lunata was identified from the PDA colony growth. The growth was fast and turned from white to black toward the periphery. Brown, septate conidiophores, arising singly or in groups or sometimes branched were found with spores in a bunch of 5–6. Conidia were brown, endless, lighter, three- to five-celled, and more or less fusiform, typically bent or curved at the center, with one or two cells enlarged. The conidia measured from 20–32 × 7.5–13 μm. Two isolates were sent to ITCC and IARI and identified with ID Nos. 10,186.1 and 10,187.16. They were identified as C. lunata (Figure 6).

3.4.4. Helminthosporium oryzae
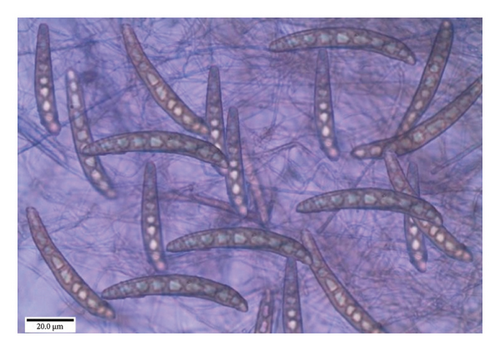
The study revealed that the colonies on PDA exhibited a slow-growing pattern, with a growth rate of 80 mm in 10 days. The observed coloration ranged from dark to slightly black, gradually transitioning to a cottony texture toward the margin, yellowish-gray at the center, and dark gray at the margin. The mycelium manifested as light to dark in culture, with short and long conidiophores, septate, simple or branched, and bearing conidia successively on the new growing tips. Furthermore, the conidia were dark brown to olivaceous brown, fusiform, straight, or curved, with 5–11 pseudosepta and a prominent hilum at the base.
The conidia measure ranged from 37–125.6 × 10.5–17.5 μm. Based on the observations, the fungus was identified as H. oryzae (Figure 7).
3.4.5. Trichoconis padwickii
The colonies observed on the PDA medium exhibited moderate growth, measuring 70 mm in just 6 days. These colonies were characterized by a slightly zonated, thickly felted, and gray appearance, gradually lightening toward the outward regions. Upon examination of the reverse side of the PDA plate, a black coloration was observed, which became lighter in the outward direction. In their early stage, the mycelia displayed septate, profusely branched, and hyaline features, which gradually matured to become creamy yellow. The conidiophores exhibited a swollen apex of 4–6 μm diameter, while the smooth conidia were found to be slightly straight or curved, straw-colored to golden brown, smooth to echinulate, and had three to four transverse septa, often accompanied by 1–2 septa in the beak. The dimensions of the conidia were measured to be 50–125 × 9–19 μm. Based on these observations, the fungus was identified as T. padwickii (Figure 8).

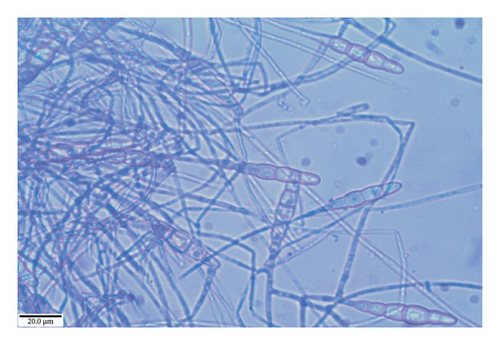
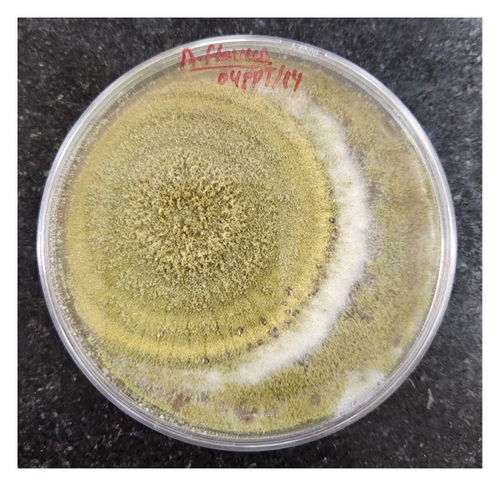
3.4.6. Aspergillus flavus
The morphological characteristics of A. flavus were also examined on the PDA medium. The colony exhibited a grayish-powdery appearance and a fast growth rate. The conidial heads were yellowish green in color and turned brownish at the edges. The conidiophores were 300–500 μm or less than 1 mm long, with a diameter of 15–35 μm, and the vesicle was globose to subglobose. Phialides were cylindrical tapering to distinct necks measuring 5–8.5 μm seriate in size. The conidia were globose and minutely accumulated, measuring 2.5–5.3 μm. Based on these characteristics, the fungus was identified as Aspergillus flavus (Figure 9).
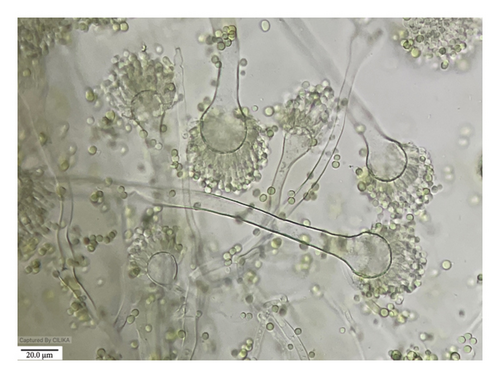
3.4.7. Rhizopus sp.
Similarly, the colony of Rhizopus sp. on the PDA medium was initially white and cottony and had well-developed rhizoids at nodes. The sporangiophores arose in clusters and were irate, aseptate, yellowish to light brown, rhizoidal, connected to sporangiophores, measuring 629.5–1200 × 5.5–15.7 μm. Sporangia were globose, dark brown to black, apparently subglobose after maturity, and columellate on dehiscence, measuring 91.5–165 μm. The sporangiospores were round to ovule, hyaline or grayish-brown, one-celled smooth, and measured 6.8–9.4 μm in diameter. Based on these features, it was identified as Rhizopus sp. (Figure 10).

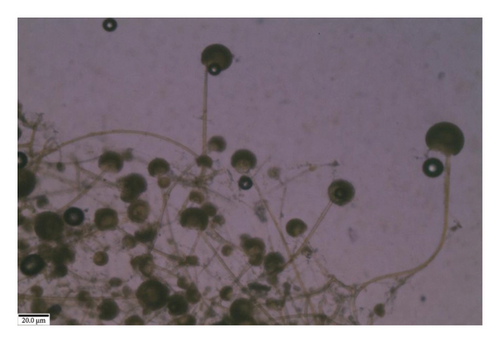
3.4.8. Penicillium sp.
Lastly, the colony of Penicillium sp. on PDA medium was velvety with areal mycelium and had a very fast-growing and ashy color. The reverse color of the plate was yellow to brownish. The conidiophores were smooth vesiculate, containing phialides, and the conidia were globose to subglobose, measuring 3–4 × 2.5–3.5 μm. Phialides are cylindrical, 5.5–8 × 2–2.5 μm (Figure 11).
3.5. The Impact of Seed Inoculation on Seed Health and Quality
The present study offers insight into the impact of seed inoculation with various fungal pathogens on seed health and quality parameters. Healthy seeds of different rice varieties (Hema, Jyotirmayee, Swarna, Lalat, and Annapurna) were artificially inoculated with fungi such as F. pallidoroseum, F. fujikuroi, T. padwickii, C. lunata, and H. oryzae. The effects on germination, seedling VGI I and II, speed of germination, and seed health were investigated. The findings reveal that different fungi have variable effects on seed quality parameters.
In the Hema variety, T. padwickii induced the highest reduction in germination (16.33%), followed by F. fujikuroi (14.80%). Additionally, seedling vigor was notably compromised, particularly with T. padwickii inoculation, significantly reducing seedling dry weight. Seed rot incidence was prominent, with T. padwickii causing the highest rate (15.8%), followed by F. fujikuroi (14.3%). Root discoloration, indicative of fungal infection, reached a maximum of 40% due to C. lunata inoculation (Table 1).
| Pathogens | Seed quality parameters | Seed health | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Germination % | Root length (cm) | Shoot length (cm) | Dry weight (gm) | SVI | % reduction | SVII | % reduction | % seed rot | % root symptoms | % shoot symptoms | ||
| Inoculated | Reduction % | |||||||||||
| F. pallidoroseum | 87 | 11.22 | 12.4 | 11.85 | 0.0892 | 2109.75 | 21.5 | 7.764 | 22.70 | 10.5 | 25 | 6.5 |
| F. fujikuroi | 83.5 | 14.80 | 11.475 | 10.375 | 0.0791 | 1824.47 | 32.11 | 6.610 | 34.19 | 14.3 | 23 | 8.5 |
| C. lunata | 84 | 14.29 | 12.725 | 10.875 | 0.0783 | 1982.4 | 26.24 | 6.581 | 34.48 | 13.6 | 40 | 9.5 |
| H. oryzae | 86 | 12.24 | 12.75 | 10.125 | 0.065 | 1967.25 | 26.81 | 5.59 | 44.35 | 12.3 | 15 | 8.5 |
| T. padwickii | 82 | 16.33 | 11.05 | 10.35 | 0.0585 | 1754.8 | 34.73 | 4.797 | 52.24 | 15.8 | 35 | 5 |
| Control | 98 | 14.475 | 12.95 | 0.1025 | 2687.65 | — | 10.045 | — | ||||
- Abbreviations: SVI = seedling vigor index I, SVII = seedling vigor index II.
Similarly, data in Table 2 revealed that, in the Jyotirmayee variety, C. lunata exhibited the most detrimental impact on germination (18.88%), significantly reducing seedling vigor and dry weight. Seed rot incidence was substantial, particularly with C. lunata (17.6%), while F. pallidoroseum showed the lowest incidence (9.2%). Notably, H. oryzae induced prominent shoot discoloration (10%).
| Pathogens | Seed quality and health parameters | Seed health | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Germination % | Root length (cm) | Shoot length (cm) | Dry weight (gm) | SVI | Reduction % | SVII | Reduction % | % seed rot | % root symptoms | % shoot symptoms | ||
| Inoculated | Reduction % | |||||||||||
| F. pallidoroseum | 87.5 | 10.71 | 12.375 | 10.4 | 0.109 | 1992.81 | 24.75 | 9.5375 | 14.18 | 9.2 | 23.5 | 9 |
| F. fujikuroi | 84.5 | 13.78 | 12.85 | 9.6 | 0.0878 | 1897.02 | 28.37 | 7.4191 | 20.31 | 13.5 | 22 | 8.5 |
| C. lunata | 79.5 | 18.88 | 12.725 | 10.875 | 0.0783 | 1876.2 | 29.15 | 6.2288 | 33.09 | 17.6 | 37 | 8.5 |
| H. oryzae | 85 | 13.27 | 13.65 | 11.4 | 0.0890 | 2129.25 | 19.60 | 7.5692 | 18.69 | 11.8 | 36.5 | 10 |
| T. padwickii | 85.5 | 12.76 | 12.375 | 10.4 | 0.109 | 1866.03 | 29.54 | 5.9465 | 36.12 | 12.3 | 23.5 | 9 |
| Control | 98 | 13.95 | 13.075 | 0.095 | 2648.45 | — | 9.31 | — | ||||
- Abbreviations: SVI = seedling vigor index I, SVII = seedling vigor index II.
For the Lalat variety, all fungi led to reduced seed germination compared to the control, with F. fujikuroi inducing the most substantial reduction. Additionally, seedling vigor was significantly compromised, particularly with T. padwickii inoculation. Seed rot and seedling blight symptoms were observed, with F. fujikuroi causing the highest seed rot incidence (19.3%) and T. padwickii inducing the most severe root discoloration (81.5%) (Table 3).
| Pathogens | Seed quality and health parameters | Seed health | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Germination % | Root length (cm) | Shoot length (cm) | Dry weight (gm) | SVI | Reductio n % | SVII | Reduction % | SGI | % seed rot | % root symptoms | % shoot symptoms | ||
| Inoculated | Reduction % | ||||||||||||
| F. pallidoroseum | 85 | 10.53 | 13.65 | 10.8 | 0.0952 | 2078.25 | 8.84 | 8.092 | 16.89 | 17.09 | 11.7 | 40.5 | 9 |
| F. fujikuroi | 76 | 20.00 | 12.825 | 11.17 | 0.0906 | 1824 | 20.00 | 6.885 | 29.28 | 16.9 | 19.3 | 30.5 | 8.5 |
| C. lunata | 84 | 11.58 | 11.55 | 11.02 | 0.0810 | 1896.3 | 16.82 | 6.804 | 30.12 | 19.85 | 13.4 | 38 | 7 |
| H. oryzae | 86 | 9.47 | 12.8 | 11.42 | 0.0980 | 2082.92 | 8.64 | 8.428 | 13.44 | 18.81 | 10.8 | 38 | 12 |
| T. padwickii | 80 | 15.7 | 13.65 | 10.8 | 0.0952 | 1528 | 32.98 | 5.352 | 45.03 | 15.9 | 16.7 | 81.5 | 9 |
| Control | 95 | 11.675 | 12.32 | 0.1025 | 2280 | — | 9.737 | 26.93 | |||||
- Abbreviations: SGI = speed of germination index, SVI = seedling vigor index I, SVII = seedling vigor index II.
The data presented in Table 4 show that the Annapurna variety, F. fujikuroi, causes a notable reduction in germination (16.58%) and seedling vigor, with significant decreases in seedling dry weight. Seed rot incidence was pronounced, especially with F. fujikuroi (17.7%) and C. lunata (13.1%). Root discoloration was prominent, particularly with F. pallidoroseum (59%) and F. fujikuroi (56%). In the case of the Swarna variety, all the fungi reduced the seed germination compared with the control (90%); the maximum of 81% reduction was by F. pallidoroseum, which was 10% lower than the control.
| Pathogens | Seed quality parameters | Seed health | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Germination % | Root length (cm) | Shoot length (cm) | Dry weight (gm) | SVI | Reduction % | SVII | Reduction % | SGI | % seed rot | % root symptoms | % shoot symptoms | ||
| Inoculated | Reduction % | ||||||||||||
| F. pallidoroseum | 85 | 11.92 | 13.92 | 12.35 | 0.0825 | 2232.95 | 14.59 | 7.012 | 35.40 | 24.99 | 12.5 | 59 | 29.5 |
| F. fujikuroi | 80.5 | 16.58 | 13.725 | 12 | 0.0852 | 2070.86 | 20.79 | 6.858 | 36.82 | 24.06 | 17.7 | 56 | 10.5 |
| C. lunata | 84 | 12.95 | 14.025 | 11.4 | 0.0926 | 2135.7 | 18.31 | 7.778 | 28.35 | 27.34 | 13.1 | 33 | 6 |
| H. oryzae | 84 | 12.95 | 13.75 | 11.95 | 0.0980 | 2158.8 | 17.43 | 8.232 | 24.10 | 28.4 | 11.4 | 40 | 7 |
| T. padwickii | 86.5 | 10.36 | 9.1 | 10.35 | 0.0798 | 1682.42 | 35.65 | 6.902 | 26.41 | 26.46 | 9.3 | 35.5 | 6 |
| Control | 96.5 | 14.775 | 12.32 | 0.1125 | 2614.66 | — | 10.85 | — | 28.04 | ||||
- Abbreviations: SGI = speed of germination index, SVI = seedling vigor index I, SVII = seedling vigor index II.
In the Swarna variety, T. padwickii caused the highest reduction in SVII (19.37%), while F. pallidoroseum showed the least reduction (8.78%). F. fujikuroi had the lowest germination index (19.48%). T. padwickii induced the most seed rot (15.3%), while F. fujikuroi resulted in the least (12.1%) (Table 5). Root discoloration was most severe with F. fujikuroi (56%), and shoot discoloration was highest with F. pallidoroseum (12.5%), followed by F. fujikuroi (10%). These findings underscore the diverse impacts of fungal inoculation on rice seed health and quality.
| Pathogens | Seed quality parameters | Seed health | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Germination % | Root length (cm) | Shoot length (cm) | Dry weight (gm) | SVI | Reduction % | SVII | Reduction % | SGI | % seed rot | % root symptoms | % shoot symptoms | ||
| Inoculated | Reduction % | ||||||||||||
| F. pallidoroseum | 81 | 10.00 | 12.025 | 10.11 | 0.08 | 1793.17 | 13.99 | 6.48 | 28.0 | 20.35 | 14.8 | 23 | 12.5 |
| F. fujikuroi | 82.5 | 8.33 | 13.125 | 9.925 | 0.0874 | 1901.62 | 8.78 | 7.210 | 19.88 | 19.48 | 12.1 | 56 | 10 |
| C. lunata | 83 | 7.78 | 12.025 | 10.67 | 0.0758 | 1884.1 | 9.62 | 6.291 | 30.09 | 21.05 | 13.4 | 32 | 5.5 |
| H. oryzae | 82.5 | 8.33 | 12.975 | 9.875 | 0.0747 | 1885.12 | 9.57 | 6.162 | 31.52 | 21.22 | 14.6 | 40.5 | 5 |
| T. padwickii | 81.5 | 9.44 | 11.425 | 9.2 | 0.0635 | 1680.93 | 19.37 | 5.175 | 42.49 | 20.88 | 15.3 | 52 | 4 |
| Control | 90 | 12.4775 | 10.68 | 0.1 | 2084.8 | — | 9.0 | — | 25.83 | ||||
- Abbreviations: SGI = speed of germination index, SVI = seedling vigor index I, SVII = seedling vigor index II.
4. Discussion
The identification of fungal pathogens that are associated with rice seeds is crucial for the effective management of diseases in rice crops. The types of fungi commonly associated with rice seeds can vary widely, ranging from less harmful pathogens [31] to major ones that can significantly impact rice production [31–34]. Our studies have revealed some interesting contrasts. We found that the most frequently isolated pathogen in nonsterilized stored seed was A. flavus, and T. padwickii in sterilized seed; on the other hand, in freshly harvested seed, the most frequently isolated pathogen was C. lunata in both sterilized and nonsterilized seeds. Reference [35] found H. oryzae to be the most frequently isolated pathogen; in contrast, it was the least frequently isolated pathogen. F. fujikuroi and F. pallidoroseum were also frequently isolated pathogens, following T. padwickii and C. lunata, irrespective of the rice cultivar and conditions, which supports previous research by [32]. F. fujikuroi leads to reduced rice stands [35] and causes discoloration of rice seeds [36]. A. flavus and Rhizopus sp., considered surface contaminants, were commonly observed as the most predominant fungi in rice seed, specifically in nonsterilized stored rice varieties. Penicillium and Aspergillus are reported from stored seeds [37–40], and Aspergillus sp. has been found to cause deterioration in stored grains [40, 41]. IIyas and Javaid [42] isolated F. moniliforme and B. oryzae from discolored seeds in deep rice water in the Sialkot district of Pakistan. Various fungi associated with rice seed have been reported by [17, 43–50]. These pathogens exhibited various characteristic growth patterns on PDA, with distinct morphological features [51]. C. lunata and H. oryzae displayed rapid and slow growth, respectively, along with unique colony characteristics consistent with previous reports by [37, 38, 52], which reported an association between C. lunata and other pathogens from rice. As reported by previous researchers, T. padwickii showed moderate growth and identifiable morphological traits, confirming its association with rice seeds. Additionally, A. flavus, Rhizopus sp., and Penicillium sp. were identified with typical growth patterns and conidial characteristics. Association of T. padwickii (Alternaria padwickii) with rice seeds is also reported by [37, 39].
Furthermore, the study focuses on the impact of seed inoculation with various fungal pathogens on seed health and quality parameters. The results indicate that T. padwickii and F. fujikuroi significantly reduced germination rates and seedling vigor of the Hema variety. A similar study was published by Gopalakrishnan et al. [53], which showed reduced germination and discoloration in plants inoculated with Sarocladium oryzae. Similarly, C. lunata significantly impaired germination and seedling vigor in the Jyotirmayee variety. At the same time, T. padwickii caused a widespread reduction in seed germination and low seedling vigor in the Lalat variety. A study by [51, 54] recorded a reduction in germination, seedling vigor, shoot length, root length, and root weight in treated rice cultivars than in local check rice varieties. In the Annapurna variety, F. fujikuroi caused a notable reduction in germination rates and compromised seedling vigor. Bora and Robin Gogoi [55] reported that F. moniliforme and B. oryzae reduced the germinability of seeds. The Swarna variety also showed vulnerability to fungal infections, significantly reducing seed health parameters, including germination rates and seedling vigor. Islam et al. [56] observed abnormal rice seedlings using paper towels. The above workers reported seed infection by F. fujikuroi, T. padwickii, and Curvularia sp., confirming the current investigation.
5. Conclusion
Diseases transmitted through seeds can significantly impact seed health and quality, highlighting the importance of high-quality seeds for productivity. This study identifies several seed-borne fungi, including F. pallidoroseum, F. fujikuroi, C. lunata, T. padwickii, H. oryzae, A. flavus, Rhizopus sp., and Penicillium sp. in rice varieties based on their morphological characteristics from infected seeds, and the study also examines their impact on seed health and quality. The prevalence of seed mycoflora across various rice varieties highlights the diversity and complexity of fungal communities within stored, freshly harvested, sterilized, and nonsterilized rice seeds. The analysis of mycoflora association across different rice varieties showed varying levels of fungal infection. The Hema variety had the highest seed mycoflora infection rate at all conditions. These pathogens exhibited variable effects on seed germination, seedling vigor indices, and overall seed health and quality, with T. padwickii, C lunata, and F. fujikuroi significantly reducing germination rates and seedling vigor across multiple varieties.
Seed health and quality management are crucial for crop productivity. Identifying and controlling fungal infections early is critical to minimize the adverse effects on seed germination and seedling vigor. Further research is needed to evaluate the impact of different control measures on seed health and quality, aiding farmers in managing disease threats and increasing production.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization of the study: M.W.H. and M.K.M.; guided data analysis and interpretation: M.K.M.; investigation and drafted the original manuscript: M.W.H. and F.F.; methodology: M.W.H., M.K.M., and D.A.; language improvement: A.M.; reviewed and edited the manuscript: M.K.M.
Funding
The authors thank the Department of Plant Pathology at Odisha University of Agriculture and Technology for support and resources during this study. Special appreciation goes to Dr. Mihir Kumar Mishra for his mentorship. The authors also acknowledge the Indian Council of Agricultural Research (ICAR) for providing a fellowship.
Acknowledgments
The authors thank the Department of Plant Pathology at Odisha University of Agriculture and Technology for support and resources during this study. Special appreciation goes to Dr. Mihir Kumar Mishra for his mentorship. The authors also acknowledge the Indian Council of Agricultural Research (ICAR) for providing a fellowship. I also acknowledge that portions of this manuscript are derived from the work completed during my master’s thesis at Orissa University of Agriculture and Technology (OUAT), Odisha, under the supervision of Dr. Mihir Kumar Mishra. The thesis, titled “Seed mycoflora of rice and their management,” was deposited in the public domain as part of the Indian Council of Agriculture Research (ICAR) initiative and can be accessed via the following link: [https://krishikosh.egranth.ac.in/server/api/core/bitstreams/68b17948-8ef2-4b59-a388-6bb6b869894b/content].
Supporting Information
The supplementary result includes a list of identified pathogens, their morphological features, and tables for seed mycoflora association studies.
Open Research
Data Availability Statement
The authors verify that the data supporting the findings of this study are provided within the article and as supplementary result files.




