Tree Species Richness and Diversity Among Selected Urban Forest Types in Lilongwe City, Malawi
Abstract
Urban vegetation plays a crucial role in achieving Sustainable Development Goal (SDG) 11 by providing ecosystem services in cities. In support of this goal, this study aimed at determining the present urban tree species richness and diversity in Lilongwe City, Malawi, across six forest types: cemeteries, institutional lands, parks and recreation centers, residential areas, riverine areas, and roadside/avenues. Stratified random sampling was employed and 498 sample plots were laid. A total of 4031 trees were recorded, representing 166 species, with five species common to all forest types. Tree species richness and diversity were determined using Rẻnyi diversity profiles in Biodiversity R. Residential areas exhibited 87 tree species, with highest species richness, while Riverine areas had 15 tree species. Rẻnyi diversity profiles indicated that residential forests were the most diverse, albeit with an uneven distribution, primarily due to the dominance of Mangifera indica. Similarly, other forest types showed less horizontal diversity profiles, suggesting disturbance. An even species distribution signifies a healthier ecosystem essential for sustaining its services. Therefore, the findings imply that all the six forest types are disturbed. Hence, the study underscores the need for effective management across all forest types and provides valuable insights for urban planning. In addition, there is a call for a comprehensive study to estimate carbon stock potential and emissions, particularly in industrial areas, to ensure the holistic sustainability of Lilongwe’s urban environment.
1. Introduction
Urban vegetation remains the most pivotal ecosystem for the provision and support of ecosystem services in cities [1, 2]. Urban forest is a forest or a collection of trees that grows within a city or town. The care and management practices for urban forests are referred to as urban forestry. Urban forests largely contribute to the attainment of Sustainable Development Goal (SDG) 11 which states that “Make cities and human settlements inclusive, safe, resilient, and sustainable” [3]. Trees and forests in urban and peri-urban areas offer numerous goods and ecosystem services, significantly enhancing the livelihoods and well-being of city residents [4]. For instance, urban forest plays an important role in fulfilling the four basic services of provisioning, regulating, supporting, and sociocultural services [2].
However, due to increased urbanization in Malawi, particularly in Lilongwe City, urban forests have been degraded due to the subjection of the existing forest types to other perceived land use types. As such, urban areas are increasingly becoming more prone to climate change impacts. This could be due to urban heat island effects and exacerbated effects of drought and extreme weather events resulting from man-made impervious cover from highly constructed physical structures [4]. In response to this, most developed countries have put in place strategies to reduce or deal with the impacts of climate change in most cities. Of paramount importance have been the reduction of greenhouse gas emissions and the promotion of environmentally planned cities to allow the existence of an aspect of nature. Well-planned cities could emanate from balanced spaces for both built-up areas and existing urban vegetation in order to adapt to climate change impacts. As such a detailed inventory of the existing situation using either direct field approach or GIS and remote sensing techniques can greatly aid in the development of well-planned cities [5].
As opposed to developed nations, it has been a great challenge for the developing nations to develop and implement sustainable urban forests, which can positively contribute to the reduction of climate change impacts in the cities. Sub-Saharan Africa, and Malawi in particular, is one of the countries facing such climate change impacts [6]. Therefore, it has been suggested that urban forestry is a major strategy for Malawi to focus on since the country had faced recurrent challenges, which has resulted in so many impacts almost annually for the past two (2) decades in most cities [6].
The drawback to coming up with a solution is attributed to a manifold of issues which already impinges development that include fewer local expatriates in urban forestry and climate change issues, lack of evidence-based research on urban tree species richness and diversity, economic woes, low levels of technology, and lack of political will and population increase. Consequently, Malawian cities are increasingly facing unexpected disasters emanating from the impacts of climate change. For instance, in 2015, 2017, 2018, and 2023, there were persistent disasters of stormy winds and floods in the cities of Lilongwe, Mzuzu, Blantyre, Zomba, and Karonga and the meteorological department issued a warning on most of such incidences then [7]. However, there is no direction towards any efforts in assessing the potential of the urban forestry, which could have made a contribution in addressing these challenges. Hence, this study has brought scientific information on the level of urban forestry in order to develop mitigation strategies to the challenges resulting from climate change through sustainable urban forestry management in the face of the rapid growth of Malawian cities.
Since the country’s democratization in 1994, Lilongwe City has experienced significant urbanization, with rapid growth in the 2000 s [8]. According to UN-HABITAT [6], almost 60–75% of the four main cities of Malawi are in informal settlements with an average of 65% of the urban population living in informal settlements. In recent years, Malawi has continuously been facing an increase in the frequency and intensity of disasters in its cities [7]. Most of which are linked to climate change and variability and manifestation of poor planning, poor drainage systems, inadequate and unregulated waste disposal, and settlement in high-risk areas [6]. The 2023 Tropical Cyclone Freddy disaster has greatly signified the consequences of urban forest degradation as Blantyre City and Mulanje municipality were greatly affected [7]. Efforts to reduce or counteract climate change impacts in Malawian cities have for so long neglected the contributions of urban forestry. There was no substantial research conducted or proposed to account for an inventory of urban forests, the urban vegetation cover changes, and their contributions to climate change mitigation in the cities particularly in Lilongwe City.
So far, the studies which have been conducted concentrated on species composition and diversity [9], mapping and analyzing LULC using GIS and remote sensing [5, 10], peoples’ perception of urban forests, and a focus on surface temperature changes associated with LULC change [11]. Even so, no research was performed in Lilongwe City to account for its tree species richness and diversity to analyze the potential and the gap. In addition, the 2018 National Forestry Inventory (NFI) conducted by the Department of Forestry in conjunction with PERFORM/USAID completely left out the aspect of urban forestry. This in turn could have an impact on the decision-making process toward better strategies to address urban forests management, which are the key strategies for mitigation and adaptation to climate change impacts in urban areas.
It is against this background that this study was conducted to determine the present urban tree species richness and diversity in Lilongwe City, Malawi. Specifically, the study was executed to (1) identify the tree species richness existing in different urban forest types in Lilongwe City, and (2) determine the tree species diversity existing in different urban forest types in Lilongwe City.
2. Materials and Methods
2.1. Study Area
The study was conducted in Lilongwe district, which harbors the capital city of Malawi, located in the central region of Malawi between latitude 13.59 south and longitude 33.47 east (Figure 1). Its elevation ranges from 1000 to 1200 m above sea level, with a primarily flat terrain (GoM, 2010). The city has four major sectors, namely, Lumbadzi, Capital Hill, Kanengo, and Old Town [10].
The city’s climate is characterized by two distinctive seasons: hot–wet season from November to March, and the cool–dry season from April to October. The city experiences an average annual rainfall between 800 and 1000 mm and a mean annual temperature of about 20°C–22.5°C . The district covers an area of 615,900 ha, of which, 37,786 ha is delineated as the main city. In terms of geology, the region is mostly composed of metamorphic and plutonic rocks, which are topped by alluvium and soil made of worn metamorphic and plutonic rocks [8].
In 2005, Lilongwe City experienced a substantial increase in urban growth, which was further enhanced by the repositioning of government offices from Blantyre City to Lilongwe. The current city’s population is estimated at 1.1 million, with an estimated annual growth rate of 4% [12]. With regard to social infrastructure and fundamental urban services, nearly 76% of settlement areas are deemed unsafe. This presents a significant threat, leaving residents vulnerable to natural calamities such as floods and fires [8].
In addition, the city acts as the central region’s main hub for agricultural products’ markets. The primary industrial region is Kanengo in the north, which is the main center for light industry operations such as food processing, maize storage, and tobacco sales and storage, among many other industrial activities. Parks and recreation centers also contribute significantly to the city’s economy. Even so, the unemployment rate is estimated at 16% leading to approximately 25% of the inhabitants being poor [8].
2.2. Data Collection Methods
A stratified random sampling method was employed in order to obtain the required samples. This sampling procedure ensures that homogeneous units, which capture key subgroups and minority groups of tree communities, are created and hence improve the precision of the study [13]. The strata were determined according to the existing informal urban land use types specifically where urban forests fall [8, 9]. According to informal categorization, urban forest types were categorized based on the purpose of forest types as outlined in Table 1 [8].
| ID | Urban forest type | Description |
|---|---|---|
| 1 | Parks and recreation | Green spaces open to the public with set rules and regulations meant biodiversity and ecosystem and recreation and esthetic, i.e., public parks, sanctuaries, botanical gardens, and golf courses |
| 2 | Riverbanks/riverine | They act as buffer zones for aquatic uses and other issues such as sand collection and cultivation, e.g., along Lilongwe and Lingadzi Rivers |
| 3 | Roadside/street/avenue | Trees along the roads, these are for ornamental purposes/orchard trees etc. |
| 4 | Households/residential | Trees planted within household areas, tree species that do not pose a hazard to the infrastructure |
| 5 | Graveyards/cemeteries | Burial sites under the city council or traditional/local authorities |
| 6 | Institution land | Private and public areas within institutions with green spaces including schools, offices, and lodges |
In brief, in residential areas, random sampling was performed by considering population sizes. Residential areas were categorized as low-, medium-, and high-density areas. Two areas were selected in both low and medium areas, while three areas were selected in the high-density area. In each area, four houses were purposively sampled from the houses with a considerable number of trees [9]. A complete tree inventory was performed accordingly at each sampled household.
For urban street trees, roads were sampled as determined through Google Earth Map and verified by the LCC authorities or the Malawi Roads Fund Administration (MRFA). First, random sampling was performed to select the required number of streets. Eleven avenues were identified. The first two were the major streets of ≥ 1000 m across the city and hence were automatically included. Then, three avenues were purposively selected from the remaining nine. In each avenue/road, sample plots were laid within a stretch of 20 m long on both sides of the road/street at a regular interval of 500 m.
For riverine/riverbank forests, Lilongwe City has two major rivers that pass through it [8]. Hence, two major rivers were identified and purposively selected as Lilongwe and Namanthanga Rivers, respectively. A distance of 20 m (riparian zone) for each selected river was sampled. Along each riverbank, sample plots were laid within a stretch of 20 m long on both sides of the river at a regular interval of 500 m within the city.
Lastly, four institutional areas were randomly selected with a subcategorical consideration of each for offices, schools, churches, and lodges. No sample plots were laid in the identified areas, rather the area for each institution was measured, and a complete tree inventory was performed. Table 2 and Figure 2 summarize the sampled areas.
| No. | Forest type | Sample plots and locations |
|---|---|---|
| 1 | Cemeteries | Plots of 0.01 ha (City Council, GVH 18, Nsungwi, and Chilinde) |
| 2 | Institutions | Plots of 0.04 ha (Capital Hill, MIM, Ufulu Gardens, and Lutheran Church-Area 22) |
| 3 | Parks and recreation | Plots of 0.04 ha (botanical garden, sanctuary, golf course, and Dzenza Forest Reserve) |
| 4 | Residential | High (Area 57-Chinsapo, Area 23, and Area 21-Chilinde), medium (Area 17-Institute and Area 18), and low (Area 10 and Area 12): 4 houses were sampled in each area |
| 5 | Riverine | Every 500 m along the Lilongwe River |
| 6 | Roads/avenues | Every 500 m along (M1 Road-6miles to Lumbadzi, Bypass-6miles to Kanengo, Chayamba Drive, Airport Road, and Mzimba Avenue) |
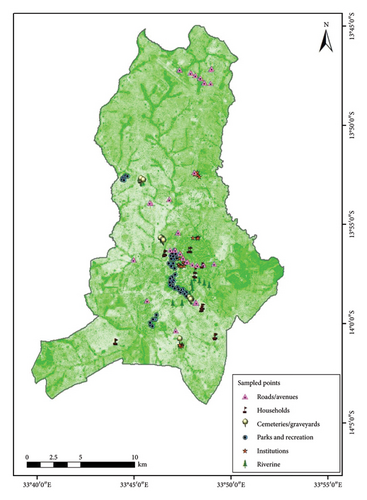
In each sample plot or sampled area, GPS coordinates were taken and a full inventory was conducted for all tree species as required. According to FAO [4], a tree is well-defined as any perennial woody plant possessing one main trunk, or in the case of coppice, with numerous trunks, possessing a more or less definite crown. In this case and for the purpose of this study, a tree was regarded as such excluding bamboos, palms, and other woody plants meeting the criteria in question. Thus, in each sampling plot, an inventory of trees taller than 1.5 m and of diameter at breast height (DBH) (1.3 m from the ground) of greater than or equal to 5 cm was performed. Trees in each sampling plot were identified up to species level with the help of a plant taxonomist and guidelines of plants of Malawi and nearby countries [16] as well as the Malawi species code list developed by the Forest Research Institute of Malawi (FRIM) and the National Herbarium. The sample of all unidentified tree species specimens was collected, pressed, dried, mounted, and deposited in the Herbarium of FRIM with a code number on the description sheet. Furthermore, tree identification relies on taxonomic classification, which organizes trees into hierarchical categories: kingdom, phylum (division), class, order, family, genus, and species. This system enables accurate identification and comparison by grouping trees based on shared characteristics, aiding botanists, ecologists, and foresters in research, conservation, and sustainable management practices [9].
2.3. Data Analysis
Tree species richness and diversity were assessed using Rẻnyi diversity profiles in BiodiversityR. [17]. The functionality of BiodiversityR has been thoroughly explained by Missanjo et al. [18]. In summary, BiodiversityR is a software that performs comprehensive biodiversity analyses, while Rẻnyi diversity profiles are curves that provide insights into species richness and evenness. The shape of the profile indicates evenness, with the initial position on the left-hand side representing species richness. A profile that starts at a higher level indicates greater richness. The main advantage of Rẻnyi diversity profiles is that they allow for easy comparison of sites in terms of diversity. If the profile for one site is consistently above that of another, the site with the higher profile is more diverse.
3. Results and Discussion
3.1. Tree Species Richness in Urban Forest Types in Lilongwe City
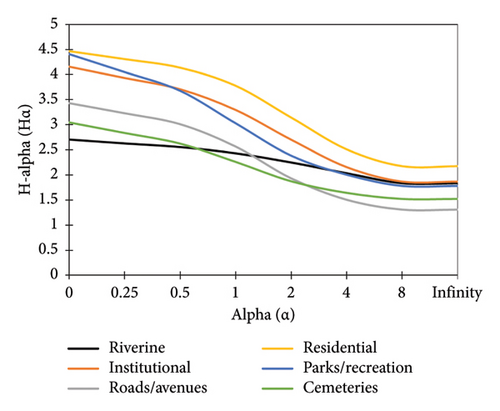
The Rẻnyi diversity profiles for the six forest types in Lilongwe City are presented in Figure 3 and a summary of tree species encountered in each forest type is presented in Table 3.
| Forest type | Number of sample plots | Stems per ha | Number of tree species recorded |
|---|---|---|---|
| Parks and recreation | 44 | 1472 | 82 |
| Cemeteries | 20 | 934 | 21 |
| Institutional | 22 | 824 | 64 |
| Riverine | 84 | 361 | 15 |
| Residential | 28 | 290 | 87 |
| Roads/avenue | 300 | 150 | 31 |
| Total | 498 | 4031 |
- Note: 498 is the total number of sample plots laid and 4031 is the total number of stems/trees recorded/sampled.
A total of 498 sampled plots were laid in all the six forest types constituting a total record of 4031 individual trees encountered (Table 3). The number of tree species was 165 which were recorded in all six forest types (Table A1). Interestingly, of the total tree species recorded, only five tree species (Gmelina arborea, Khaya anthotheca, Rauvolfia caffra, Senegalia polyacantha, and Senna spectabilis) were recorded in the six forest types. Each of the six urban forest types had its own tree species, which are not shared with any of the forest types. In this respect, 38 tree species were recorded in parks/recreation forests, 37 tree species were recorded in residential forests, 15 tree species were recorded in institutional forests, 4 tree species (Vachellia karroo, Ficus exasperate, Ficus natalensi, and Tecomaria capensis) were recorded in road/avenue forests, while one tree species (Afzelia quanzensis) was recorded in the cemetery forests.
Furthermore, the most dominant family consisting of at least four species was identified (Table 4). These families constituted 64.44% of the total tree species documented in the six urban forest types in Lilongwe City.
| No. | Family | Number of tree species | Percentage (%) |
|---|---|---|---|
| 1 | Leguminosae | 29 | 17.47 |
| 2 | Fabaceae | 13 | 7.83 |
| 3 | Combretaceae | 10 | 6.02 |
| 4 | Moraceae | 9 | 5.42 |
| 5 | Rubiaceae | 8 | 4.82 |
| 6 | Annonaceae | 6 | 3.61 |
| 7 | Meliaceae | 6 | 3.61 |
| 8 | Bignoniaceae | 5 | 3.01 |
| 9 | Euphorbiaceae | 5 | 3.01 |
| 10 | Anacardiaceae | 4 | 2.41 |
| 11 | Ebenaceae | 4 | 2.41 |
| 12 | Myrtaceae | 4 | 2.41 |
| 13 | Rutaceae | 4 | 2.41 |
The parks and recreation and residential area forests had the highest species number probably due to the protection they receive and that most of these forests lie in Miombo woodland forest areas [19] in addition to being supplemented by planting of exotic tree species [9]. Less number of tree species was recorded in institutional and road/avenue forests. This could be a result of the loss of most trees due to construction as the land is cleared and the planted trees are mostly chosen for a specific purpose, which is mostly ornamental. This also concurs with what the authors in [9, 20, 21] concluded that there is a great anthropogenic influence in these land use types, hence a smaller number of tree species as the choice is in accordance with the owner’s interests.
The similarity of most tree species being found across all the forest types is attributed to the indigenous forest type of area being Miombo woodland [8]. Most species are of indigenous type as they have been naturally established [19], whereas, Afzelia quanzensis, which is found only in cemeteries, could be a true reflection of this species being sacred in Malawian culture.
3.2. Tree Species Diversity in Urban Forest Types in Lilongwe City
The results on tree species diversity (from Rẻnyi diversity profiles value), which were analyzed based on richness and evenness levels in each forest type, showed that the residential forest had a higher Hα (4.47) at 0-alpha followed by the parks/recreation forest (Hα = 4.41) (Figure 3). The riverine forest had the lowest Hα (2.71) at 0-alpha. This indicates that the residential forest had a higher tree species richness and evenness, based on Rẻnyi diversity profiles, followed by the parks/recreation forest, while the riverine forest had the least tree species’ Rẻnyi diversity index value (richness and evenness). Households have proved to harbor a considerable number of trees in accordance with similar studies conducted in Zomba City, Malawi [9], and Kumasi City, Ghana [22].
The results show that the Rẻnyi diversity profile for residential forests is higher than those of the other five forest types (Figure 3). This is an indication that residential forests are more diverse than the other forest types. However, the shape of the profile for the residential forest is less horizontal. This is an indication that the proportions of the individual tree species are not evenly distributed. This was attributed to the dominance of one species (such as Mangifera indica). According to studies [9, 22] in Malawi and Ghana, respectively, residential areas indeed have a higher number of species. However, they are less diverse because the choices of the tree species are mostly determined by the owners. It has been observed that most tree species in households are those which can provide food/fruits such as Mangifera indica. This fruit tree was found to be dominant in almost all the households of the residential forest type.
According to other studies [9], Mangifera indica, Psidium guajava, Persia americana, and Carica papaya were the dominant species in households, signifying their purposes for food beyond their values as beautification of the homesteads. Similarly, the study [23] concluded that tree households’ spaces in most cities of developing countries were planted for a variety of purposes, including fruits, nuts, fuel wood, vegetables, fodder, shade, and windbreak. For this reason, the authors in [24] argue that trees and their seed networks have implications for genetic diversity with regard to a study on urban fruit trees conducted in Yaounde, Cameroon.
Similarly, the shapes of the diversity profiles for the other five forest types are less horizontal. This means that the proportional of the individual tree species were not relatively evenly distributed [25]. An even distribution of individual plant species in an ecosystem signifies a healthier or good ecosystem where few plant species are not dominating the community/vegetation [26]. As stated by the authors in [25], a denser, functionally diverse, and well-adapted community of urban trees could provide more ecosystem services that are more resilient to the increasing risks of biophysical disturbances induced by environmental changes. Even with the limitations of available space for infrastructure and buildings, there are still plenty of opportunities for planting trees, even though forest-like tree densities are not appropriate for all land use types. For instance, the authors in [25] suggest that through hybrids of traditional and green infrastructure designs, it may be possible to increase tree density and diversity by using soils that are good for load bearing and root development in sidewalks. McKinney [20] concurs that, such alterations to street tree growth environments may make it easier to plant a wider variety of species, including more indigenous tree species; in essence, these kinds of indigenous tree species preservation and promotion initiatives can prevent the loss of regional biotic distinctiveness. On the other hand, modifications to landscaping techniques can present chances for spontaneous regrowth [27].
Therefore, the present results suggest that all the six forest types are disturbed and need appropriate management. This is in line with the findings of a similar study carried out in Kumasi, Ghana, by the authors in [21], which showed that changes in land use and land cover between 2007 and 2017, which are primarily related to infrastructure development in most cities, have significantly decreased the richness and diversity of urban tree species. However, the major challenge remains in the minimal understanding of drivers of diversity about different types of urban forests that make up the entire urban forest ecosystem, which represent differences in tree planting and establishing techniques, ownership, and maintenance [28–32]. For instance, in a study conducted, by the authors in [2], in cities of Lilongwe and Mzuzu, Malawi, it was found that there is negligible evidence that urban green spaces and green setup are integrated into decision-making protocols at the national level. The planning and strategy documents created by the city councils of Mzuzu and Lilongwe did, however, prioritize developing and expanding urban green space and green infrastructure. Therefore, the analysis suggests that if the numerous advantages of urban green spaces and green infrastructure are to be realized, there has to be better institutional coordination and policy coherence across national level sectors that are impacted [2].
4. Conclusion
The study has revealed that the residential forest in Lilongwe City had higher tree species richness and was also more diverse than the other five forest types. However, there was an uneven distribution of species in all the six forest types. An even species distribution signifies a healthier ecosystem essential for sustaining its services. Therefore, the findings imply that all the six forest types are disturbed. Hence, the study underscores the need for effective management across all forest types and provides valuable insights for urban planning, in order to achieve SDG 11 which states that “Make cities and human settlements inclusive, safe, resilient, and sustainable.” Therefore, the study recommends that entrusted institutions and authorities for urban planning and management should have joint efforts in making sure that there is sustenance and joint development and management of urban forests for better realization of eco-city construction and their inherent benefits.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was funded by the World Bank Group through the Africa Center of Excellence (ACE), Climate Smart Agriculture and Biodiversity Conservation (SABC), Haramaya University, Ethiopia.
Acknowledgments
The authors are greatly indebted to Steven Mphamba for his great work in the field during the data collection exercise especially on species identification.
Appendix A
| No. | Species name | Family | Cemeteries | Institutions | Recreations | Residential | Riverine | Roads |
|---|---|---|---|---|---|---|---|---|
| 1 | Adansonia digitata | Malvaceae | N | N | N | Y | N | N |
| 2 | Adenia gummifera | Passifloraceae | N | N | Y | N | N | N |
| 3 | Afzelia quanzensis | Fabaceae | Y | N | N | N | N | N |
| 4 | Albizia amara | Fabaceae | N | Y | Y | N | N | N |
| 5 | Albizia antunesina | Fabaceae | N | N | Y | N | N | N |
| 6 | Albizia glaberrima | Fabaceae | N | N | Y | N | N | N |
| 7 | Albizia harveyi | Fabaceae | N | N | Y | Y | N | Y |
| 8 | Albizia lebbeck | Fabaceae | N | Y | Y | Y | N | Y |
| 9 | Albizia versicolor | Fabaceae | N | N | Y | N | N | Y |
| 10 | Annona senegalensis | Annonaceae | N | N | Y | N | N | N |
| 11 | Annona squamosa | Annonaceae | N | N | N | Y | N | N |
| 12 | Annona stenophylla | Annonaceae | N | N | N | Y | N | N |
| 13 | Araucaria araucana | Araucariaceae | N | N | N | Y | N | N |
| 14 | Archontophoenix alexandrae | Arecaceae | N | N | N | Y | N | N |
| 15 | Bauhinia petersiana | Leguminosae | N | N | Y | N | N | N |
| 16 | Bauhinia tomentosa | Leguminosae | N | N | Y | N | N | Y |
| 17 | Bauhinia variagata | Leguminosae | N | N | N | Y | N | N |
| 18 | Boscia angustifolia | Capparaceae | Y | N | Y | N | N | N |
| 19 | Boscia salicifolia | Capparaceae | N | N | Y | N | N | N |
| 20 | Brachystegia spiciformis | Leguminosae | N | Y | Y | N | N | N |
| 21 | Brachystegia stipulata | Leguminosae | N | Y | Y | N | N | N |
| 22 | Broussonetia papyrifera | Moraceae | N | N | N | Y | N | N |
| 23 | Burttdavya nyasica | Rubiaceae | N | N | Y | N | N | N |
| 24 | Callistemon viminalis | Myrtaceae | N | Y | N | Y | N | N |
| 25 | Carica papaya | Caricaceae | N | N | N | Y | N | Y |
| 26 | Caryota urens | Arecaceae | N | N | N | Y | N | N |
| 27 | Casimiroa edulis | Rubiaceae | N | N | N | Y | N | N |
| 28 | Cestrum nocturnum | Solanaceae | N | N | N | Y | N | N |
| 29 | Citrus limonium | Rutaceae | N | Y | N | Y | N | N |
| 30 | Citrus reticulata | Rutaceae | N | N | N | Y | N | N |
| 31 | Citrus sinensis | Rutaceae | N | N | N | Y | N | N |
| 32 | Clerodendrum capitatum | Lamiaceae | N | N | Y | N | N | N |
| 33 | Clerodendrum rotundifolium | Lamiaceae | N | N | Y | N | N | N |
| 34 | Combretum adenogonium | Combretaceae | Y | Y | Y | Y | N | N |
| 35 | Combretum collinum | Combretaceae | Y | Y | Y | Y | N | N |
| 36 | Combretum molle | Combretaceae | Y | Y | Y | N | N | N |
| 37 | Combretum apiculatum | Combretaceae | N | Y | N | N | N | N |
| 38 | Combretum pentagonum | Combretaceae | N | Y | Y | N | N | N |
| 39 | Combretum zeyheri | Combretaceae | N | Y | Y | Y | N | Y |
| 40 | Commiphora caerulea | Burseraceae | N | N | N | Y | N | N |
| 41 | Commiphora edulis | Burseraceae | N | N | N | Y | N | N |
| 42 | Commiphora zanzibarica | Burseraceae | N | N | Y | N | N | N |
| 43 | Cordia africana | Boraginaceae | N | Y | N | N | N | N |
| 44 | Cossonia arborea | Araliaceae | N | Y | Y | N | N | N |
| 45 | Dalbergia inteo | Leguminosae | N | N | Y | N | N | N |
| 46 | Dalbergia melanoxylon | Leguminosae | N | N | Y | Y | N | N |
| 47 | Dalbergia nitidula | Leguminosae | N | Y | N | N | N | N |
| 48 | Delonix regia | Leguminosae | N | Y | N | Y | N | Y |
| 49 | Dichrostachys cinerea | Leguminosae | N | Y | Y | N | N | N |
| 50 | Dichrostachys glomerata | Leguminosae | N | N | Y | N | N | N |
| 51 | Diospyros mespiliformis | Ebenaceae | Y | N | Y | N | N | N |
| 52 | Diospyros lycioides | Ebenaceae | N | N | Y | N | N | N |
| 53 | Diospyros mespiliformis | Ebenaceae | N | N | Y | N | N | N |
| 54 | Diplorhynchus condylocarpon | Apocynaceae | N | Y | Y | N | N | N |
| 55 | Dombeya burgessiae | Malvaceae | N | Y | N | N | N | N |
| 56 | Dombeya rotundifolius | Malvaceae | N | N | Y | N | N | N |
| 57 | Dracaena steudneri | Dracaenaceae | N | Y | N | Y | N | N |
| 58 | Ehretia divariata | Boraginaceae | N | N | Y | N | N | N |
| 59 | Ekebergia benguelensis | Meliaceae | Y | N | N | Y | Y | N |
| 60 | Ekebergia capensis | Meliaceae | N | Y | Y | N | N | N |
| 61 | Encephalartos gratus | Zamiaceae | N | Y | N | N | N | N |
| 62 | Entada abyssinica | Fabaceae | N | Y | N | N | N | N |
| 63 | Erythrina abyssinica | Leguminosae | N | Y | N | Y | N | N |
| 64 | Eucalyptus camaldulensis | Myrtaceae | N | Y | N | N | Y | N |
| 65 | Eucalyptus saligna | Myrtaceae | N | Y | N | Y | N | Y |
| 66 | Euclea racemosa | Ebenaceae | N | N | Y | N | N | N |
| 67 | Euphorbia tirucalli | Euphorbiaceae | N | Y | N | Y | N | N |
| 68 | Faurea racemosa | Proteaceae | N | Y | N | N | Y | N |
| 69 | Feretia aeruginescens | Proteaceae | N | N | Y | N | N | N |
| 70 | Ficalhoa laurifolia | Theaceae | N | N | Y | N | N | N |
| 71 | Ficus benjamina | Moraceae | N | N | N | Y | N | Y |
| 72 | Ficus bussei | Moraceae | N | N | N | Y | N | N |
| 73 | Ficus exasperata | Moraceae | N | N | N | N | N | Y |
| 74 | Ficus natalensis | Moraceae | N | N | N | N | N | Y |
| 75 | Ficus sur | Moraceae | N | N | N | Y | N | N |
| 76 | Ficus sycomorus | Moraceae | N | Y | N | Y | N | N |
| 77 | Ficus thonningii | Moraceae | N | N | N | Y | N | N |
| 78 | Fraxinus excelsior | Oleacea | N | N | N | Y | N | N |
| 79 | Friesodielsia obovata | Annonaceae | N | Y | Y | N | N | N |
| 80 | Gardenia jovis-tonantis | Rubiaceae | N | Y | N | N | N | N |
| 81 | Gardenia volkensii | Rubiaceae | N | Y | N | N | N | N |
| 82 | Gmelina arborea | Rubiaceae | Y | Y | Y | Y | Y | Y |
| 83 | Holarrhena pubescens | Apocynaceae | N | N | Y | N | N | N |
| 84 | Jacaranda mimosifolia | Bignoniaceae | N | Y | N | Y | N | Y |
| 85 | Jatropha carcus | Euphorbiaceae | N | N | N | Y | N | N |
| 86 | Karomia speciosa | Lamiaceae | N | N | Y | N | N | N |
| 87 | Keetia gueinzii | Rubiaceae | N | N | Y | N | N | N |
| 88 | Khaya anthotheca | Meliaceae | Y | Y | Y | Y | Y | Y |
| 89 | Kigelia africana | Bignoniaceae | N | N | N | Y | N | N |
| 90 | Lagerstroemia indica | Lythraceae | N | N | N | Y | N | N |
| 91 | Lannea discolor | Anacardiaceae | N | Y | Y | N | N | N |
| 92 | Leucaena leucocephala | Leguminosae | N | N | N | Y | N | N |
| 93 | Mangifera indica | Anacardiaceae | Y | Y | Y | Y | Y | N |
| 94 | Markhamia obtusifolia | Bignoniaceae | Y | Y | Y | Y | N | N |
| 95 | Markhamia zanzibarica | Bignoniaceae | N | Y | N | N | N | N |
| 96 | Maytenus heterophylla | Celastraceae | N | N | Y | Y | N | N |
| 97 | Melia azedarach | Meliaceae | N | Y | N | Y | N | Y |
| 98 | Monotes africanus | Dipterocarpaceae | N | N | Y | N | N | N |
| 99 | Moringa oleifera | Moringaceae | N | N | N | Y | N | N |
| 100 | Moringa stenopetala | Moringaceae | N | N | N | Y | N | N |
| 101 | Morus alba | Moraceae | N | N | N | Y | N | N |
| 102 | Mystroxylon aethiopicum | Celastraceae | N | N | Y | N | N | N |
| 103 | Neoboutonia africana | Euphorbiaceae | N | Y | N | N | N | N |
| 104 | Ochna puberula | Ochnaceae | N | N | Y | N | N | N |
| 105 | Parkia filicoidea | Leguminosae | N | N | N | Y | N | N |
| 106 | Peltophorum africanum | Leguminosae | N | N | Y | N | N | N |
| 107 | Pericopsis angolensis | Leguminosae | N | Y | Y | Y | N | N |
| 108 | Persia americana | Lauraceae | N | N | N | Y | N | N |
| 109 | Philenoptera bussei | Leguminosae | N | Y | Y | N | N | N |
| 110 | Philenoptera violecea | Leguminosae | N | Y | Y | Y | N | Y |
| 111 | Phoenix reclinata | Arecaceae | N | Y | N | N | N | N |
| 112 | Piliostigma thonningii | Leguminosae | Y | Y | N | Y | N | Y |
| 113 | Pinus oocarpa | Pinaceae | N | Y | N | N | N | N |
| 114 | Plumeria rubra | Euphorbiaceae | N | N | N | Y | N | N |
| 115 | Plunus persia | Rosaceae | N | Y | N | N | N | N |
| 116 | Polyalthia longifolia | Annonaceae | N | N | N | Y | N | N |
| 117 | Prunus persica | Rosaceae | N | N | N | Y | N | N |
| 118 | Pseudolachnostylis maprouneifolia | Euphorbiaceae | N | N | Y | N | N | N |
| 119 | Psidium guajava | Myrtaceae | N | N | N | Y | Y | N |
| 120 | Psydrax livida | Rubiaceae | N | N | Y | N | N | N |
| 121 | Pterocarpus angolensis | Leguminosae | N | N | Y | Y | N | N |
| 122 | Pterocarpus rotundifolius | Leguminosae | N | Y | Y | Y | N | N |
| 123 | Pyracantha coccinea | Rosaceae | N | N | N | Y | N | N |
| 124 | Rauvolfia cafra | Apocynaceae | Y | Y | Y | Y | Y | Y |
| 125 | Rawsonia lucida | Flacourtiaceae | N | N | Y | N | N | N |
| 126 | Rhus longipes | Anacardiaceae | N | N | N | Y | N | N |
| 127 | Rourea orientalis | Connaraceae | N | N | Y | N | N | N |
| 128 | Schefflera myriantha | Araliaceae | N | N | Y | N | N | N |
| 129 | Scherebera trichoclada | Oleaceae | N | N | Y | N | N | N |
| 130 | Sclerocarya birrea | Anacardiaceae | Y | N | N | Y | N | N |
| 131 | Senegalia polyacantha | Fabaceae | Y | Y | Y | Y | Y | Y |
| 132 | Senna petersiana | Leguminosae | N | N | Y | N | N | N |
| 133 | Senna siamea | Leguminosae | N | Y | Y | Y | Y | Y |
| 134 | Senna spectabilis | Leguminosae | Y | Y | Y | Y | Y | Y |
| 135 | Solanum macranthum | Solanaceae | N | Y | N | N | N | Y |
| 136 | Spathodea campanulata | Bignoniaceae | Y | N | Y | Y | N | Y |
| 137 | Steganotaenia araliacea | Apiaceae | N | Y | Y | N | N | Y |
| 138 | Strychnos cocculoides | Loganiaceae | N | Y | Y | N | N | N |
| 139 | Strychnos potatorum | Loganiaceae | Y | Y | Y | N | N | N |
| 140 | Strychnos spinosa | Loganiaceae | Y | Y | Y | Y | N | N |
| 141 | Syzvgium guineense | Loganiaceae | N | N | N | Y | Y | N |
| 142 | Syzygium jambos | Myrtaceae | N | N | N | Y | N | N |
| 143 | Tamarindus indica | Myrtaceae | N | N | Y | N | N | N |
| 144 | Tecomaria capensis | Leguminosae | N | N | N | N | N | Y |
| 145 | Terminalia catappa | Combretaceae | N | N | N | Y | N | N |
| 146 | Terminalia mollis | Combretaceae | N | N | N | Y | N | N |
| 147 | Terminalia sericea | Combretaceae | Y | N | Y | N | N | Y |
| 148 | Terminalia stenostachya | Combretaceae | N | Y | N | N | N | N |
| 149 | Thespesia garckeana | Malvaceae | N | N | N | Y | N | N |
| 150 | Thevetia peruviana | Asteraceae | N | N | Y | Y | N | N |
| 151 | Toona ciliata | Meliaceae | N | Y | Y | Y | N | Y |
| 152 | Trichilia emetica | Meliaceae | N | N | Y | Y | N | N |
| 153 | Uvaria lucida | Annonaceae | N | N | Y | N | N | N |
| 154 | Vachellia amythethophylla | Fabaceae | N | N | Y | N | N | N |
| 155 | Vachellia galpinii | Fabaceae | N | Y | Y | Y | Y | Y |
| 156 | Vachellia goetzei | Fabaceae | N | N | Y | N | Y | N |
| 157 | Vachellia karroo | Fabaceae | N | N | N | N | N | Y |
| 158 | Vangueria infausta | Rubiaceae | N | Y | N | N | N | Y |
| 159 | Vernonia amygdalina | Asteraceae | Y | N | N | Y | Y | N |
| 160 | Vernonia myriantha | Asteraceae | N | N | Y | Y | N | N |
| 161 | Vitex mombassae | Verbenaceae | N | N | Y | N | N | N |
| 162 | Vitex payos | Verbenaceae | N | Y | Y | N | N | N |
| 163 | Zanha africana | Sapindaceae | N | N | N | Y | N | N |
| 164 | Zanthoxylum chalybeum | Rutaceae | N | N | Y | N | N | N |
| 165 | Ziziphus mauritiana | Rhamnaceae | N | N | Y | Y | N | N |
- Note: Y, presence; N, absence. Bold represents present in all forest types. Italic represents present only in one specific forest type.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




