A Comparative Assessment of Carbon Stock Potential and Regeneration Status Between Natural and Plantation Forests in Southwest Ethiopia
Abstract
Forests play a key role in combating climate change by sequestering and storing carbon from the atmosphere through photosynthesis. This study compares the carbon stock potential and regeneration status between natural and plantation forests in the Derashe special district, Southwest Ethiopia. The study involved collecting of data from 90 20 × 20 m plots for trees, 5 × 5 m plots for seedlings and saplings, and 1 × 1 m plots for soil, litter, herb and grass, aboveground biomass and carbon stock were measured using allometric equations, while belowground biomass was calculated based on the ratio of belowground to aboveground biomass. Soil organic carbon (SOC) was determined from organic carbon content in three soil layers (0–10 cm, 10–20 cm, and 20–30 cm). The regeneration potential was assessed by counting seedlings, saplings, and mature trees. Data analysis was performed using two independent t-tests and one-way ANOVA. The results showed that the average total biomass carbon density of natural forests (271.57 tons·ha−1) was higher than that of plantation forests (259.23 tons·ha−1). There was no significant difference in aboveground biomass carbon, belowground biomass carbon, and deadwood biomass carbon between the two forest types. However, significant differences (p < 0.05) were found in SOC, litter, herb and grass biomass carbon, and stump deadwood biomass carbon. The total number of seedlings, saplings, and mature trees were 127, 107, and 813 in natural forests, and 169, 62, and 1030 in plantation forests, respectively. Both forest types exhibited poor regeneration, as the number of seedlings and saplings was lower compared to mature trees, indicating forest degradation. The Gardula forest stores a total of 530.8 tons ha-1 carbon. In general, both plantation and natural forests in Gardula have significant carbon storage potential and contributing to national and global efforts to mitigate climate change.
1. Introduction
Global warming and climate change are critical issues driven by increasing carbon emissions, primarily from greenhouse gases (GHGs) like CO2. High concentrations of CO2, a heat-trapping gas, significantly raise Earth’s temperature, leading to global warming [1]. Urbanization, unplanned development, and deforestation contribute to the continuous accumulation of GHGs in the atmosphere [2]. The Kyoto Protocol has addressed these concerns, emphasizing the need to reduce carbon emissions [3].
Forests play a crucial role in mitigating climate change by sequestering and storing carbon through photosynthesis. They store about one-fourth of the global terrestrial carbon, making them vital for the global carbon cycle [4]. Forests provide numerous environmental benefits, including erosion control and significant carbon storage both above and below ground [5]. The world’s forests are estimated to store over 650 billion tons of carbon, with 44% in aboveground biomass (AGB), 11% in dead wood, and 45% in forest soils [6]. Afforestation and reforestation are effective climate change mitigation strategies, enhancing carbon storage in biomass and soil [7]. Increasing planted forests can boost carbon sequestration, reduce soil erosion, and support biodiversity [8]. Plantations can alleviate deforestation pressure on natural forests, connecting fragmented landscapes and providing alternative livelihoods for forest-fringe communities [9].
Forest regeneration is essential for species survival and ecosystem sustainability. It depends on various environmental conditions, such as forest floor conditions and nutrient cycling [10]. Sustainable forest management requires understanding regeneration dynamics and factors influencing canopy tree species [11]. Regeneration ensures the continuous supply of young trees to replace mature ones, maintaining forest health and productivity [12].
Ethiopia has one of the largest forest resources in the Horn of Africa, with 53.1 million hectares of woody vegetation, including 12.5 million hectares of forestland and 40.6 million hectares of other woodlands [13, 14].
The Gardula forest comprised of both natural and plantation forests. Healthy forests require a diverse age structure, with robust regeneration crucial for maintaining ecosystem stability and providing future generations of trees [15]. However, the Gardula forest exhibits low density of seedlings and saplings, despite a significant number of mature trees. This threatens the forest’s long-term health, resilience, and carbon sequestration potential. The scarcity of young trees in Gardula, potentially driven by anthropogenic pressures such as cattle grazing and other human activities [16], indicates a stressed ecosystem [17]. This impedes the forest’s capacity to act as an effective carbon sink, particularly since young, actively growing trees are vital for biomass accumulation and long-term carbon storage [18]. Therefore, research into the specific regeneration status and carbon sequestration potential of the two forests is crucial to inform management strategies that promote forest recovery, biodiversity, and long-term carbon sequestration.
Moreover, limited research has been conducted on the comparative study of carbon stock potential and regeneration status between natural and plantation forests in Ethiopia. There is ongoing debate within the scientific community regarding which type stores more carbon dioxide. This study aims to fill research gaps by evaluating these aspects and their contributions to climate change mitigation.
2. Materials and Methods
2.1. Description of the Study Area
This study was conducted in Gardula Forest, located in the Derashe Special District of South Ethiopia Regional State. Gardula Forest is situated between 5°39′78″N latitude and 37°01′56″E longitude (Figure 1). The forest covers an area of 1987 ha, with 1635 ha being plantation forest and 352 ha being natural forest (Figure 1). The natural forest, found to the north and south of the plantation forest, is dominated by Syzygium guineense, Prunus africana and Myrica salicifolia species. The plantation forest, established in 1977, is dominated by Eucalyptus globulus, Cupressus lusitanica and Juniper procera and is protected by the government and local communities.
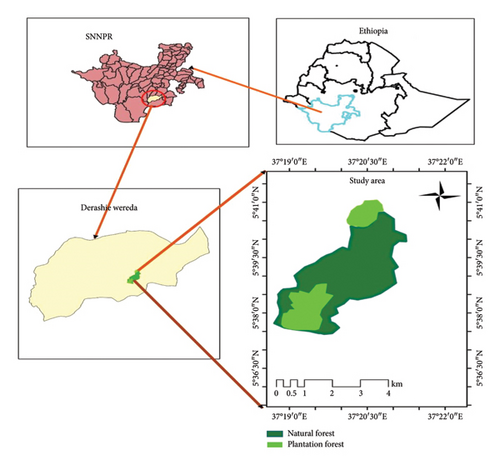
The altitude of Gardula Forest ranges from 1140 to 2614 m above sea level. The mean annual rainfall in the area varies from 600 to 1600 mm [19]. The agro-climate of the study area is categorized into highland (17.24%), midland (34.17%), and lowland (48.61%). The annual maximum and minimum temperatures range from 26°C to 28°C and 11°C–14°C, respectively [19]. The soil types in the study area include vertisols, characterized by swelling and cracking features, and cambisols, formed from volcanic ashes [20].
2.2. Data Collection Methods
2.2.1. Sampling Techniques
A systematic sampling technique was employed to estimate the carbon stock potential of both natural and plantation forests. The study area includes two natural forest regions: Shokollo Forest (88 ha) to the north and Naga Forest (264 ha) to the south. Following Eshetu and Hailu [21]; the study site was classified into three altitudinal gradients: lower altitude (LA; 2267–2346 m), mid-altitude (MA; 2347–2425 m), and high altitude (HA; 2426–2505 m) for natural forests, and (LA; 2172–2296 m), (MA; 2297–2420 m), and (HA; 2421–2546 m) for plantation forests.
Transects were laid along the altitude gradient in both natural and plantation forests to capture vegetation heterogeneity. A total of 10 transects (five in plantation forests and five in natural forests) were established, oriented east to west, with a 400 m interval between transects. Main sampling plots were positioned along the transect lines at 100 m intervals. Ninety plots (45 in natural forests and 45 in plantation forests) were established along the transects, with each plot located 50 m away from the borders to avoid edge effects.
In each transect, 20 × 20 m (400 m2) main plots were established for woody vegetation data and biomass inventory, identified using GPS and a compass (Figure 2). In each plot, the diameter at breast height (DBH ≥ 2.5 cm) of all tree species was measured using a diameter tape and caliper. The height of woody species was measured with a Suunto Hypsometer, a calibrated eucalyptus stick, and visual estimation [22]. The height distribution of trees was classified into four classes: Class I (0–10 m), Class II (10–20 m), Class III (20–30 m), and Class IV (30–40 m). The altitude of each main plot was recorded using GPS. At the center and each corner of the main plot, nested 1 × 1 m (1 m2) subplots were established for collecting litter, grass, herb, and soil (samples (Figure 2). The DBH measurements were categorized in to 8 classes (5–20; 20–40; 40–60; 60–80; 80–100; 100–120; 120–140; and > 140 cm) following [22].
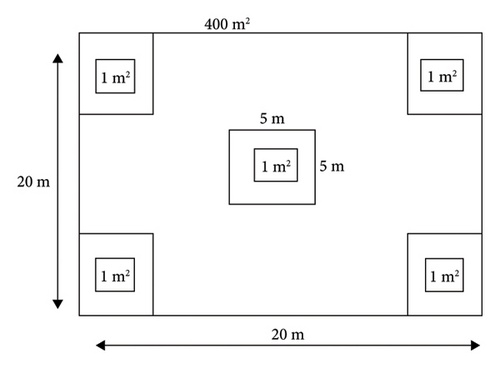
All plant species in each quadrant were counted and recorded, with local tree names noted. For species difficult to identify in the field, herbarium specimens were collected, dried, and sent to the National Herbarium at Addis Ababa University. Scientific names were determined using the Flora of Ethiopia and Eritrea [23–25] and Useful Trees and Shrubs for Ethiopia [26].
2.2.2. Carbon Stock Data Collection and Analysis
2.2.2.1. AGB and Carbon Stock Estimation
The AGB of trees was measured by recording the DBH and height, following standard carbon inventory procedures [27]. Allometric equations, which rely on nondestructive and easily measurable tree metrics, were used for estimation [28]. In each sample plot, the DBH and height of woody live plant species were recorded, with only trees having a DBH ≥ 2.5 cm included [16]. Tree height was measured using a combination of a Suunto clinometer and a graduated stick.
The AGB of all trees in the study sites was estimated using an allometric equation developed by Chave et al., [29]; based on aboveground tree biomass for different tropical climatic regions. The biomass estimate was converted to tons of carbon, and a scaling factor was applied to estimate carbon stocks per hectare. The biomass of all trees sampled in each plot was then summed to estimate the total carbon stock in each sampled plot [30].
2.2.2.2. Belowground Biomass (BGB) and Carbon Stock Estimation
Collecting samples for BGB (roots) is challenging due to the need for excavation. Therefore, BGB was estimated using a root-to-shoot ratio [32] and the biomass was converted to tons per hectare. The BGB was 20% of the AGB [33]. This method is recommended for tropical or humid forests [31]. Carbon stock of below ground biomass was calculated by multiplying the biomass with the default carbon fraction of 0.47 [34].
2.2.2.3. Dead Wood Biomass and Carbon Stock Estimation
The biomass and carbon stock of dead wood were calculated based on the type of dead wood present in the study area, which included stump dead wood, fallen dead wood, and standing dead wood. However, standing dead wood was not found in the 90 plots surveyed.
The DBH and height of dead trees were measured. For fallen dead wood, the diameter was measured at both the base (maximum diameter) and the top (minimum diameter), and the length was recorded [27]. Dead wood was categorized into downed dead wood and stump dead wood. The decomposition level of downed dead wood was assessed using the “machete test,” which classifies wood into three categories based on density: Category 1—when struck by a machete, the wood emits a sound; Category 2—when struck, the machete slightly penetrates the wood, indicating an intermediate state; and Category 3—when struck, the wood disintegrates into pieces, indicating advanced decomposition. The diameter and height of standing stump dead wood were measured using a measuring tape.
The total biomass of dead wood (TBDW) in a given plot was determined by summing up the BSSDW and fallen or down dead wood of biomass (BLDDW).
Carbon content of dead wood in a given plot was calculated by multiplying TBDW with default carbon fraction of 0.47 as outlined by IPCC [35].
2.2.2.4. Biomass and Carbon Stock of Leaf, Litter, Herbs, and Grass (LHG)
2.3. Soil Organic Carbon (SOC) Estimation
SOC was determined from samples collected at specified depths (0–10, 10–20, and 20–30 cm) as prescribed by Subedi et al., [36]. Soil samples were taken from the four corners and the center of the 1 m2 sample frame. Equal weights of each layer’s soil samples from five nested plots were homogenized and composited for SOC analysis. Soil samples bulk density were collected using a core sampler.
2.3.1. Total Carbon Stock of the Study Site
2.4. Statistical Analysis
Descriptive statistics, including sample mean, variance, minimum and maximum values, frequency tables, and graphs, were used to describe the carbon stocks, regeneration potential of natural, and plantation forests. Comparisons of mean variations in carbon stock and regeneration status were determined using a two-sample t-test at a significance level of p ≤ 0.05. The significant effects of altitude on carbon stock and regeneration potential in both natural and plantation forests were tested using a linear regression model. Data analysis was performed using Microsoft Excel and SPSS.
3. Results
3.1. Vegetation Characteristics
The total numbers of woody plant individuals were 813 in the natural forest and 1030 in plantation forest. The density of woody species was greater in the plantation forest (576.67 trees ha−1) than in the natural forest (448.33 trees ha−1). Across sampling sites, tree species number and density varied with forest types. The species frequency distribution showed that Syzygium guineense 211 (25.95%), followed by Myrica salicifolia 128 (15.74%), in the natural forest, and Eucalyptus globulus 661 (64.17%) followed by Cupressus lusitanica 186 (18.06%) in plantation forest were the most frequent tree species. The species with the least occurrence in the study site was Ficus vasta 1 (0.1%) in natural forest and Juniperus procera has a frequency of 22 (2.14%) in plantation forest, respectively.
3.2. DBH and Height Distribution of Woody Species
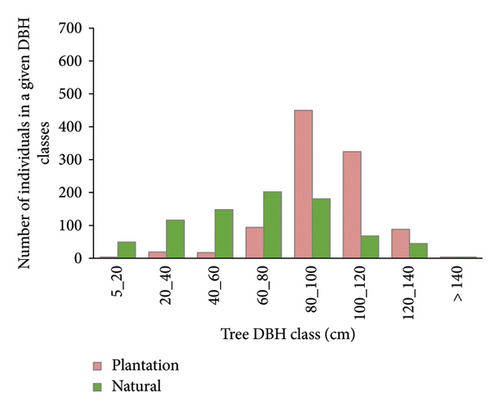
DBH class 60–80 cm had the highest density of trees with 202 trees ha−1 (24.85%) whereas DBH > 140 cm had the lowest density with 4 trees ha−1 (0.49%) in natural forest. On the other hand, DBH class 80–100 had the highest density of trees with 480 trees ha−1 (46.60%) while DBH 5–20 and > 140 had the lowest density with 4 trees each ha−1 (0.39%) in plantation forest (Figure 3). The overall pattern of diameter class exhibited irregular shaped distribution. However, this pattern might not describe an equal pattern in population dynamics and recruitment processes of a given individual species. Still, this indicates the highest frequency in middle diameter classes and a gradual decrease toward both sides.
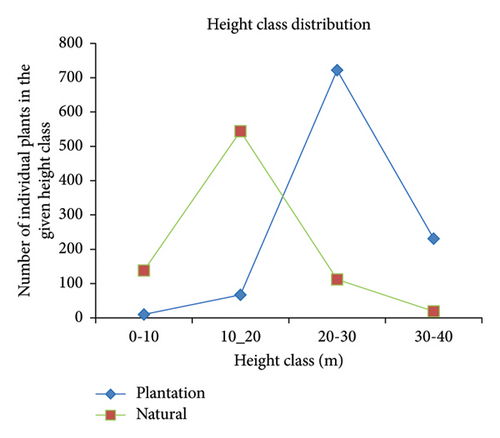
In the natural forest, the highest number of trees was recorded in height Class II (66.91%), followed by Class I (16.97%), Class III (13.78%), and Class IV (2.34%). Conversely, in the plantation forest, the highest number of trees was recorded in height Class III (70.10%), followed by Class IV (22.43%), Class II (6.50%), and Class I (0.97%) (Figure 4). The classification of height classes was based on the height data collected in the sampling area. This indicates that the plantation forest had a higher number of taller trees compared to the natural forest (Figure 4).
3.3. Regeneration Status of the Two Forest Types
In the natural forest, 127 seedlings (12.13%), 107 saplings (10.22%), and 813 mature woody species (77.65%) were recorded. In the plantation forest, there were 169 seedlings (13.54%), 62 saplings (4.91%), and 1030 mature woody species (81.55%) were counted (Table 1). Mature woody species were the most abundant in both forest types, while saplings were the least common. The seedling-to-mature and sapling-to-mature ratios were 0.16 and 0.13 in the natural forest, and 0.16 and 0.06 in the plantation forest, respectively. These findings suggest that natural forests have a higher potential for regeneration compared to plantation forests. Saplings in plantation forests were only found in plots with felled trees and open canopies, indicating limited regeneration in other areas.
| Forest type | Scientific name | Family | Seedling | Sapling | Mature |
|---|---|---|---|---|---|
| Natural forest | Allophylus abyssinicus (Radlk.) Gilg | Sapindaceae | 0 | 0 | 21 |
| Annona senegalensis pers. | Annonaceae | 0 | 0 | 10 | |
| Apodytes dimidiata E.Mey. ex Arn. | Metteniusaceae | 0 | 0 | 4 | |
| Arundinaria alpina K.Schum | Poaceae | 0 | 0 | 6 | |
| Bersama abyssinica Fresen. | Melianthaceae | 4 | 5 | 19 | |
| Couroupita guianensis Aubl. | Lecythidaceae | 0 | 0 | 6 | |
| Croton macrostachyus Hochst. ex Delile | Euphorbiaceae | 5 | 4 | 29 | |
| Diospyros abyssinica (Hiern) F.White | Ebenaceae | 0 | 0 | 32 | |
| Dombeya torrida (J.F.Gmel.) | Malvaceae | 7 | 2 | 39 | |
| Dovyalis abyssinica (A.Rich.) Warb. | Salicaceae | 0 | 0 | 9 | |
| Ekebergia capensis Sparrm. | Meliaceae | 0 | 0 | 11 | |
| Enclea natalensis (Oliv.) | Myrtaceae | 0 | 0 | 11 | |
| Euphorbia ampliphylla Pax | Euphorbiaceae | 0 | 0 | 6 | |
| Ficus carica L. | Moraceae | 0 | 0 | 7 | |
| Galpinia saxifraga N.E.Br. | Lythraceae | 0 | 0 | 4 | |
| Grewia villosa Willd. | Malvaceae | 0 | 0 | 9 | |
| Hagenia abyssinica (Bruce) J.F.Gmel. | Rosaceae | 19 | 16 | 60 | |
| Hypericum revolutum Vahl | Hypericaceae | 0 | 0 | 2 | |
| Maesa lanceolata Forssk. | Primulaceae | 0 | 0 | 8 | |
| Millettia ferrea (Vahl) Wight and Arn. | Fabaceae | 0 | 0 | 7 | |
| Myrica salicifolia Hochst. ex A.Rich | Myricaceae | 14 | 11 | 64 | |
| Myrsine africana L | Primulaceae. | 1 | 0 | 0 | |
| Myrsine melanophloeos (L.) R.Br. | Primulaceae | 0 | 0 | 8 | |
| Nuxia congesta R.Br. ex Fresen. | Stilbaceae | 0 | 0 | 6 | |
| Olea europaea L. | Oleaceae | 7 | 7 | 21 | |
| Olinia rochetiana A.Juss. | Oliniaceae | 0 | 0 | 4 | |
| Phoenix reclinata Jacq. | Arecaceae | 0 | 0 | 26 | |
| Phytolacca dodecandra L’Hér | Phytolaccaceae | 0 | 0 | 5 | |
| Polyscias fulva (Hiern) Harms | Araliaceae | 0 | 0 | 10 | |
| Prunus Africana (Hook.f.) Kalkman | Rosaceae | 30 | 26 | 127 | |
| Rubus apetalus Poir. | Rosaceae | 0 | 0 | 2 | |
| Schefflera abyssinica (Hochst. ex A.Rich.) | Araliaceae | 0 | 0 | 8 | |
| Syzygium guineense (Willd.) DC. | Myrtaceae | 40 | 36 | 208 | |
| Tectona grandis L.f. | Lamiaceae | 0 | 0 | 2 | |
| Ziziphus mucronata Willd. | Rhamnaceae | 0 | 0 | 22 | |
| Total | 127 | 107 | 813 | ||
| Plantation forest | Cupressus lusitanica Mill. | Cupressaceae | 29 | 10 | 220 |
| Eucalyptus camaldulensis Dehnh | Myrtaceae | 26 | 11 | 112 | |
| Eucalyptus globulus Labill. | Myrtaceae | 69 | 25 | 581 | |
| Eucalyptus citriodora Hook. | Myrtaceae | 40 | 14 | 95 | |
| Juniperus procera Hochst. ex Endl. | Cupressaceae | 5 | 2 | 22 | |
| Total | 169 | 62 | 1030 | ||
3.4. AGB and BGB and Carbon Stock
The results showed that the mean AGB of woody species was 394.2 ± 131.6 tons·ha−1 for natural forest and 379.2 ± 69.3 tons·ha−1 for plantation forest (Table 2). The maximum and minimum AGB was 1177.7 and 54 tons·ha−1 for natural forest, and 770.2 and 173.4 tons·ha−1 for plantation forest, respectively. Natural forest had higher AGB than plantation forest.
| Forest type | Altitude class | Altitudinal range | No of plots | Different carbon pools in tons ha−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AGB | AGC | BGB | BGC | LHGC | SOC | Dead wood | TOC | TCO2eq | |||||
| DDWC | SDWC | ||||||||||||
| Natural | Lower | 2172–2296 | 8 | 359.7 | 169.1 | 71.9 | 33.8 | 3.3 | 39.8 | 6.7 | 0.77 | 253.4 | 929.9 |
| Middle | 2297–2420 | 15 | 539.6 | 253.6 | 107.9 | 50.7 | 3.4 | 40.4 | 6.7 | 0.72 | 355.6 | 1305.1 | |
| Upper | 2421–2546 | 22 | 283.2 | 133.2 | 56.7 | 26.6 | 3.1 | 37.0 | 5.1 | 0.71 | 205.7 | 755.0 | |
| Plots | 394.2 ± 131.6 | 185.3 ± 61.8 | 78.8 ± 26.3 | 37.1 ± 12.4 | 3.3 ± 0.1 | 39.0 ± 1.8 | 6.15 ± 0.9 | 0.73 ± 0.03 | 271.6 ± 76.4 | 996.6 ± 281.3 | |||
| Plantation | Lower | 2267–2346 | 20 | 370.4 | 174.1 | 74.1 | 34.8 | 4.2 | 37.4 | — | 0.33 | 251.2 | 921.7 |
| Middle | 2347–2425 | 15 | 452.6 | 212.7 | 90.5 | 42.5 | 4.6 | 37.2 | — | 0.31 | 297.3 | 1091.0 | |
| Upper | 2426–2504 | 10 | 314.9 | 148.0 | 63.0 | 29.6 | 4.4 | 37.0 | 9.6 | 0.30 | 229.3 | 841.4 | |
| Mean + Std | 379.3 ± 69.3 | 178.3 ± 32.6 | 75.9 ± 13.9 | 35.7 ± 6.5 | 4.4 ± 0.2 | 37.2 ± 0.2 | 3.7 ± 2.8 | 0.31 ± 0.01 | 259.2 ± 34.7 | 951.4 ± 127.4 | |||
| p-value | 0.7 | 0.7 | 0.7 | 0.7 | 0.01∗∗ | 0.2 | 0.00∗ | 0.7 | 0.7 | ||||
The mean BGB was 78.8 ± 26.3 tons·ha−1 for natural forest and 75.9 ± 13.9 tons·ha−1 for plantation forest (Table 2). The maximum and minimum BGB was 235.53 and 10.79 tons·ha−1 for natural forest, and 154.0 and 34.7 tons·ha−1 for plantation forest, respectively. BGB was also higher in natural forest than in plantation forest.
The mean AGC density and CO2 equivalent were 185.3 ± 61.8 and 677 ± 227 tons·ha−1, respectively, for natural forest, and 178.3 ± 32.6 and 654.4 ± 119.6 tons·ha−1, respectively, for plantation forest (Table 2). The maximum and minimum carbon densities per plot were 553.50 and 25.36 tons·ha−1, with corresponding CO2 equivalents of 2031.4 and 93.1 tons·ha−1, respectively, for natural forest. For plantation forest, the maximum and minimum carbon estimates were 362.0 and 81.5 tons·ha−1, with corresponding CO2 equivalents of 1328.5 and 299 tons·ha−1, respectively. AGC was higher in natural forest (185.3 tons·ha−1) than in plantation forest (178.3 tons·ha−1), likely due to the DBH of the tree species.
The mean belowground carbon (BGC) density and CO2 equivalent were 37.1 ± 12.4 and 136.2 ± 45.5 tons·ha−1, respectively, for natural forest, and 35.7 ± 6.5 and 130.7 ± 23.9 tons·ha−1, respectively, for plantation forest (Table 2). The maximum and minimum carbon densities per plot were 110.7 and 5.1 tons·ha−1, with corresponding CO2 equivalents of 406.3 and 18.6 tons·ha−1 for natural forest, and 72.4 and 16.3 tons·ha−1, with corresponding CO2 equivalents of 265.7 and 59.8 tons·ha−1 for plantation forest, respectively. BGC was higher in natural forest than in plantation forest (Table 2).
3.5. Biomass and Carbon Stock Estimation in Dead Wood
The mean biomass and carbon density stored in logged trees (stumps) and their corresponding CO2 equivalents were 1.6 ± 0.99, 0.7 ± 0.03, and 2.7 ± 2.05 tons·ha−1 for natural forest, and 0.7 ± 0.63, 0.3 ± 0.01, and 1.1 ± 1.07 tons·ha−1 for plantation forest, respectively. The CO2 equivalent of stump dead wood in natural forest was significantly higher than in plantation forest (p ≤ 0.05) (Table 2).
The mean biomass, carbon density, and CO2 equivalent stored in downed dead wood were 13.4 ± 11.2, 6.2 ± 0.9, and 22.6 ± 13.1 tons·ha−1 for natural forest, and 8.7 ± 4.2, 3.7 ± 2.8, and 13.6 ± 9.7 tons·ha−1 for plantation forest, respectively. Fallen trees were found in 12 out of 45 plots in natural forest and 5 out of 45 plots in plantation forest (Table 2). The carbon density of downed dead wood in natural forest was significantly higher than in plantation forest (p ≤ 0.05).
3.6. Estimation of Carbon Stock in LHG
The mean value of biomass, carbon density stored in LHGs biomass and CO2 equivalent were 7.0 ± 2.1, 3.3 ± 0.1 and 12.1 ± 3.7 tons·ha−1 for natural forest but 9.4 ± 2.9, 4.4 ± 0.2 and 16.2 ± 5.1 tons·ha−1 for plantation forest, respectively. The maximum and minimum carbon densities per plot were 5.44- and 1.65-tons ha−1 for natural forest and 6.75 and 1.31 tons·ha−1 carbon density for plantation forest, respectively. However, LHG biomass was found in all plots for natural and plantation forests except plot 10 of natural forest. The corresponding mean CO2equivalent of LHG biomass was 12.1 and 16.2 tons·ha−1 for the natural forest and plantation forest respectively (Table 2). On the other hand, the mean value of carbon density of LHG for natural forest 3.3-ton ha−1 was significantly lower than plantation forest 4.4 tons·ha−1 (p ≤ 0.05).
3.7. SOC
The average bulk density of soil was 1.23 g/cm3 for natural forest and 1.17 g/cm3 for plantation forest. Mean SOC stock and CO2 equivalent were 39.4 ± 5.7 and 144.4 ± 20.7 tons ha−1 for natural forest, and 37.2 ± 3.9 and 136.3 ± 14.3 tons·ha−1 for plantation forest. The mean maximum and minimum SOC densities were 54.7 and 30.1 tons·ha−1 for natural forest, and 43.4 and 29.0 tons·ha−1 for plantation forest. SOC was higher in natural forest than in plantation forest (Figure 5). Bulk density increased with soil depth while SOC decreased with increasing depth (Figure 5).
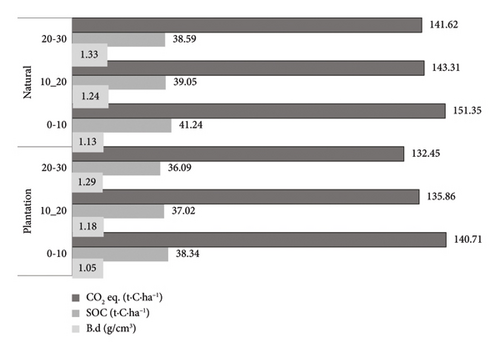
3.8. Carbon Stock in All Carbon Pools
In the natural forest, biomass carbon storage is distributed as follows: aboveground biomass constitutes 68.04%, BGB accounts for 13.61%, leaf litter and herb biomass make up 1.21%, deadwood biomass represents 2.26%, stump deadwood comprises 0.34%, and soil contains 14.55% of the carbon stock. In contrast, the plantation forest shows a slightly different distribution: AGB includes 68.63%, BGB encompasses 13.73%, leaf litter and herb biomass consist of 1.70%, deadwood biomass holds 1.42%, stump deadwood makes up 0.22%, and soil comprises 14.34% of the carbon. The most substantial carbon stocks were observed in the AGB, followed by the soil carbon pool, BGB, deadwood biomass, leaf litter biomass, and lastly, stump deadwood in both forest types.
3.9. The Mean Carbon Density Comparisons Between Natural and Plantation Forests in All Carbon Pools
A comparative analysis of the Gardula forest’s carbon pools showed that the mean carbon density for both AGC and BGC was higher in natural forests than in plantation forests (Table 2). However, the observed differences were not statistically significant (p ≥ 0.05), which may be due to the species heterogeneity in natural forests and the variation in tree height and DBH within plantation forests. The SOC of the natural forest was greater than that of the plantation forest, and this difference was significant, possibly owing to the different decomposition rates between the two forest types. Similarly, the carbon density in both downed deadwood and stump deadwood of the natural forest was greater than that of the plantation forest, with the difference being significant in stump deadwood and insignificant in downed deadwood (Table 2). Conversely, the carbon density of litter, herbs, and grass in the plantation forest was greater than in the natural forest, and this difference was significant, which might be attributed to the different vegetation types of the forests.
3.10. Carbon Stocks of Different Pools Along Altitudinal Variation
In this study, it was observed that the total carbon stock amount at each forest type showed variation with altitudinal gradient. As shown in Table 2 the total carbon was higher at middle altitude in both natural and plantation forests. The higher altitudinal range takes lowest portion of carbon stock. The middle altitude forests hold more carbon than lower altitude forests, which in turn hold more than higher altitude forests, and this pattern is consistent across both natural and plantation forest types.
3.11. The Correlation Between Carbon Stock With Altitude
The statistical analysis showed that the relation between carbon stock pools with altitude. AGC and BGC carbon showed weak inverse relation with elevation (R = −0.203; p = 0.035) (p ≤ 0.05). Down deadwood carbon showed positive weak correlation whereas total carbon also showed negative weak correlation with altitude (R = 0.210; p = 0.048 and R = −144; p = 0.041) (p ≤ 0.05), respectively. On the other hand, litter, grass and herb carbon, SOC and stump deadwood carbon showed no correlation with altitude (Table 3).
| Parameter | Carbon pools | R value | p value |
|---|---|---|---|
| Plot elevation | AGC | −0.203∗ | 0.035 |
| BGC | −0.203∗ | 0.035 | |
| SOC | −0.052 | 0.626 | |
| CLHG | −0.081 | 0.446 | |
| CDDW | 0.210∗ | 0.048 | |
| CSDW | −0.066 | 0.535 | |
| TC | −0.144∗ | 0.041 |
- ∗Correlation is significant at p ≤ 0.05 level (2-tailed).
4. Discussions
4.1. Vegetation Characteristics
The study area is classified as dry evergreen Afromontane forest, characterized by vegetation type, elevation (2172–2546 m above sea level), and mean annual precipitation (600–1600 mm per year) [41]. Dominant woody species in natural forests include Syzygium guineense, Myrica salicifolia, and Allophylus abyssinicus, while Eucalyptus globulus, Cupressus lusitanica, and Eucalyptus citriodora dominate plantation forests. These findings align with studies in Humbo Forest [42] and Shawo Forest [43], where Syzygium guineense was also dominant, likely due to its adaptation to local environmental conditions. The average tree density was 448.33 trees ha−1 in natural forests and 576.67 trees ha−1 in plantation forests, higher than in Sekele-Mariam forest (264 trees ha−1) [44] and Achera natural forest (392.5 trees ha−1) [45], but lower than in Setema District (587 trees ha−1) [46].
Wood species from natural and plantation Gardula forests were classified into DBH and height classes to represent size and age distribution. Of the 813 and 1030 individual trees recorded in natural and plantation forests, respectively, less than 3% were in the < 40 cm DBH class, and more than 97% were in the > 40 cm DBH class (Figure 3), indicating dominance by older trees. This is in line with the finding of Guluma [47] and Tesfaye et al. [48]. In terms of height, 66.91% of woody species in natural forests were in the 10–20 m class, and 70.10% in plantation forests were in the 20–30 m class. Tree density decreased in the lower and higher height classes but increased in the medium height classes in both forest types (Figure 4). These results are consistent with findings from Zonba natural forest [49] and Furi forest [47].
4.2. Regeneration Status of the Two Forest Types
The regeneration status of Gardula forest is generally poor, but both natural and plantation forests require increased attention due to illegal activities affecting their regeneration. Both forest types are in a poor regeneration rate, with significantly lower number of seedlings than saplings than mature trees, consistent with findings from Bradi forest [50]. Low numbers of seedlings and saplings may be due to seed predation, grazing, browsing, and lack of safe sites for seed recruitment, dormancy requirements, litter accumulation, pathogens, species specificity, and moisture stress. Some species may have alternative propagation and reproduction adaptations besides seed germination [51].
4.3. AGB and BGB and Carbon Stock
The AGC amounts in this study fall within the global range for tropical rainforests (95–527.85 tons ha−1) [52]. AGC was higher in natural forests (185.28 tons ha−1) than in plantation forests (178.24 tons ha−1). Studies by Gobena [46]; Besar et al., [1]; and Kendie et al., [53] reported higher carbon stocks in natural forests, attributing this to the greater diversity and age of trees (Table 4). Natural forests maintain more woody species and carbon sinks [57]. The diversity and structure of trees in natural forests significantly impact biomass and carbon storage, unlike the younger, uniform planted forests. Additionally, forest stand structure, composition, topography, elevation, and microclimate variations contribute to changes in AGB carbon [51].
BGC stocks showed similar trends to AGCSs. The average density of BGC stocks was higher in natural forests than in plantation forests AGB and BGB contributed 68.04% and 13.61% in natural forests, and 68.63% and 13.73% in plantation forests, respectively, of the total biomass. This finding aligns with Gobena [46]; indicating higher BGC sequestration in natural forests. The BGB carbon stock in this study was within the global average for tropical forests (10–45 Mg ha−1) [58]. Roots are crucial for soil organic matter (SOM), influencing soil microbial activity and decomposition processes [59]. Fine root production and turnover play a vital role in forest carbon dynamics, contributing significantly to soil carbon [60].
The carbon in stump dead wood biomass related to the number of stumps in the plots, with higher stump density leading to more carbon. The result of this study is lower than in Gerba-Dima moist Afromontane forest [61].
Dead wood biomass carbon in Gardula forest was lower than in Gesha humid Afromontane forest [6] but higher than in Dorze Ayira Natural Forest [62]. This may be due to fewer plots containing dead wood and firewood collection reducing dead wood in the study area.
The LHG biomass in Gardula forest falls within the global range of 2–16 tons ha−1, with plantation forests showing higher carbon density than natural forests, likely due to slower leaf decomposition. These results align with Edegu Forest [63] and are higher than Sekele-Mariam forest [44], Dorze Ayira Natural Forest [62], Shawo Forest [43], and the community Forest of Danaba [64]. However, they are lower than Mount Zequalla Monastery [65] and Humbo Forest [42]. Variations can be attributed to decomposition rates influenced by climatic factors, forest vegetation, and climate [65].
The SOC values in Gardula forest align with the IPCC [35] guidelines of 20–300 tons ha−1 but are lower than those in Shawo Forest [43], Danaba District [64], and Mount Zequalla Monastery [65] (Table 4). This discrepancy may be due to low SOM content, bulk density, and environmental factors like slope and temperature affecting decomposition rates. The rugged terrain may also cause early litter flow, reducing organic matter decomposition. SOC was highest in the upper soil layer due to rapid litter accumulation and decomposition, with carbon content decreasing with depth, consistent with other studies [64].
4.4. Variation of Carbon Stock Along Altitudinal Gradient
Altitude significantly influences biomass and carbon stocks in forest ecosystems [66]. This study found that carbon pools varied with altitude, showing an inverse correlation with AGC and BGC, but no correlation with litter and humus carbon (LHG) and SOC. Scattered vegetation at higher altitudes likely explains this pattern, consistent with findings by Bogale et al. [67] and Marín-Spiotta1 and Sharma, [68]; who observed a negative correlation between tree biomass and altitude. Similarly [69], reported a decrease in biomass carbon storage with increasing altitude, while Feyissa et al., [63] noted an increase in live biomass carbon with altitude.
Maximum carbon stocks in tree biomass were stored at intermediate altitudes. In natural forests, this included AGC, BGC, LHG, and SOC, while in plantation forests, it included AGC, BGC, and LHG. Lower and higher altitudes exhibited lower carbon storage levels. Specifically, dead wood at lower elevations contained more carbon in downed dead wood and stumps. Natural forests had higher carbon content at upper and middle elevations, whereas plantation forests had more carbon at higher elevations. Middle elevations in plantation forests had a greater abundance of stump dead wood.
Yohannes [70] suggested that higher stem density in productive forests at intermediate altitudes, compared to higher and lower elevations, could explain this distribution. Reduced human settlements and disturbances at these altitudes may also contribute. Higher elevations, characterized by gentle slopes, are more suitable for farmland, resulting in lower carbon storage. Consequently, vegetation becomes more scattered, and large-diameter trees at breast height (DBH) and height (H) are less common at upper elevations compared to middle and lower elevations.
Topographic differences cause litter and soil to roll down from the highest to the lowest elevation. However, favorable environmental conditions and the prevalence of most plant species in the middle section result in high biomass and carbon reserve values. These findings on carbon storage density at average elevations are consistent with other research in Ethiopia [64, 70, 71].
Future research should investigate the impact of grazing on phenology, functional strategies, and plant-animal interactions within forests. Seedling and young tree populations are often found in areas with cut trees and exposed stumps, suggesting that the tree canopy influences the growth of these younger plants. High canopy gap fractions (low canopy cover) benefit the regeneration of light-demanding species [72].
5. Conclusions
This study in Gardula Forest, Southwest Ethiopia, compared carbon stock and regeneration in natural and plantation forests. Natural forests showed higher average total biomass carbon density (271.57 tons ha−1) than plantation forests (259.23 tons ha−1), though not statistically significant. Natural forests had significantly greater carbon density in soil organic carbon, stump dead wood, and downed dead wood, likely due to greater tree diversity and age. Plantation forests, however, exhibited higher carbon density in litter, herbs, and grass, possibly due to vegetation differences and decomposition rates. Both forest types displayed “fair” regeneration, with seedlings and saplings outnumbered by mature trees, suggesting potential degradation from illegal activities. These results highlight natural forests’ superior carbon sequestration, particularly in soil pools, and plantation forests’ potential for enhanced carbon storage through litter management, underscoring the need for targeted conservation strategies.
5.1. Recommendations
To improve carbon sequestration and the overall health of the Gardula Forest ecosystem, it is essential to prioritize the conservation of natural forests, given their higher carbon stocks compared to plantation forests. Measures should be implemented to facilitate healthy regeneration while addressing and mitigating human-induced challenges that hinder this process. Research is necessary to identify factors that limit regeneration in both natural and plantation forests, with particular attention to regeneration patterns near stumps in plantations. Additionally, enhancing carbon sequestration in plantation forests can benefit from exploring strategies such as increasing species diversity and refining management practices. The impact of grazing on forest dynamics should also be studied to develop approaches that minimize its adverse effects on regeneration. Understanding the relationship between canopy cover, subsidiary species regeneration, and the effects of microclimate and topography on carbon accumulation across altitudes is crucial for effective management. These recommendations, grounded in the study’s findings, provide a comprehensive framework for optimizing carbon stocks, addressing climate change, and ensuring the sustainability of the Gardula Forest ecosystem.
Consent
All authors have given their consent and agreed to publish the research in the Journal of New Forests.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Shetie Gatew interpreted the results, analyzed the data, provided comments, and edited the manuscript. Mekonnen Zerihun: conceptualized, designed, conducted the fieldwork, analyzed the data, and drafted the manuscript. All authors read, revised, and approved the final manuscript.
Funding
The research received financial support from Arba Minch University, College of Natural and Computational Sciences (Reference No. GOV/AMU/Biol/2021).
Acknowledgments
We express our heartfelt gratitude and appreciation to Arba Minch University, Department of Biology, for their financial support. Additionally, we extend our thanks to the Department of Environment, Forestry, and Climate Change Regulation of the Derashe Woreda for their contributions in various ways. Special recognition goes to the Gamo Zone of Women and Children’s Affairs for supporting this research.
Open Research
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.




