Iron Exposure Effects on Seedling Growth of Enterolobium cyclocarpum (Jacq.) Griseb. and Albizia chinenesis (Osbeck) Merr on Water Culture Media
Abstract
Iron (Fe) is a trace element and metal crucial to plant growth and development. However, it can become toxic at high concentrations, potentially harming plants. Information related to the tolerance or sensitivity of forest plants to Fe exposure can be the basis for selecting species to be planted on land with high Fe content. This study aims to determine the growth response and tolerance of Parota (Enterolobium cyclocarpum) and Red sengon (Albizia chinensis) seedlings to Fe exposure. A completely randomized design (CRD) was used in this study, with Fe treatments consisting of nine concentration levels (0, 0.25, 0.5, 1, 1.25, 1.5, 1.75, and 2 mM). Observed parameters included growth metrics (height, root length, dry weight of roots, shoots, and total plants), chlorophyll content, photosynthesis rate, malondialdehyde (MDA) content, and tolerance index. The results showed that Fe exposure significantly affected the growth of E. cyclocarpum and A. chinensis seedlings. For E. cyclocarpum, a Fe concentration of 0.25 mM resulted in the best growth in all growth parameters, whereas, for A. chinensis, Fe exposure reduced all growth parameters. Fe exposure did not significantly impact either species chlorophyll content or photosynthesis rate. The MDA content in E. cyclocarpum seedlings peaked at 1.75 mM Fe, with no significant difference between 1 to 2 mM concentrations. In contrast, for A. chinensis, MDA content increased significantly at 1.25 mM Fe. The tolerance index of E. cyclocarpum seedlings increased significantly at 0.25 mM Fe concentration, but decreased with increasing Fe concentration, while A. chinensis showed a decrease in tolerance index with increasing Fe concentration. This finding suggests that E. cyclocarpum is able to provide greater tolerance to Fe exposure compared to A. chinensis seedlings. E. cyclocarpum can also be recommended as one of the species for rehabilitation and reclamation of marginal lands with high Fe content.
1. Introduction
Soil types in Indonesia, such as Oxisols and Ultisols, are classified as acidic due to very intensive soil weathering [1, 2]. High Fe content is also typically found in acid sulfate soils, acid clay soils, and peat soils [3, 4]. Additionally, acid soils can result from human activities, including mining [5, 6]. Acidic soils cause elements such as Al, Fe, Zn, Mn, Cd, and Cu to dissolve, negatively affecting plant growth, especially when these elements are in high concentrations [3, 7]. Al and Fe can form strong bonds with phosphate in acidic soils, resulting in decreased phosphate availability and absorption by plants [8, 9]. This condition can inhibit nutrient absorption, causing plant nutrient deficiencies [10]. Therefore, Fe availability can limit plant growth and productivity [11, 12].
Iron (Fe) is an essential heavy metal in trace elements and is widely distributed in various soil types [13, 14]. Fe is one of the first microelements identified as playing a crucial role in plant growth and development due to its involvement in various vital plant activities [15, 16]. Fe is important in plant metabolic and physiological processes, such as photosynthesis, respiration, DNA synthesis, acting as a cofactor for various enzymes, and in the biosynthesis of primary and secondary metabolites, as well as nitrogen fixation [12, 17, 18]. In addition to being an essential microelement required by plants, Fe can also be toxic at high concentrations, inhibiting plant growth and potentially causing plant death [19, 20]. The high availability of Fe in the soil is a significant issue for the productivity of some crops [21, 22]. Furthermore, high Fe availability poses challenges in revegetating marginal lands, such as post-mining coal lands [23, 24]. This issue is a critical factor in determining the species of plant species used in reclamation activities, where selected species must have a high level of adaptation to the surrounding environment, especially to stresses present in marginal lands, including high Fe concentrations [25, 26].
Parota (Enterolobium cyclocarpum) and Red sengon (Albizia chinensis), both belonging to the Fabaceae family, are recognized as pioneer species, fast growing, and widely used for construction materials, firewood, animal feed, and medicinal uses [27, 28]. Fabaceae species, including E. cyclocarpum and A. chinensis are able to symbiotize with rhizobium bacteria, thus being able to fix nitrogen from the atmosphere [29, 30]. This makes fabaceae species including these two species often chosen to be planted in reclamation or rehabilitation of marginal lands [31, 32]. However, the reclamation or rehabilitation of marginal lands often fails because the selected species are not tolerant to environmental stresses, one of which is Fe stress. This research will provide information related to the tolerance or sensitivity of forest plants to Fe exposure, especially in Fabaceae species. The results of this study can also be the basis for the selection of the use of E. cyclocarpum and A. chinensis species when they will be planted on land with high Fe content. This study aims to determine the growth response and tolerance of Parota (Enterolobium cyclocarpum) and Red sengon (Albizia chinensis) seedlings to Fe exposure.
2. Materials and Methods
2.1. Seed Preparation and Culture Media
The seeds of E. cyclocarpum and A. chinensis were germinated by following the method of Algofar and Purnamaningsih [33] and Salim et al. [34], where for 15 min the seeds are soaked in hot water (80°C), then the seeds are picked up and soaked again with water (25–30°C) for 24 h. After the dormancy-breaking process, the seeds are sown in a media tub that already contains zeolite media. Seeds are maintained until they grow (± 2 weeks) until two to three leaves appear. Seed maintenance is carried out by watering twice a day by looking at the humidity of the growing media. The sprout container is placed in a place that is not exposed to direct sunlight until the sprouts appear. Meanwhile, the culture medium used is a solution of distilled water that has been dissolved with several nutrients. The nutrients used consist of macro and micronutrients with the composition according to Sopandi [35] and Salim et al. [34]: 1.5 mM Ca(NO3)2·4H2O, 1.0 mM NH4NO3, 1.0 mM KCl, 0.4 mM MgSO4·7H20, 1.0 mM KH2PO4, 0.50 ppm MnSO4·H2O, 0.02 ppm CuSO4·5H2O, 0.05 ppm ZnSO4·7H2O, 0.50 ppm H3BO3, 0.01 ppm (NH4)6 Mo7O24·4H2O. For Fe exposure, use ferrous sulfate heptahydrate (FeSO4·7H2O).
2.2. Seedling Transplantation and Adaptation
E. cyclocarpum and A. chinensis seedlings that were ready to wean (already had 2–3 leaves) were transferred to containers containing media. This weaning and seedling adaptation method refers to the research of Salim et al. [34], where each seedling is weaned into a container that has been equipped with a serophome and has been given a hole. The seedlings were placed in the serophome holes wrapped in cotton so that the seedlings could stand upright. This seedling acclimation was carried out for 14 days. After the acclimation, the seedlings were moved back into a container that already contained new media and several Fe concentration treatments, ranging from 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.74, and 2 mM. During the acclimation and Fe exposure experiment, media conditions were maintained at pH 4. pH adjustment was performed weekly by adding 1 N HCl to lower the pH and 1 N KOH to raise the pH. Media addition was done when the volume of media in the container began to decrease. The growth medium was replaced after 14 days to maintain optimal seedling growth. Seedlings were maintained for up to one month in the greenhouse with the daily temperature of 29°C–35°C and relative humidity of 60%–90%, light intensity of 6.200–49.600 lux, and photoperiod of 40%–41%.
2.3. The Observed Parameters
2.4. Chlorophyll and Carotenoid Analysis
2.5. MDA Lipid Peroxide Analysis
2.6. Data Analysis
This study used a one-factor completely randomized design (CRD), namely Fe concentration. The Fe concentration used consisted of 9 levels, namely 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, and 2 mM. Each treatment was repeated 3 times and each replicate consisted of 3 seedling units. The data analysis used was ANOVA test then continued with Duncan multiple’s range test (DMRT) at 95% confidence level (α = 5).
3. Result
3.1. Growth of E. cyclocarpum and A. chinensis on Fe Exposure
Growth is one of the parameters that is easily measured with the aim of seeing the response to the effect of the treatment given (Fe exposure) on E. cyclocarpum and A. chinensis seedlings. Growth parameters measured in this study include height, root length, root dry weight, shoot dry weight, and total plant dry weight. The results of the analysis indicate that Fe exposure significantly affects all growth parameters of E. cyclocarpum and A. chinensis seedlings (Table 1). For E. cyclocarpum seedlings, 0.25 mM Fe exposure resulted in the best growth, as evidenced by increases in all observed growth parameters. This shows that E. cyclocarpum species can still tolerance Fe exposure up to a concentration of 0.25 mM and even show a positive response to the growth of E. cyclocarpum seedlings. However, higher concentrations of Fe (0.75–2 mM) reduced the growth of E. cyclocarpum across all parameters. This shows that the higher Fe concentration has a negative impact on the growth of E. cyclocarpum seedlings. In contrast, A. chinensis seedlings exhibited a decrease in all growth parameters with increasing Fe concentrations. This shows that A. chinensis species tend to be intolerant of Fe exposure, so that the presence of Fe exposure has a negative impact on the growth of A. chinensis seedlings. Notably, the height, root dry weight, shoot dry weight, and total biomass of A. chinensis seedlings declined dramatically when exposed to 0.5 mM Fe. The different growth responses between E. cyclocarpum and Albizia chinensis species indicate different tolerance and sensitivity to Fe exposure, and it is very clear that high Fe concentrations (1.5–2 mM) reduce the growth of both E. cyclocarpum and A. chinensis species (Figure 1).
| Seedling | Fe concentration (mM) | Parameters | ||||
|---|---|---|---|---|---|---|
| Height (cm) | Root length (cm) | Root dry weight (g) | Shoot dry weight (g) | Total dry weight (g) | ||
| E. cyclocarpum | 0 | 12.17 ± 3.05ab | 32.58 ± 9.75b | 0.76 ± 0.28b | 2.73 ± 0.56bc | 3.49 ± 0.08bc |
| 0.25 | 14.00 ± 20.09a | 41.62 ± 11.93a | 1.45 ± 0.25a | 3.98 ± 0.36a | 5.43 ± 0.61a | |
| 0.5 | 10.86 ± 3.07bc | 28.86 ± 7.18b | 0.80 ± 0.06b | 3.10 ± 0.35b | 3.9 ± 0.30b | |
| 0.75 | 9.94 ± 1.82c | 30.50 ± 5.11b | 0.75 ± 0.10b | 2.29 ± 0.66cd | 3.04 ± 0.80cd | |
| 1 | 6.04 ± 1.22d | 29.50 ± 9.06b | 0.60 ± 0.07b | 1.83 ± 0.23ce | 2.43 ± 0.29de | |
| 1.25 | 2.59 ± 2.18e | 14.32 ± 3.21c | 0.46 ± 0.14bc | 2.07 ± 0.19d | 2.53 ± 0.34de | |
| 1.5 | 0.87 ± 0.24e | 10.02 ± 1.29c | 0.36 ± 0.06ed | 1.52 ± 0.32e | 1.88 ± 0.34ef | |
| 1.75 | 1.16 ± 0.59e | 9.30 ± 2.38c | 0.20 ± 0.04ef | 1.39 ± 0.18e | 1.59 ± 0.25fg | |
| 2 | 1.07 ± 0.47e | 8.12 ± 0.86c | 0.16 ± 0.04f | 0.90 ± 0.24f | 1.06 ± 0.27g | |
| p-value | < 0.0001∗∗ | < 0.0001∗∗ | < 0.0001∗∗ | < 0.0001∗∗ | < 0.0001∗∗ | |
| A. chinensis | 0 | 16.68 ± 2.91a | 43.96 ± 0.13a | 0.42 ± 0.13a | 1.25 ± 0.36a | 1.67 ± 0.52a |
| 0.25 | 15.15 ± 3.96a | 36.44 ± 0.08b | 0.35 ± 0.08a | 1.07 ± 0.42a | 1.42 ± 0.50b | |
| 0.5 | 5.58 ± 2.14b | 34.26 ± 0.03b | 0.13 ± 0.03b | 0.35 ± 0.08b | 0.48 ± 0.09c | |
| 0.75 | 4.79 ± 1.48bc | 21.86 ± 0.03c | 0.13 ± 0.03b | 0.32 ± 0.05b | 0.45 ± 0.08c | |
| 1 | 3.29 ± 1.36cd | 21.52 ± 0.04c | 0.11 ± 0.04bc | 0.22 ± 0.09b | 0.33 ± 0.13c | |
| 1.25 | 2.05 ± 1.82de | 18.08 ± 0.04cd | 0.07 ± 0.04bc | 0.15 ± 0.07b | 0.22 ± 0.10c | |
| 1.5 | 1.79 ± 0.10de | 16.30 ± 0.04cd | 0.07 ± 0.04bc | 0.14 ± 0.03b | 0.21 ± 0.04c | |
| 1.75 | 0.56 ± 0.49e | 13.94 ± 0.01d | 0.06 ± 0.01bc | 0.12 ± 0.03b | 0.18 ± 0.03c | |
| 2 | 0.38 ± 0.27e | 12.22 ± 0.01d | 0.03 ± 0.01c | 0.07 ± 0.04b | 0.10 ± 0.05c | |
| p-value | < 0.0001∗∗ | < 0.0001∗∗ | < 0.0001∗∗ | < 0.0001∗∗ | < 0.0001∗∗ | |
- Note: means ± standard deviation, the letter behind the number shows a significant difference in the DMRT test results at the 5% level.
- ∗∗significance at the 1% level.


3.2. Chlorophyll Content
Chlorophyll is a green pigment found in chloroplast and plays an important role in the physiological process of photosynthesis [41] and chlorophyll content is also able to reflect the photosynthetic ability of a plant [42]. The analysis showed that Fe exposure did not significantly affect the chlorophyll content of E. cyclocarpum and A. chinensis seedlings, and Fe exposure only had a significant effect on the carotenoid content of E. cyclocarpum seedlings (Table 2). Chlorophyll values in E. cyclocarpum seedlings fluctuated at various Fe concentrations. Exposure to 1 mM Fe in E. cyclocarpum seedlings showed the highest chlorophyll a, total chlorophyll, and carotenoid values compared to other concentrations. This shows that E. cyclocarpum seedlings are able to maintain and even increase the chlorophyll value under the pressure of Fe exposure. Meanwhile, in A. chinensis seedlings, the chlorophyll content value in the control treatment (Fe 0 mM) was the highest compared to other concentrations. This indicates that in A. chinensis seedlings, Fe exposure is able to reduce the chlorophyll value, although the decrease is not consistent. This also indicates that A. chinensis species tend to be more sensitive and show a less positive response to Fe exposure than E. cyclocarpum species.
| Seedlings | Fe concentration (mM) | Parameters | |||
|---|---|---|---|---|---|
| Chlorophyll a (mg/g) | Chlorophyll b (mg/g) | Total chlorophyll (mg/g) | Carotenoids (mg/g) | ||
| E. cyclocarpum | 0 | 3.83 ± 0.32bc | 2.25 ± 0.20bc | 6.08 ± 0.51bc | 1.25 ± 0.08c |
| 0.25 | 3.88 ± 0.28bc | 2.31 ± 0.29bc | 6.19 ± 0.55abc | 1.30 ± 0.14bc | |
| 0.5 | 4.23 ± 0.03abc | 2.67 ± 0.27a | 6.74 ± 0.01ab | 1.42 ± 0.01ab | |
| 0.75 | 4.22 ± 0.06abc | 2.53 ± 0.15ab | 6.75 ± 0.10ab | 1.42 ± 0.03ab | |
| 1 | 4.32 ± 0.11a | 2.54 ± 0.03ab | 6.87 ± 0.12a | 1.46 ± 0.03a | |
| 1.25 | 3.97 ± 0.20abc | 2.30 ± 0.14bc | 6.27 ± 0.33abc | 1.33 ± 0.05abc | |
| 1.5 | 4.15 ± 0.22abc | 2.40 ± 0.10abc | 6.55 ± 0.31abc | 1.38 ± 0.08abc | |
| 1.75 | 3.92 ± 0.34abc | 2.31 ± 0.22bc | 6.23 ± 0.56abc | 1.36 ± 0.12abc | |
| 2 | 3.82 ± 0.15bc | 2.18 ± 0.09c | 5.60 ± 0.24c | 1.29 ± 0.03bc | |
| p-value | 0.0567ns | 0.0652ns | 0.0569ns | 0.0435∗ | |
| A. chinensis | 0 | 2.84 ± 0.51a | 1.64 ± 0.31a | 4.48 ± 0.12a | 0.94 ± 0.82a |
| 0.25 | 2.72 ± 0.47ab | 1.48 ± 0.23ab | 4.20 ± 0.07ab | 0.89 ± 0.71ab | |
| 0.5 | 2.01 ± 0.24b | 1.13 ± 0.14b | 3.15 ± 0.13b | 0.75 ± 0.39b | |
| 0.75 | 2.34 ± 0.26ab | 1.37 ± 0.14ab | 3.87 ± 0.05ab | 0.89 ± 0.44ab | |
| 1 | 2.78 ± 0.15ab | 1.55 ± 0.07ab | 4.32 ± 0.05ab | 0.93 ± 0.18ab | |
| 1.25 | 2.71 ± 0.54ab | 1.57 ± 0.34ab | 4.29 ± 0.41ab | 1.11 ± 0.87ab | |
| 1.5 | 2.75 ± 0.36ab | 1.56 ± 0.19ab | 4.32 ± 0.07ab | 0.92 ± 0.55ab | |
| 1.75 | 2.38 ± 0.15ab | 1.34 ± 0.07ab | 3.72 ± 0.05ab | 0.78 ± 0.22b | |
| 2 | 2.65 ± 0.64ab | 1.51 ± 0.40ab | 4.16 ± 0.17ab | 0.90 ± 1.02ab | |
| p-value | 0.2727ns | 0.2991ns | 0.3257ns | 0.3817ns | |
- Note: means ± standard deviation, the letter behind the number shows a significant difference in the DMRT test results at the 5% level. Leaf sampling for chlorophyll analysis was conducted at the end of the observation period (after 30 days).
- ∗significance at the 5% level, ns: not significance at the 5% level.
3.3. Photosynthesis Rate
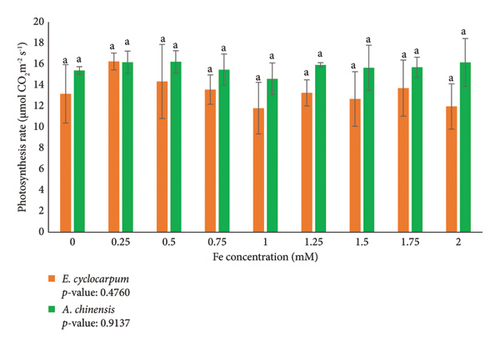
Photosynthesis is one of the physiological parameters that is generally measured to determine the physiological response of plants to a treatment. According to the analysis, Fe exposure treatment did not significantly affect the photosynthesis rate of E. cyclocarpum and Albizia chinensis seedlings (Figure 2). The photosynthesis value of E. cyclocarpum and A. chinensis after Fe exposure varied and fluctuated (Figure 2). Each Fe concentration did not show significant differences in E. cyclocarpum and A. chinensis seedlings. A 0.25 Fe concentration was able to increase the photosynthesis value of E. cyclocarpum species by approximately 16.26 μmol CO2·m−2·s−1, while in A. chinensis, 0.5 mM Fe exposure was able to increase photosynthesis by 26.23 μmol CO2·m−2·s−1. Increasing Fe concentration can reduce the value of photosynthesis in both species although not consistently. Increasing Fe concentration can reduce the value of photosynthetic rate in both species although not consistently. The decrease in photosynthetic rate values in E. cyclocarpum and A. chinensis seedlings occurred at a concentration of 1 mM.
3.4. MDA Content
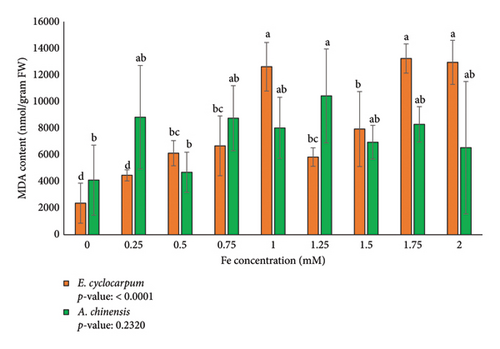
MDA content is a parameter used as a marker of lipid peroxides in plant tissues that increase due to oxidative stress [43] and especially to determine plant responses to abiotic and biotic stress [44]. The analysis showed that Fe exposure had a significant effect on MDA content only in E. cyclocarpum seedlings (Figure 3). In E. cyclocarpum seedlings, MDA content consistently increased from 0 to 1 mM Fe concentration, decreased at 1.25 mM, and significantly increased again at higher concentrations up to 2 mM. The highest MDA content was observed at 1.75 mM Fe exposure (13,240.57 nmol/gram FW) and 2 mM Fe exposure (12,945.67 nmol/gram FW). This indicates that high Fe content can increase the MDA content of Enterolobium cyclocarpum seedlings although the increase is not consistent. Meanwhile, in A. chinensis seedlings, the MDA content fluctuated considerably at different Fe concentrations, and significantly increased at Fe concentration of 1.25 mM (10,431.79 nmol/gram FW). The MDA content of E. cyclocarpum was higher than that of A. chinensis at Fe concentrations of 1, 1.5, 1.75, and 2 mM. These findings suggest that E. cyclocarpum and A. chinensis seedlings showed different responses and impacts to Fe toxicity.
3.5. Tolerance Index
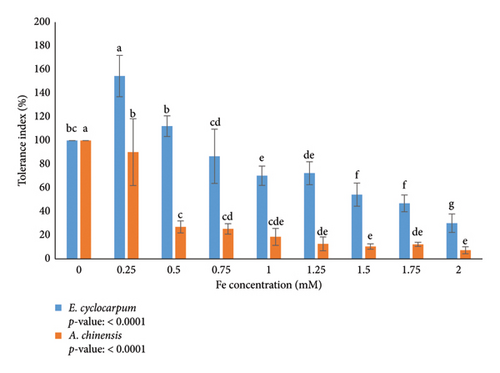
Tolerance index is a parameter used to measure the tolerance ability of a plant to abiotic stress. Each plant is sensitive to heavy metal exposure, especially Fe. According to the analysis, Fe exposure had a significant effect on the tolerance index of E. cyclocarpum and A. chinensis seedlings (Figure 4). The tolerance index of Enterolobium cyclocarpum species is higher than that of A. chinensis seedlings (Figure 4). This suggests that E. cyclocarpum species have a greater tolerance to Fe exposure than A. chinensis seedlings. In the E. cyclocarpum seedling, the tolerance index increased significantly to 154.52% at a concentration of 0.25 mM. At 0.25 and 0.5 mM concentrations, the tolerance index of E. cyclocarpum was higher than the control (0 mM). However, the seedling tolerance index decreased significantly by increasing the Fe concentration from 0.75 to 2 mM. While in the A. chinensis seedlings, the tolerance index decreased significantly with increasing Fe concentration (100% (0 mM) sampai 7.33% (2 mM)).
4. Discussion
Fe is an essential microelement that is very important for plant growth and development, which significantly affects plant productivity due to its vital role in physiological and metabolic processes [45, 46]. The results showed that Fe exposure significantly affected the growth of E. cyclocarpum and A. chinensis seedlings (Tables 1 and 2). In E. cyclocarpum seedlings, exposure to 0.25 mM Fe increased seedling growth, indicating that Fe is required by plants in sufficient amounts to support growth and development [47]. Some studies showed that there was an increase in growth with the application of Fe in low concentrations, namely in basil (Ocimum basilicum) [48], corn (Zea mays) [11], and rice with Basmati-55 cultivar [12]. Meanwhile, increasing Fe concentration (0.5–2 mM) can reduce the growth of E. cyclocarpum seedlings, indicating that higher Fe concentrations can be toxic to plants. This toxic effect is also observed in A. chinensis seedlings, where increasing Fe concentration (0–2 mM) results in reduced plant growth. The same results were reported by Salim et al. that the species of Calliandra calothyrsus and Leucaena leucocephala seedlings (fabaceae family) experienced a decrease in growth along with increasing Fe concentration [49]. Several studies have shown that Fe toxicity can significantly inhibit plant growth [22, 50]. The inhibition of growth due to Fe toxicity can also result from disrupting nutrient absorption processes, particularly nitrogen and phosphorus, which are essential for cell division [51–53]. Fe toxicity can lead to osmotic imbalance, enzymatic changes, and disruption of cell structure, inhibiting plant growth [54]. Roots are the first organ to interact with heavy metals, making them the most sensitive to heavy metal exposure, especially Fe [55]. This effect can be observed with increasing Fe concentrations from 0.25 to 2 mM in E. cyclocarpum and from 0 to 2 mM in A. chinensis, which caused a decrease in root length (Table 1). Similar results were reported by Vu et al. [22], indicating that increasing Fe concentrations from 0 to 200 ppm can reduce the root length of two varieties of rice seedlings. Root inhibition occurs when the root tip is in direct contact with high concentrations of Fe [14, 56]. One of the adaptations of plant tolerance to Fe exposure is high biomass production [57]. High plant growth can result in high biomass production and help plants reduce the impact of Fe toxicity [58]. This can be seen in the results of this study which showed an increase in biomass production or dry weight (roots, shoots, and total) of E. cyclocarpum seedlings at Fe concentrations of 0.25 and 0.5 mM compared to the control treatment (0 mM) (Table 2). This is also similar to the results reported by Liu et al. [59], who found that Fe can increase the dry weight of roots and shoots in rice plants under certain conditions. However, an increase in Fe concentration can reduce biomass production or dry weight (roots, shoots, and total) of E. cyclocarpum seedlings. Meanwhile, in A. chinensis seedlings, an increase in Fe concentration from 0 to 2 mM can reduce biomass or dry weight (roots, shoots, and total) of seedlings. The decrease in biomass or dry weight was likely caused by excessive Fe uptake, which led to reduced shoot and root growth, leading to a decrease in plant dry weight accumulation [50].
Fe is one of the elements that play a role in metabolic processes, including chlorophyll biosynthesis [60]. Fe also plays a role in maintaining chloroplast’s structural and functional integrity [46, 61]. Fe exposure showed no significant effect on the chlorophyll content of E. cyclocarpum and A. chinensis seedlings (Table 2). The research results by Ifie et al. [62] also reported that exposure to Fe at 100 and 400 mg L−1 did not significantly affect the chlorophyll content of cowpea (Vigna unguiculata). In E. cyclocarpum seedlings, exposure to 1 mM Fe increased the content of chlorophyll a, total chlorophyll, and carotenoids. The high value of chlorophyll at some concentrations is quite high, indicating one of the plant’s adaptation responses to Fe exposure. However, a Fe concentration of 2 mM reduced the chlorophyll content, although this reduction did not show significant differences compared to several other Fe concentrations. Meanwhile, in A. chinensis seedlings, Fe exposure reduced the content of chlorophyll a, b, total chlorophyll, and carotenoids, although the decrease was not significant. Frąszczak et al. [20] also reported that applying Fe can reduce the chlorophyll content of Lactuca sativa. Excessive Fe concentration can cause a reduction in chlorophyll and carotenoid content due to the inhibition of chlorophyll synthesis, damage to protein molecules, and chlorophyll degradation, leading to a decrease in chlorophyll pigments [63, 64]. This is likely the cause of the decrease in chlorophyll content in both seedlings tested, especially at high Fe concentrations. Chlorophyll is important in photosynthesis, particularly light reactions [50]. A decrease in chlorophyll content suggests an impact on inhibiting and reducing the photosynthetic process [65, 66].
About 80% of Fe in leaves is located in chloroplasts, which are crucial for the photosynthesis process [67, 68]. This indicates that Fe plays a significant role in photosynthesis [69]. Fe is a critical component of cellular redox homeostasis, mediating light reactions in photosynthesis [70]. Additionally, Fe is involved in the electron chain transfer process during photosynthesis [71, 72]. In photosynthesis, two or three Fe atoms are found in molecules directly related to photosystem II (PS II), 12 atoms in photosystem I (PSI), five atoms in the cytochrome complex, and two atoms in the ferredoxin molecule [67]. This underscores the importance of Fe in photosynthesis [13, 44]. Fe exposure did not result in a significant difference in the photosynthetic rate of E. cyclocarpum and A. chinensis seedlings, with the photosynthesis values varied and fluctuating (Figure 2). These results are also similar to those reported by Salim et al. [49] where Fe exposure did not significantly affect the photosynthetic rate of C. calothyrsus and L. leucocephala seedlings. At a Fe concentration of 0.25 mM, the photosynthesis rate of E. cyclocarpum seedlings increased, while in A. chinensis seedlings, exposure to 0.5 mM Fe increased photosynthesis. This suggests that the plants can still adapt and tolerate Fe exposure at these concentrations, as indicated by the highest photosynthesis values compared to other concentrations [73]. Fe toxicity can cause oxidative stress and catalyze the formation of free radicals and reactive oxygen species (ROS), which can interfere with and inhibit photosynthesis [3, 73].
Lipid peroxides, measured by MDA production, indicate heavy metal toxicity in plants [74]. MDA, an end product of lipid oxidative modification, induces oxidative stress, leading to cell membrane damage [75], reduced growth [76], and potential plant death. Fe exposure had a significant effect on the tolerance index of E. cyclocarpum and A. chinensis seedlings (Figure 3). Specifically, MDA content increased steadily from 0 to 1 mM Fe, decreased at 1.25 mM Fe, and increased significantly at 2 mM Fe. The highest MDA content was observed at 1.75 mM Fe, with no significant difference between 1 and 2 mM Fe concentrations. Conversely, MDA content in A. chinensis seedlings fluctuated at different Fe concentrations, peaking significantly at 1.25 mM Fe. Rajonandraina et al. [75] similarly observed a sharp increase in MDA content under Fe toxicity conditions. This rise in lipid peroxide levels is attributed to oxidative stress induced by Fe toxicity, which catalyzes ROS formation [13, 76]. Oxidative stress disrupts plant metabolic and physiological processes, inhibiting cytoplasmic enzymes, damaging cell structures, and reducing growth [3, 13]. While increasing Fe concentration generally leads to higher MDA accumulation in various plants [77], not all high Fe concentrations result in elevated MDA content. This is related to the defense of each species when exposed to heavy metals, especially Fe. Plants are able to develop enzymatic and nonenzymatic antioxidant defense mechanisms to respond to oxidative stress caused by exposure to heavy metals, especially Fe [21, 78]. Enzymatic antioxidant defense mechanisms include: superoxide activity (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPX), and others, which play a role in ROS detoxification, while several nonenzymatic antioxidant compounds including ascorbic acid, phenolics, fatty acids, amino acids, carotenoids, glutathione, and others play a role in nonenzymatic ROS scavengers [78, 79]. The results of research by Novianti et al. [78] reported that iron exposure treatment from low to high concentrations was able to increase SOD activity. According to Hasanuzzaman et al. [79], the increase in enzymatic activity including SOD, catalase, and APX is the main mechanism of plants in dealing with oxidative stress caused by heavy metal exposure.
Each plant species can develop distinct tolerance mechanisms to heavy metal exposure, depending on its inherent capacity [80]. Environmental conditions significantly influence plant tolerance to heavy metals [81]. Fe exposure had a significant effect on the tolerance index of E. cyclocarpum and A. chinensis seedlings (Figure 4). The tolerance index of E. cyclocarpum increased at 0.25 mM Fe. However, it decreased notably with higher Fe concentrations, indicating that elevated Fe levels can diminish the tolerance of E. cyclocarpum seedlings and subsequently reduce their growth. Similarly, Rasafi et al. [82] found that the tolerance index of wheat plants increased at 50 mg/L Fe but decreased with further increases in Fe concentration. In contrast, A. chinensis experienced a decrease in tolerance index with increasing Fe concentration. This is consistent with findings in other species of the same family (fabaceae) such as L. leucocephala and C. calothyrsus, which experienced a decrease in tolerance index with increasing Fe concentration [49]. Plants can manage Fe toxicity by regulating Fe2+ uptake and converting it into insoluble forms at the root tip [21, 79]. In addition, there is retention of Fe in root cell vacuoles and plastids to reduce translocation to the navel and partitioning of excess Fe in old tissues to prevent damage to young tissues [83]. In addition, plants can develop enzymatic and nonenzymatic antioxidant defense mechanisms when exposed to heavy metals especially Fe [21].
5. Conclusion
Fe exposure significantly influenced all growth parameters of E. cyclocarpum and A. chinensis seedlings. Fe concentration of 0.25 mM was the optimal concentration to stimulate the growth of E. cyclocarpum seedlings, while concentrations between 1.5 and 2 mM had a negative impact on the growth of E. cyclocarpum seedlings. Meanwhile, an increase in Fe concentration can reduce the growth of A. chinensis seedlings. Fe exposure in both species showed no significant effect on chlorophyll content and photosynthetic rate. MDA content increased with Fe exposure; in E. cyclocarpum species, it had a significant increase when the Fe concentration was 1.75 mM, while in A. chinensis species, it had a significant increase when the Fe concentration was 1 mM. The tolerance index of E. cyclocarpum seedlings increased significantly at 0.25 mM Fe concentration, but decreased with increasing Fe concentration, while A. chinensis showed a decrease in tolerance index with increasing Fe concentration. This finding suggests that E. cyclocarpum is able to provide greater tolerance to Fe exposure compared to A. chinensis seedlings. Meanwhile. A. chinensis species responded negatively to Fe exposure as indicated by a decrease in growth with increasing Fe concentration. Enterolobium cyclocarpum species can also be recommended as one of the species for rehabilitation and reclamation of marginal lands that have high Fe content.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This research was supported by the Ministry of Research, Technology, and Higher Education of the Republic of Indonesia through the PMDSU (Program Magister Menuju Doktor untuk Sarjana Unggul) program, based on Research Grant Agreement No. 1/E1/KP.PTNBH/20 dated March 18, 2020, and No. 1/E1/KP.PTNBH/2021.
Acknowledgments
We would like to express our sincere gratitude to the National Research and Innovation Agency (BRIN) for their invaluable support and for the postdoctoral fellowship. The facilities and resources provided by BRIN significantly contributed to the successful completion of this paper. We are also grateful for the discussions and guidance provided by our colleagues and mentors throughout this research.
Open Research
Data Availability Statement
This article contains all the main contributions of the study, and further information can be requested from the corresponding author.




