Enhancing Thermal Properties of Co3O4 Nanoparticles Through Optimized Mn-Doping Concentrations
Abstract
Herein, a facile co-precipitation technique was employed to prepare cobalt oxide (Co3O4) and Co3O4 nanoparticles (NPs) with manganese (Mn) doping. The morphologies and crystalline structures of the as-prepared NPs were characterized by X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), differential thermal analysis (DTA), thermogravimetric analysis (TGA), and Brunauer–Emmett–Teller (BET) techniques. XRD measurements showed that Co3O4 (19.42 nm) and Mn-doped Co3O4 (14.02 nm) NPs were successfully synthesized on average, and they exhibit a face-centered cubic crystalline structure. The SEM images also depicted the spherical morphology of Co3O4 NPs, with Mn particles clustering together. FTIR spectroscopy further confirmed the presence of Mn within the doped Co3O4 NPs. TGA/DTA analysis revealed boosted thermal stability in Mn-doped Co3O4 NPs compared to Co3O4 NPs. Moreover, the BET surface areas of Co3O4 and Mn-doped Co3O4 NPs were 534.013 and 712.741 m2/g, respectively, indicating an increase in surface area due to Mn doping. Consequently, Mn doping enhances both surface area and thermal stability, rendering Mn-doped Co3O4 NPs desirable for nanomaterial applications. This study shows that modulating the Mn doping concentration in the Co3O4 NPs offers superior surface area and thermal stability compared to the prepared Co3O4 NPs.
1. Introduction
In recent years, nanotechnology has emerged as one of the most significant and exciting frontiers across all scientific fields. It enables the industrialization of novel materials and devices with atomic precision. Typically, there are three primary methods for synthesizing nanoparticles (NPs): chemical [1, 2], physical [3, 4], and biological (or green) methods [5–11].
Recently, significant attention has been given to metal oxide NPs due to their distinct optical, electronic, and magnetic characteristics, which often diverge from those of bulk materials [12–16]. The morphology and characteristics of nanocomposites strongly depend on the chemical composition, dimension, configuration, and dispersion of the particles [12]. The synthesis of NPs is a complex interplay of various factors that can influence their final properties. Understanding and controlling these parameters is crucial for tailoring NPs for specific applications, from drug delivery to catalysis. Each factor, whether it be pH, temperature, the choice of salt or solvent, or reaction time, plays a pivotal role in defining the characteristics and behaviors of the resulting NPs [17].
Various metal oxides, such as ruthenium oxide (RuO), manganese oxide (MnO), nickel oxide (NiO), copper oxide (CuO), zinc oxide (ZnO), and cobalt oxide (Co3O4), stand out as some of the most formidable materials [18, 19]. Co3O4 is a well-known p type semiconductor with exceptional physical and chemical characteristics [20, 21]. It finds application in various fields such as sensors [22], fuel cells [23], electrochemistry [24], supercapacitors [25, 26], and water treatment [27–29].
Various techniques have been employed to synthesize Co3O4 NPs, like the sol-gel method [30], co-precipitation [31], thermal decomposition [32], solvothermal [33, 34], and hydrothermal method [35]. Previously, the doping of transition metals (e.g., chromium (Cr), Mn, zinc (Zn), Cu, silver (Ag), etc.) onto metal oxides (e.g., Co3O4, ZnO, CuO, etc.) was studied to modify their optical properties and mechanical properties [12, 36–41]. Among transition metal ions, Mn is the most beneficial dopant because it enables fine-tuning the structural and optical properties of Co3O4-based materials [42–45]. Moreover, its ability to adopt variable oxidation states, affordability, and nontoxic nature make it a good candidate for doping Co3O4 [46, 47].
Doping the assembly of Mn ions onto Co3O4 leads to a notable enhancement in magnetic, structural, and electrical properties [48, 49]. Within the spinel structure of Co3O4, determining the oxidation state and the positioning of cation ions between the octahedral and tetrahedral lattice sites can be quite challenging [50, 51]. This is why there are various oxidation states observed for both Co and Mn cations in the case of Mn-doped Co3O4 [52]. These properties, along with the synergistic effect between Co3O4 (high oxidation potential) and Mn (rapid electron transport), have attracted widespread attention for Mn-doped Co3O4 for their improved optical properties [53, 54], making Mn-doped Co3O4 NPs suitable in a diverse range of applications such as supercapacitors, sensors, batteries, fuel cells, electro-photocatalysis, magnetic materials, and electromagnetic shielding [55, 56]. In general, Mn-doped Co3O4 spinel nanocomposite has high electronic conductivity, good thermal stability, and a thermal expansion coefficient [57].
This study focused on enhancing the thermal properties of Co3O4 by doping Mn. We employed the Co-precipitation method to synthesize both Co3O4 and Mn-doped Co3O4 NPs. For both samples, numerous characterization techniques were utilized, including X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), differential thermal analysis (DTA), thermogravimetric analysis (TGA), and Brunauer–Emmett–Teller (BET) techniques. As a result, Mn doping enhances both surface area and thermal stability, offering superior characteristics in these aspects compared to the Co3O4 NPs prepared in this study.
2. Materials and Methods
2.1. Materials
All chemicals and reagents, including cobalt (II) chloride hexahydrate (CoCl2.6H2O, 99.5%, Neolab Ltd), Mn chloride (MnCl2, 98%, Loba Chemical Pvt. Ltd), sodium hydroxide (NaOH, 99.8%, Alphax Chemical Industry, India), sodium carbonate (Na2CO3, 99.8%, UNI-CHEM), and ethanol (99.5%, ECOCHEM), were utilized without additional purification and were of analytical grade. The solutions were prepared using distilled water.
2.2. Preparation of Co3O4
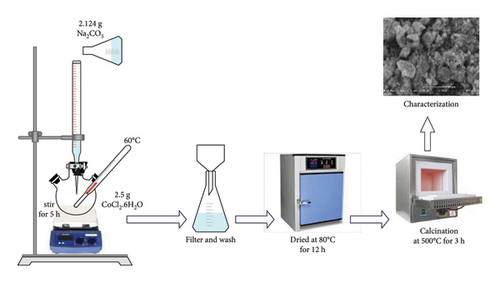
Co3O4 NPs have been prepared by employing the co-precipitation approach (Figure 1). Initially, the CoCl2.6H2O (2.5 g) precursor was dissolved in 250 mL of distilled (DI) water and stirred for 20 min, resulting in a pink-colored solution. Subsequently, 2.124 g of Na2CO3 (1 M) were added, and the mixtures were stirred for 5 h at 60°C, forming a light purple precipitate. Afterward, the precipitated sample was washed three times with DI water and ethanol to remove impurities. The precipitated sample was dried at 80°C for 12 h in an oven to dry it completely. Finally, the dried sample was calcinated in an electric furnace at 500°C for about 3 h to obtain black crystalline Co3O4 [27, 34].
2.3. Preparation of Mn-Doped Co3O4 NPs
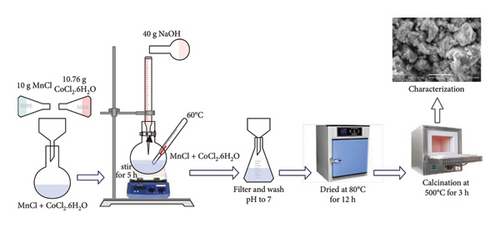
Manganese-doped Co3O4 NPs were synthesized with a simple co-precipitation technique (Figure 2). Initially, 10 g of MnCl2 and 10.76 g of CoCl2.6H2O were separately dissolved in 100 mL of DI water in two different beakers. Subsequently, 40 g of NaOH suspension were dissolved in 200 mL of DI water. The solutions of MnCl2 and CoCl2.6H2O were then combined in a single beaker, followed by the slow addition of NaOH suspension to the mixture using a co-precipitation method with continuous stirring. This process resulted in a noticeable color change after a few minutes. The solution was subsequently filtered to adjust its pH to 7. Finally, the resulting solution underwent drying in a vacuum oven, followed by grinding and calcination in a furnace for about 3 h at 600°C to obtain Mn-doped Co3O4 [45, 48].
2.4. Characterization
The XRD analysis of samples was conducted using the XRD-7000 X-ray diffractometer from SHIMADZU. Measurements were carried out in the 2θ angle range of 10°–80° at room temperature. Morphological investigations of samples were performed using a SEM (SEM, InspectF50). Functional group analysis was further conducted using FTIR (FT-IR 6660, JASCO MODEL) in the wavelength range of 400–4000 cm−1. Thermal behavior analysis was also carried out by TGA coupled with DTA. The surface areas of the synthesized NPs were determined using version 11.0 of Quanta Chrome Nova Win.
3. Results and Discussion
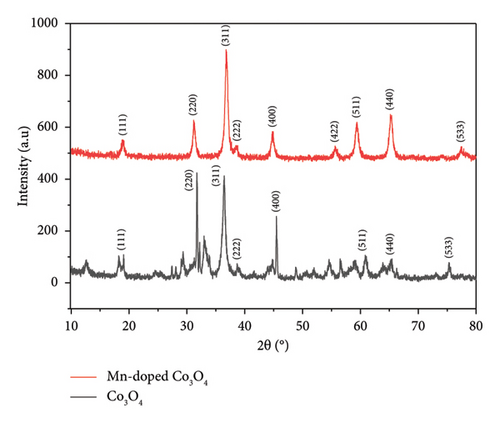
3.1. XRD Analyses
3.2. SEM Analysis
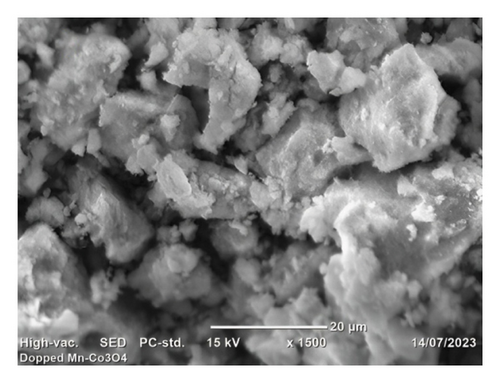
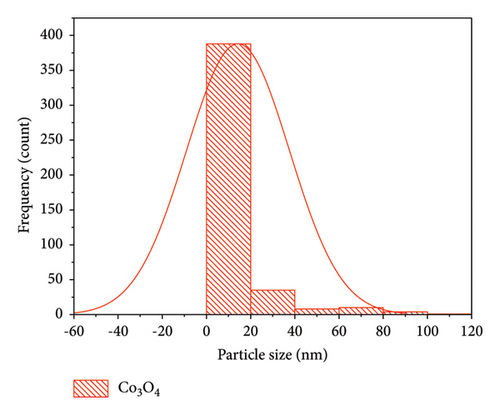
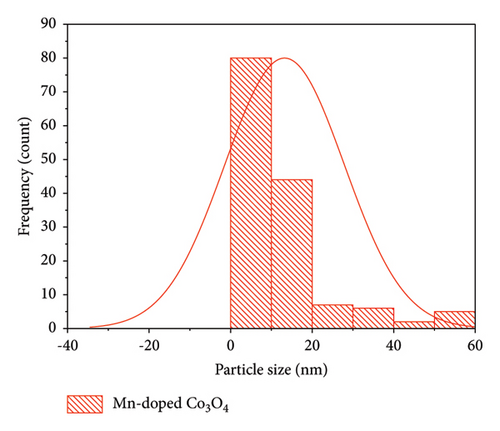
Morphological analyses provide valuable insights into how dopants affect the surface properties of host oxides. Particle morphology plays a crucial role in various physicochemical characteristics such as chemical reactivity and adsorption, both of which heavily rely on surface area. In Figure 4, SEM images depict Co3O4 NPs alongside Mn-doped Co3O4 NPs. These images reveal the spherical shape of Co3O4 NPs with the observed clustering of Mn. Additionally, the particle size of Mn-doped Co3O4 nanocomposites is notably reduced, as indicated in Figure 4(b), due to the inclusion of Mn ions into the Co3O4 lattice, as corroborated by XRD. Moreover, SEM images show that the average particle sizes of Co3O4 and Mn-doped Co3O4 are 14.27 nm and 13.23 nm, respectively. ImageJ software (version 1.8.0_112) was used to analyze the diameter of the as-synthesized nanomaterials in the SEM images via a histogram plot of particle size distribution (Figure 5).

3.3. BET Surface Area Analysis
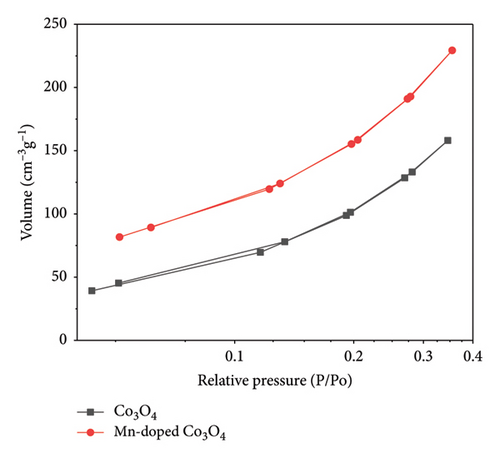
Figure 6 depicts the nitrogen adsorption and desorption isotherms of Co3O4 and Mn-doped Co3O4 samples, confirming their classification as microporous materials (IUPAC type II) [39]. The BET approach was used to calculate the total surface area of the as-prepared NPs. The surface areas of Co3O4 and Mn-doped Co3O4 NPs were determined and are presented in Table 1. The BET surface area results show a significant increase due to Mn-doping. This indicates that the produced nanomaterials have a high surface area, a finding confirmed by XRD, which showed a decrease in crystallite size leading to an increased surface area. This increase in surface area and porosity of the NPs can be attributed to the thermal decomposition of Mn species during the calcination process.
| Sample | Synthesis method | Surface area (m2/g) | Pore volume (cc/g) | Pore size (Å) | Reference |
|---|---|---|---|---|---|
| Co3O4 | Sol-gel | 24.3 | 0.057 | 158 | [30] |
| Co3O4 | Co-precipitation | 53.066 | 0.07425 | 13.85 | [36] |
| Co3O4 | Co-precipitation | 80.217 | 0.250 | 3.338 | [59] |
| Mn-doped Co3O4 | Hydrothermal | 82.2 | [44] | ||
| Co3O4@CNTs | Ultrasonic | 88.215 | [42] | ||
| Mn-Co3O4@CNTs | Ultrasonic | 100.8 | [42] | ||
| Co3O4 | Co-precipitation | 534.013 | 0.1314 | 1.838 | This work |
| Mn-doped Co3O4 | Co-precipitation | 712.741 | 0.2077 | 1.838 | This work |
3.4. FTIR Analysis
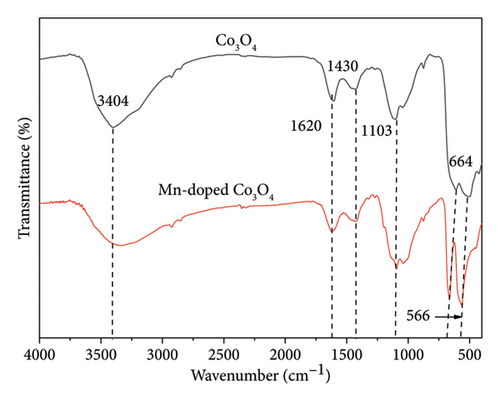
FTIR analysis was conducted to characterize the functional groups of the synthesized catalysts, with absorption spectra representing the bonding and stretching vibrations of Co3O4 and Mn-doped Co3O4 NPs across the wavelength range of 400–4000 cm−1 (Figure 7). In Co3O4 NPs, sharp peaks at 512 and 612 cm−1 correspond to the OB3 and ABO vibrations in the spinel lattice, indicating octahedral and tetrahedral holes occupied by Co2+ and Co3+ ions. In Mn-doped Co3O4 nanocomposites, the spectra exhibit sharp peaks associated with metal oxide stretching vibrations of Mn-O and Co-O. Strong peaks at 566 and 664 cm−1 are attributed to O−2 ⟶ Co2+/Mn ion bonding vibrations. These stretching vibrations observed in the spectra are characteristic of metal oxides in spinel structures. Additionally, absorption peaks at 3404, 3334, and 1620 cm−1 are attributed to water molecules and O-H bonding present on the surface of pure Co3O4 and other nanocomposites. Minor peaks at 1427 cm−1 and others are attributed to carbon dioxide present on the surface [14, 48]. The peak at 2328 cm−1 represents C = O vibrations due to the heat treatment of metal oxide [48]. The FTIR spectra observed in this study closely align with previously reported findings [18, 36, 58, 60, 61].
3.5. TGA Analysis
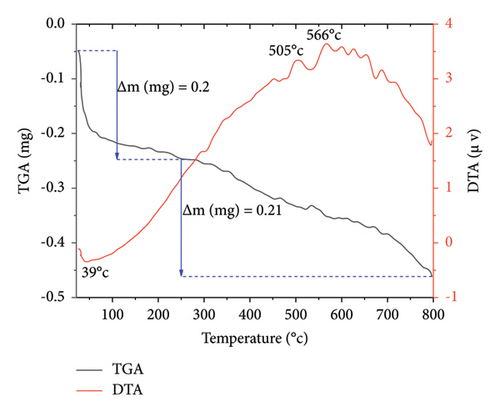
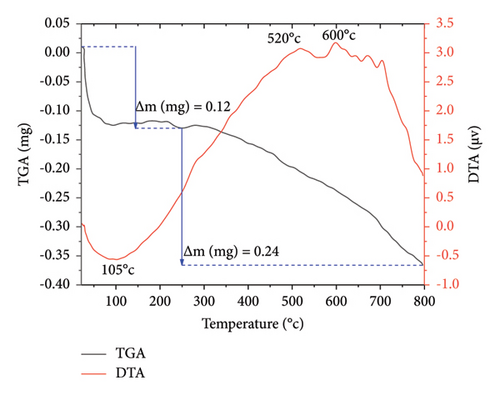
The thermal stability of Co3O4 and Mn-doped Co3O4 NPs was investigated through TGA/DTA analysis. Figure 8 illustrates the TGA/DTA curves of both Co3O4 and Mn-doped Co3O4 NPs, with a sample mass of approximately 10 mg in a corundum crucible, subjected to a heating rate of 20°C/min within the temperature range of 20°C–800°C under an air atmosphere. The TGA profiles of Co3O4 and Mn-doped Co3O4 NPs reveal two stages of weight loss. In Co3O4 NPs (Figure 8(a)), the initial weight loss of 0.2 mg occurs between 25°C and 250°C, accompanied by an endothermic peak observed at 39°C, likely due to the loss of absorbed water in the sample. Subsequently, a second weight loss of 0.21 mg is observed between 250°C and 798°C, associated with the breakdown of precursor materials or residual organic ligands, as indicated by similar DTA curves at 505°C and 566°C. Similarly, in Mn-doped Co3O4 NPs (Figure 8(b)), a first mass loss of 0.12 mg is evident between 23°C and 250°C, with an endothermic peak observed at 105°C, attributed to the loss of absorbed water. This is followed by a second weight loss of 0.24 mg between 250°C and 797°C, with similar DTA curves at 520°C and 600°C, indicative of precursor material or residual organic ligand breakdown. The TGA analysis reveals a total weight loss of 4.1% for Co3O4 NPs and 3.6% for Mn-doped Co3O4 NPs. Consequently, Mn-doped Co3O4 NPs exhibit greater thermal stability compared to Co3O4 NPs.
4. Conclusion
In this paper, Co3O4 and Mn-doped Co3O4 NPs were developed via the co-precipitation method. The effect of Mn-doping on the physical properties of Co3O4 NPs was studied and addressed. The synthesized samples were analyzed using XRD, SEM, BET, FTIR, and TGA/DTA techniques. The crystal structure of Co3O4 and Mn-doped Co3O4 NPs had high phase purity, resulting in a face-centered cubic crystalline structure without any surface adsorbed impurities. However, Mn doping showed no discernible effect on the crystal structure of Co3O4, as evidenced by XRD. The average crystallite size was determined to be 19.42 nm for Co3O4 and 14.02 nm for Mn-doped Co3O4. This finding suggests that particle size decreases with Mn doping, implying an increase in the density of contact area grains, a decrease in resistivity, and an increase in conductivity. The SEM images illustrate the spherical structure of the Co3O4 NPs, with Mn particles clustered together. FTIR spectroscopy investigation reveals the presence of Mn in doped Co3O4 NPs and confirms the production of Co3O4. The TGA/DTA studies show that Mn-doped Co3O4 NPs have higher thermal stability than Co3O4 NPs. BET surface area measurements show values of 534.013 and 712.741 m2/g for Co3O4 and Mn-doped Co3O4 NPs, respectively. Thus, the addition of Mn to Co3O4 NPs enhances their surface area and thermal stability.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Acknowledgments
This work reported in this paper was supported by Bahir Dar Energy Center, Bahir Dar Institute of Technology, Bahir Dar University, Ethiopia.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.




