The IKONIA Cohort: A Tertiary Center’s Five-Year Experience in Pulmonary Hypertension Management
Abstract
Aim and Background: Pulmonary hypertension (PH) is a progressive disease that significantly impacts patient morbidity and mortality. The recent ESC/ERS Guidelines classify PH into five groups, emphasizing the need for specific treatment in certain subgroups. This study presents a 5-year follow-up of patients diagnosed with precapillary PH at our center, aiming to analyze treatment outcomes and associated clinical characteristics.
Methods: This single-center retrospective cohort study included 65 patients diagnosed with precapillary PH between January 2018 and January 2024. Comprehensive assessments including medical history, echocardiography, and laboratory tests were performed. Treatment efficacy was evaluated through echocardiographic and laboratory parameter changes.
Results: The cohort primarily consisted of patients with idiopathic pulmonary arterial hypertension, and 40% received combination therapy. Notable improvements were observed in pulmonary artery pressure, tricuspid regurgitant velocity, and hemodynamic parameters. Despite therapeutic intervention, a mortality rate of 15.4% was recorded, highlighting the complexity of PH management.
Conclusion: This study underscores the multifaceted nature of PH and the importance of a multidisciplinary approach for optimal diagnosis and management. While combination therapy shows promise in improving patient outcomes, the persistent mortality rate emphasizes the need for enhanced awareness and early diagnosis in PH.
1. Introduction
Pulmonary hypertension (PH) is a progressive disease group to which awareness has increased in our country as well as all over the world in recent years. According to the 2022 European Society of Cardiology/European Respiratory Society (ESC/ERS) Guidelines for PH, disease groups are classified as follows: Group 1, pulmonary arterial hypertension (PAH); Group 2, PH due to left heart disease (LHD); Group 3, PH due to lung disease; Group 4, chronic thromboembolic PH (CTEPH); and Group 5, PH with unclear multifactorial mechanisms. Group 2 is known as the most common subgroup while Group 1 and Group 4 include subgroups that receive specific treatment for PH [1]. Prostacyclin analogues (Beraprost, Treprostinil, Iloprost, Epoprostenol), phosphodiesterase-5 inhibitors (Sildenafil, Tadalafil), guanylate cyclase stimulators (Riociguat), prostacyclin receptor agonists (Selexipag), and endothelin receptor antagonists (ERA) (Ambrisentan, Bosentan, Macitentan), Calcium channel blockers (responders to acute vasoreactivity testing) are used to reduce pressure and resistance in the pulmonary vascular bed which causes progressive right ventricular failure and ultimately death [1]. Studies have demonstrated that the combined use of these drugs is more effective in controlling the disease, improving symptoms, and life expectancy [2–4].
In addition, despite increased awareness of the disease, the time from symptom onset to diagnosis is still longer than 2 years [5]. Therefore, it is of great importance that a multidisciplinary team consisting of physicians specialized in cardiology, pulmonology, radiology, rheumatology, nuclear medicine, and pediatric cardiology work together in diagnosing the disease. In this study, we tried to present our 5-year follow-up data of patients diagnosed with PH and given specific treatment in our hospital.
2. Methods and Patients
The present single-center cohort was designed as retrospectively and descriptive study. The data of 65 patients diagnosed with precapillary PH and received specific treatments between January 2018 and January 2024 were included in the study to be examined. The patients’ medical history, family history, detailed physical examination, detailed blood tests, 6 min walk test, and echocardiographic characteristics were recorded in detail. This study was approved by the local ethical committee. Patients followed at a medical faculty in Konya, the largest city in Central Anatolia (historically known as Iconium, which inspired the study’s name), were included.
2.1. Definition of PAH and CTEPH
Before diagnosis, patients routinely underwent lung perfusion scintigraphy, pulmonary computed tomographic angiography, abdominal ultrasonography and rheumatological evaluation. After exclusion of Group 3 and possible Group 5, patients underwent right heart catheterization. Group 2 was diagnosed in patients with left ventricular end diastolic pressure > 15 mmHg or Pulmonary Capillary Wedge Pressure (PCWP) > 15 mmHg during right heart catheterization. Chronic thromboembolic pulmonary disease is defined as symptomatic patients with mismatched perfusion defects on perfusion scan and with signs of chronic, organized, fibrotic clots on CTPA such as ring-like stenoses, webs/slits, and chronic total occlusions after at least 3 months of therapeutic anticoagulation. After measurements performed after right heart catheterization, patients with mean pulmonary artery pressure > 25 mmHg, PCWP or left ventricular end-diastolic pressure ≤ 15 mmHg, and pulmonary vascular resistance (PVR) > 3 Woods were considered to have precapillary PH, and it was decided to initiate specific treatment for these patients. Although it is recommended to start treatment in patients with PVR > 2 and mean pulmonary artery pressure > 20 mmHg in the European PH guideline published in 2022, since the treatment initiation limit in our country is PVR > 3 and mean pulmonary artery pressure > 25 mmHg, these limits are accepted as the treatment limits for our patients group [1].
In our country, PH treatment is initiated with ERA monotherapy according to national healthcare regulations. Combination therapy is introduced if the patient’s clinical status requires escalation. Treatment decisions are made based on guideline-directed therapy, disease severity, and patient response, ensuring a standardized approach within each PH group.
2.2. Statistical Methods
Descriptive statistics were reported as means ± standard deviations for continuous variables, and categorical variables were presented as frequencies and percentages. Comparisons between subgroups were conducted using the chi-square test for categorical variables and the independent samples t-test for continuous variables. A p value < 0.05 was considered statistically significant. All analyses were performed using SPSS software version 18 (SPSS Inc., Chicago, IL, USA).
3. Results
The study investigated changes in various echocardiographic and laboratory parameters among patients before and after treatment. While some parameters, such as albumin (g/L), free thyroxine (T4, ng/dL), thyroid-stimulating hormone (TSH, μIU/mL), indirect bilirubin (mg/dL), total bilirubin (mg/dL), international normalized ratio (INR), aspartate aminotransferase (AST, U/L), alanine aminotransferase (ALT, U/L), white blood cell count (WBC, × 109/L), platelet count (PLT, × 103/μL), ejection fraction (EF, %), and heart rate (HR, bpm), did not show significant differences, certain key markers were notably affected. For instance, direct bilirubin levels (mg/dL) increased post-treatment (from 0.29 ± 0.25 to 0.37 ± 0.42; p = 0.04), whereas hemoglobin (g/dL) and hematocrit (%) levels significantly decreased (hemoglobin: 13.3 ± 2.0 to 12.2 ± 2.7, p = 0.005; hematocrit: 41.3 ± 6.0 to 38.5 ± 7.5, p = 0.009). Additionally, renal function markers showed significant changes, with a decrease in glomerular filtration rate (GFR, mL/min/1.73 m2) from 84.3 ± 27.2 to 75.5 ± 32.2 (p = 0.011) and an increase in creatinine levels (mg/dL) from 0.87 ± 0.27 to 1.06 ± 0.57 (p = 0.013). Cardiovascular parameters also shifted, as the pulmonary artery-to-aorta (Pa/Ao) ratio increased from 1.01 ± 0.24 to 1.06 ± 0.22 (p = 0.033), and tricuspid regurgitant velocity (TRV, m/s) and systolic pulmonary arterial pressure (sPAP, mmHg) both decreased significantly (TRV: 3.9 ± 0.7 to 3.6 ± 0.82, p = 0.009; PAP: 78.9 ± 24.1 to 64.8 ± 27.7, p = 0.011). Systolic and diastolic blood pressures (mmHg) also dropped (systolic: from 124.6 ± 19.7 to 112.9 ± 21.0, p = 0.001; diastolic: from 72.7 ± 10.0 to 68.0 ± 11.3, p = 0.011), indicating favorable cardiovascular changes in response to treatment (Table 1).
| Parameters | Pretreatment | Post-treatment | p value |
|---|---|---|---|
| Albumin (g/L) | 40.5 ± 4.5 | 39.2 ± 7.3 | 0.18 |
| T4 (ng/dL) | 1.2 ± 0.26 | 1.2 ± 0.28 | 0.97 |
| TSH (μIU/mL) | 1.95 ± 1.3 | 2.09 ± 1.4 | 0.32 |
| SGOT (U/L) | 22.2 ± 12.4 (14–34) | 26.9 ± 60.3 (19–70) | 0.52 |
| SGPT (U/L) | 18 ± 13.5 (12–32) | 22.5 ± 51.5 (15–62) | 0.52 |
| WBC (× 109/L) | 7.7 ± 2.2 | 8.1 ± 3.7 | 0.32 |
| PLT (× 103/μL) | 238.6 ± 72.9 | 237 ± 85.1 | 0.877 |
| Hemoglobin (g/dL) | 13.3 ± 2 | 12.2 ± 2.7 | 0.005 |
| Hematocrit (%) | 41.3 ± 6 | 38.5 ± 7.5 | 0.009 |
| GFR (mL/min/1.73 m2) | 84.3 ± 27.2 | 75.5 ± 32.2 | 0.011 |
| Creatinine (mg/dL) | 0.87 ± 0.27 | 1.06 ± 0.57 | 0.013 |
| Pa/Ao | 1.01 ± 0.24 | 1.06 ± 0.22 | 0.033 |
| IVC (mm) | 20.08 ± 4.6 | 20.9 ± 4.2 | 0.204 |
| TRV (m/s) | 3.9 ± 0.7 | 3.6 ± 0.82 | 0.009 |
| TAPSE/PAB | 0.29 ± 0.13 | 0.33 ± 0.17 | 0.036 |
| sPAP (mmHg) | 78.9 ± 24.1 | 64.8 ± 27.7 | 0.011 |
| RV-SM (cm/s) | 12.5 ± 10.5 | 10.9 ± 2.4 | 0.31 |
| TAPSE (mm) | 18.5 ± 4.6 | 17.9 ± 4.2 | 0.38 |
| EF (%) | 58.4 ± 5.6 | 57.4 ± 5.4 | 0.157 |
| HR (bpm) | 80.7 ± 16.9 | 76.9 ± 13.5 | 0.153 |
| Diastolic blood pressure (mmHg) | 72.7 ± 10 | 68 ± 11.3 | 0.011 |
| Systolic blood pressure (mmHg) | 124.6 ± 19.7 | 112.9 ± 21 | 0.001 |
| BNP (pg/mL) | 1260.9 ± 1705.4 (462–2987) | 1668.5 ± 3237.9 (856–4532) | 0.285 |
| 6 min walking test (m) | 302.3 ± 155.1 | 320.7 ± 130.3 | 0.374 |
| Initial saturation (%) | 91.2 ± 8.2 | 91.6 ± 8.1 | 0.682 |
| Finish saturation (%) | 84.6 ± 12.9 | 84.6 ± 13.4 | 0.987 |
- Note: The bold text indicates statistically significant values.
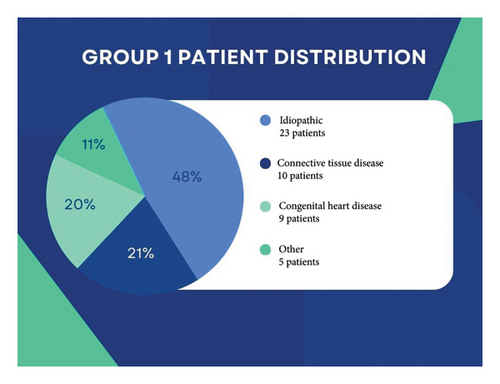
The demographic and clinical characteristics of the study population revealed an average age of 56.2 ± 18.6 years, with a female majority (75.4%). Among comorbidities, hypertension was the most common (52.3%), followed by diabetes mellitus (29.2%) and other conditions present in 24.6% of patients. Atrial fibrillation was observed in 21.5% of the population, while 38.5% exhibited right bundle branch block (RBBB). A history of hospitalization in the past year was reported by 23.1% of patients. Within the study cohort, nearly one-fourth was smokers, and liver cirrhosis was noted in 4.6% of cases. In this cohort, most patients were classified as Group I PH, although Group II PH remains the most prevalent type in clinical practice, as emphasized by the ESC/ERS guidelines (Figure 1). Precapillary PH was present in 80% of patients, and 64.6% were classified as having idiopathic PAH (IPAH). Eleven patients were followed up with CTEPH (Table 2). Twenty percent of our patients were in the low-risk group, 70% in the moderate-risk group, and 10% in the high-risk group. Three patients were receiving inhaled Iloprost treatment (μg/day).
| Parameters | |
|---|---|
| Age | 56.2 ± 18.6 |
| Sex | |
| Male | 16 (24.6%) |
| Female | 49 (75.4%) |
| Diabetes mellitus | 19 (29.2%) |
| Hypertension | 34 (52.3%) |
| Coronary artery disease | 15 (23.1%) |
| Additional disease | 16 (24.6%) |
| Smoking | 7 (10.8%) |
| Long-term oxygen therapy | 16 (24.6%) |
| One year hospitalization | 15 (23.1%) |
| Surgical history | 12 (18.5%) |
| Diuretic therapy | 30 (46.2%) |
| ACE-i | 12 (18.5%) |
| ARB | 21 (32.3%) |
| Antiaggregant | 16 (24.6%) |
| Anticoagulant | 26 (40%) |
| Betablocker | 31 (47.7%) |
| Spironolactone | 14 (21.5%) |
| Calcium channel blocker | 18 (27.7%) |
| SGLT-2 inhibitor | 5 (7.7%) |
| Digital | 6 (9.2%) |
| Atrial fibrillation | 14 (21.5%) |
| RBBB | 25 (38.5%) |
| Liver cirrhosis | 3 (4.6%) |
| Pericardial effusion | 13 (20%) |
| No mitral stenosis | 62 (95.4%) |
| Mitral regurgitation | |
| None | 39 (60%) |
| Mild | 15 (23.1%) |
| Moderate | 11 (16.9%) |
| No aortic stenosis | 65 (100%) |
| Aortic regurgitation | |
| None | 60 (92.3%) |
| Mild | 3 (4.6%) |
| Moderate | 2 (3.1%) |
| Atrial septal defect | 4 (6.2%) |
| Ventricular septal defect | 5 (7.7%) |
| ANA positive | 21 (32.3%) |
| ENA positive | 11 (16.9%) |
| Exitus | 10 (15.4%) |
| WHO group | |
| 1 | 47 (72.3%) |
| 2 | 1 (1.5%) |
| 3 | 4 (6.2%) |
| 4 | 11 (16.9%) |
| 5 | 2 (3.1%) |
| IPAH | |
| None | 42 (64.6%) |
| Vasoreactivity negative | 21 (32.3%) |
| Vasoreactivity positive | 2 (3.1%) |
| Precapillary | 52 (80%) |
| Rheumatological disease | |
| None | 54 (83.1%) |
| SLE | 2 (3.1%) |
| Scleroderma | 2 (3.1%) |
| Sjögren’s | 2 (3.1%) |
| Behçet’s | 1 (1.5%) |
| Systemic sclerosis | 3 (4.6%) |
| PAH specific treatment | |
| Macitentan | 17 (26.2%) |
| Bosentan | 8 (12.3%) |
| Riociguat | 10 (15.4%) |
| Tadalafil | 4 (6.2%) |
| Sildenafil | 2 (3.1%) |
| Selexipag | 4 (6.2%) |
| 2-pack combination | 18 (27.7%) |
| 3-pack combination | 8 (12.3%) |
Connective tissue disease (CTD)-related PH was also prevalent within the cohort, with systemic lupus erythematosus (SLE) and systemic sclerosis each comprising 3.1%, while smaller proportions included Sjögren’s syndrome, Behçet’s disease, and sarcoidosis. The primary PAH-specific treatments administered included macitentan (26.2%), bosentan (12.3%), and riociguat (15.4%), with a notable portion of patients (40%) receiving combination therapy. Of those on combination therapies, 27.7% received dual therapy and 12.3% received triple therapy regimens. Overall, the mortality rate was 15.4% (n = 10), indicating significant disease burden within this population despite therapeutic intervention (Table 2). Among the study population, one patient classified under Group 2 PH exhibited a mixed phenotype with precapillary characteristics. While Group 2 PH is generally associated with postcapillary or combined postcapillary and precapillary PH, this particular case demonstrated hemodynamic findings consistent with precapillary PH, which justified its inclusion in our cohort.
4. Discussion
The findings of this study underscore the complex nature of PH and its profound impact on patients. Our cohort predominantly consisted of individuals diagnosed with precapillary PH, with a notable proportion presenting with IPAH [6]. This aligns with existing literature, frequently identifying IPAH as a leading cause of precapillary PH, further supported by data from large studies like Kaymaz et al.‘s research, which documented similar patient distributions [7]. Additionally, the high prevalence of CTD-related PH in our cohort, including SLE and systemic sclerosis, emphasizes the multifactorial nature of PH. These findings suggest a need for clinicians to adopt a holistic approach in evaluating patients, as the underlying etiology significantly influences treatment decisions and prognostic outcomes [8]. Notably, 80% of the patients had precapillary PH, with the remaining 20% classified as combined pre- and postcapillary PH. Among the combined group, the predominant subtypes included Group 1 (PAH) and Group 2 (PH due to LHD), reflecting the multifactorial nature of the disease in this subgroup. Patients in Group 2 exhibited markedly elevated PVR, with an average value exceeding 8 Wood units, highlighting the severity of PH in this population. Similarly, patients with combined Group 3 PH demonstrated even higher PVR values, averaging over 9 Wood units, reflecting the advanced stage of disease in these individuals. The guidelines recommend initiating treatment in patients with a PVR exceeding 5 Wood units. In this subgroup of patients, we first optimize management of underlying pulmonary or cardiac conditions and closely monitor them [1]. If there is no improvement in functional capacity or if pro-BNP levels remain elevated despite these measures, we initiate PAH-specific therapy. As a first step, we start sildenafil treatment, as recommended by the guidelines. If clinical improvement remains inadequate upon close follow-up, ERA therapy is added. Importantly, we prioritize initiating treatment in a hospital setting for these patients.
The absence of intravenous prostacyclin analog therapy in our cohort warrants attention. This omission may reflect delayed initiation of treatment in high-risk patients, potentially contributing to suboptimal outcomes. This delay underscores the importance of early identification and aggressive management of high-risk patients, particularly those with advanced hemodynamic compromise. Our findings also highlight the treatment pathways undertaken for specific subgroups, particularly CTEPH. One patient underwent pulmonary balloon angioplasty, while two patients underwent pulmonary endarterectomy, demonstrating the pivotal role of interventional and surgical therapies in selected cases. Furthermore, among Group 5 PH patients, one case was attributed to sarcoidosis and another to myasthenia gravis, illustrating the heterogeneity of etiologies within this group. These findings align with the growing recognition of Group 5 PH as a challenging category requiring individualized diagnostic and therapeutic strategies.
One of the most striking aspects of our study is the efficacy of combination therapy, with approximately 40% of patients receiving dual or triple therapies. This aligns with findings from trials supporting the benefits of combination therapy in improving symptom control and long-term outcomes in PH patients [9, 10]. For instance, the GRIPHON trial demonstrated the utility of adding Selexipag to standard therapy, leading to reductions in morbidity and mortality in PAH patients [11]. In our cohort, patients receiving combination therapies experienced significant improvements in echocardiographic and laboratory parameters, suggesting a favorable reduction in right ventricular afterload—a critical factor in reducing right heart failure risk. Also our patients did not receive IV prostacyclin analogs among their treatments may be due to the delay in the treatment of high-risk patients.
Despite evidence favoring combination therapy, access remains a challenge. Our country’s unique health policies and reimbursement issues restrict the widespread adoption of dual and triple therapies, leading to lower combination therapy rates compared to other cohorts, such as those observed in the TRITON study [2]. Addressing these policy barriers could allow more patients access to comprehensive treatment regimens with proven efficacy and potentially better long-term outcomes.
The observed reductions in hemoglobin and hematocrit levels post-treatment could also reflect effective management of polycythemia, a condition that exacerbates PH complications. Notably, Kaymaz et al’s study emphasizes the burden of polycythemia and hyperviscosity in PH, underscoring how effective treatment can lead to improvements in these hematologic markers [12, 13]. In our cohort, treatment response was further supported by improvements in right heart function and the hemodynamic markers of PH. These results collectively suggest that proactive management of polycythemia may reduce disease burden, enhancing the overall therapeutic impact in PH patients [14]. When the patients’ 6 min walking test results were examined, it was seen that there was an increase after treatment, although it did not reach statistical significance.
However, it is essential to recognize that while certain markers improved, others, such as renal function indicators, revealed significant changes that require close monitoring. A reduction in GFR and an increase in creatinine levels post-treatment highlight the need for regular renal function assessments in this population, as kidney impairment can worsen PH’s clinical course and impact therapeutic decisions. This finding mirrors concerns raised in recent studies regarding renal function deterioration in PH patients, emphasizing the need for a balanced approach when managing advanced therapies that may exert renal strain [15].
Despite the therapeutic benefits observed in our cohort, the mortality rate of 15.4% raises concerns about the long-term prognosis of patients with PH. This figure reflects the persistent burden of PH and the inherent challenges in managing such a complex, progressive condition. A prolonged interval from symptom onset to diagnosis, often extending beyond 2 years for many patients, may partly explain the elevated mortality rate. These findings emphasize the urgency for increased awareness and education regarding PH’s early signs and symptoms among healthcare providers to enhance early diagnosis and improve patient outcomes [16].
Furthermore, our study underscores the necessity of a multidisciplinary approach to the diagnosis and management of PH. Collaboration among specialists—particularly in cardiology, pulmonology, and relevant fields—has shown to be essential in developing accurate diagnoses and effective treatment plans. This collaborative approach is consistent with current guidelines that advocate for comprehensive evaluations of patients with suspected PH. Involving a multidisciplinary team can help streamline ongoing monitoring and individualized treatment adjustments, crucial given PH’s dynamic nature and the variability in patient responses [17, 18].
4.1. Limitations
This study has several limitations. First, as a single-center study, our findings may not be fully generalizable, given that our patient population represents a specific demographic that may differ from PH populations elsewhere. Additionally, the retrospective design introduces potential biases, such as inaccuracies in medical records and missing data on baseline and follow-up characteristics. The relatively small sample size (65 patients) also limits the statistical power of our analyses, which may affect the generalizability of subgroup comparisons. The relatively small sample size (n = 65) may have limited the statistical power of our analyses, potentially reducing the ability to detect smaller effect sizes or subtle differences between subgroups. As a result, some associations may not have reached statistical significance despite potential clinical relevance. Larger, multicenter studies are needed to validate our findings and provide more robust conclusions.
Another limitation concerns the treatment thresholds and comorbidity assessment. Our therapeutic approach reflects national guidelines that differ from the ESC/ERS recommendations, which could influence the initiation and outcomes of PH therapies compared to those in other regions. Furthermore, the study did not comprehensively account for patient comorbidities, which are known to impact the course and prognosis of PH, especially among older individuals. Including a thorough comorbidity profile in future research could improve insights into the influence of these conditions on survival and functional outcomes in PH. Additionally, the retrospective design of our study limits the ability to establish causal relationships between treatment and outcomes. While we observed significant associations, these findings should be interpreted with caution, as confounding factors may influence the results. Prospective, randomized studies are needed to confirm these associations and better assess causality.
Finally, the lack of follow-up right heart catheterization is also a limitation, as this procedure provides valuable hemodynamic data that could inform a more accurate assessment of treatment response and disease progression. Incorporating follow-up catheterization in future studies could enhance our understanding of long-term outcomes in PH.
5. Conclusion
This study highlights the complex nature of PH and underscores the importance of a multidisciplinary approach in optimizing patient outcomes. Our findings suggest that early diagnosis and timely initiation of specific PH therapies are crucial in improving disease prognosis. The observed benefits of combination therapy in selected patients reinforce current guideline recommendations and support its consideration in clinical practice, particularly for those with inadequate response to monotherapy.
Despite advancements in PH management, significant challenges remain, including delayed diagnosis and limited access to advanced therapies. Clinicians should remain vigilant for early signs of PH and collaborate across specialties to ensure comprehensive evaluation and treatment. Future prospective studies with larger cohorts are needed to further validate our findings and refine treatment strategies.
Consent
All the patients allowed personal data processing, and informed consent was obtained from all individual participants included in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




