The Therapeutic Effectiveness of Segmental Lung Resection Versus Lobectomy in Patients With Isolated Lung Metastases From Colorectal Cancer: Evidence From a Retrospective Cohort Study
Abstract
Introduction: In patients with solitary pulmonary metastasis from colorectal cancer, surgical resection is considered a standard treatment protocol and is routinely performed in thoracic surgery. However, there is a paucity of studies that delve into the detailed discussion of the efficacy and safety of lobectomy and segmentectomy in the treatment of pulmonary metastatic cancer. Our study retrospectively analyzed the surgical outcomes of 62 patients from a single center and reported the findings.
Methods: A retrospective analysis was meticulously conducted on a cohort of 62 patients who underwent either lung segmental resection (n = 31) or lobectomy (n = 31) for isolated lung metastases at the department of thoracic surgery of Gansu Provincial People’s Hospital, spanning a decade from September 2015 to September 2024. The surgical data were thoroughly examined, encompassing perioperative details and patient outcomes.
Results: Both surgical approaches achieved successful resection of colorectal cancer lung metastases, without any perioperative mortality reported. In comparison to the group undergoing lung segmental resection, the cohort receiving lobectomy exhibited significantly larger tumor diameters (p < 0.001), prolonged operative durations (p < 0.001), increased intraoperative bleeding (p = 0.002), augmented drainage volume on the first postoperative day (p < 0.001), greater total postoperative drainage (p < 0.001), and elevated operative costs (p < 0.001). However, there were no statistically significant differences between the two groups regarding postoperative pain scores (p = 0.755), time to postoperative extubation (p = 0.744), time to postoperative ambulation (p = 0.742), duration of postoperative hospital stay (p = 0.870), lymph node metastasis (p = 0.671), postoperative complications (p = 0.562), survival rate (p = 0.550), recurrence-free survival rate (p = 0.450), and cumulative incidence (p = 0.321).
Conclusions: Pulmonary segmentectomy offers superior advantages in terms of operative time, intraoperative blood loss, drainage volume on the first postoperative day, total postoperative drainage volume, and surgical costs.
1. Introduction
Colorectal cancer (CRC) ranks as the third most prevalent cause of cancer-related mortality, despite advancements in adjuvant and surgical therapies, as well as multidisciplinary strategies that have significantly altered long-term survival outcomes. Nevertheless, metastatic dissemination occurs in approximately half of the patients following radical resection of the primary tumor [1]. Notably, the lungs constitute a frequent site for CRC metastasis, trailing only the liver in frequency. Pulmonary metastasis is observed in 15%–20% of CRC cases, highlighting its significant clinical impact [2]. Surgical resection of metastatic tumors has emerged as a standard therapeutic approach when indicated and is routinely practiced in thoracic surgery [3]. Case series from renowned cancer centers have demonstrated that approximately 40%–55% of patients undergoing resection of lung metastases from CRC achieve favorable long-term survival outcomes [4–6].
Among the surgical procedures for removing isolated lung metastases from CRC, lung wedge resection is the most frequently performed, followed by segmental resection and lobectomy [7]. Lung segmental resection involves a more limited resection of lung tissue compared to lobectomy, offering several advantages. These include greater preservation of lung function, reduced operative mortality, decreased intraoperative bleeding, and shorter hospital stays [8]. The randomized controlled trial conducted by Kenji Suzuki et al. in 2019 revealed comparable intraoperative and postoperative complication rates between patients undergoing segmental lung resection and those undergoing lobectomy. This suggests that, with the continuous advancements in imaging and other related diagnostic modalities, both procedures can be considered viable therapeutic options [9].
Over the past decade, accumulating literature has indicated that patients with lung metastases from CRC may also benefit from metastatic lung resection. However, the evidence supporting the resection of lung metastases from CRC is significantly less robust than that for liver metastases [10]. Few studies have delved into the efficacy and safety of lobectomy versus segmentectomy for the treatment of lung metastases. Our retrospective analysis examined 62 clinical cases of thoracoscopic treatment for solitary lung metastases from CRC performed in our hospital’s thoracic surgery department from September 2015 to September 2024. These cases were divided into a lobectomy group (31 cases) and a segmentectomy group (31 cases). We compared the efficacy and safety of the two surgical approaches and discussed their prognostic outcomes.
2. Methods
2.1. Clinical Data
We selected 62 patients with solitary lung metastases from CRC who underwent surgical treatment in the thoracic surgery department of Gansu Provincial People’s Hospital from September 2015 to September 2024. The inclusion criteria were as follows: (1) patients with a histopathological diagnosis of CRC, (2) patients with radiographic or pathological evidence of lung malignancy and positive postoperative genetic testing for Villin and CDX2, (3) patients aged 18 to 80 years who had undergone preoperative evaluations and were deemed to be able to tolerate lung surgery, and (4) patients who underwent surgery using thoracoscopic techniques. The exclusion criteria were as follows: (1) patients with multiple lung metastases detected on preoperative imaging, (2) patients with cardiopulmonary or general conditions that precluded lung surgery, (3) patients who had undergone multiple lung surgeries or had recurrent lung disease, and (4) to eliminate unnecessary bias, patients who underwent robot-assisted surgery or traditional open thoracotomy were excluded.
The study included a cohort of 62 patients, characterized by an average age of 61.06 ± 8.28 years and a male-to-female ratio of 1.58. The lobectomy group consisted of 31 patients, while the segmentectomy group comprised another set of 31 patients.
2.2. Surgical Techniques
2.2.1. Lobectomy Group
The two-port thoracoscopic technique was employed. Taking the right upper lobe as an example, the patient was anesthetized with general anesthesia and intubated with a double-lumen endotracheal tube. The patient was placed in the left lateral decubitus position, and the right surgical field was disinfected and draped. An observation port was established at the 8th intercostal space along the right axillary midline, and an operating port was established at the 4th intercostal space along the right anterior axillary line. After thoracoscopic exploration, the anterior segmental artery, posterior ascending branch artery, and upper lobe vein were freed, and a linear cutter stapler was used to transect them. Subsequently, the upper lobe bronchus was freed and transected. During the operation, a nontraumatic grasping clamp was used to gently retract the lung tissue and tumor, with care taken to avoid injuring surrounding blood vessels and nerves. The right upper lobe was resected intact, mediastinal lymph nodes were dissected, hemostasis was achieved, and the specimen was retrieved through the operating port. A chest tube was placed, the lung was inflated, and the thoracic cavity was irrigated to check for air leaks. The thoracotomy incision was closed in layers.
2.2.2. Segmentectomy Group
The two-port thoracoscopic technique was also utilized. Taking the dorsal segment of the left lower lobe as an example, the patient was anesthetized with general anesthesia and intubated with a double-lumen endotracheal tube. The patient was placed in the right lateral decubitus position, and the left surgical field was disinfected and draped. An observation port was established at the 8th intercostal space along the posterior axillary line on the left side, and an operating port was established at the 6th intercostal space along the anterior axillary line. Thoracoscopic exploration was performed, the posterior mediastinal pleura was opened, and the dorsal segmental artery, vein, and bronchus were freed. A linear cutter stapler was used to transect and close each structure. The lung was inflated to determine the intersegmental plane, and the dorsal segment was resected intact along this plane using a linear cutter stapler. The specimen was retrieved using a retrieval bag, hemostasis was thoroughly achieved, and a closed chest tube was placed. After verifying that all instruments and dressings were accounted for, the lung was inflated to ensure good re-expansion of the remaining lung tissue. The thoracotomy incision was then closed in a routine manner.
2.3. Observation Target
2.3.1. Basic Information
Basic information included gender, age, BMI, disease-free interval (DFI), adjuvant chemotherapy, preoperative clinical staging, multiple site metastases, smoking, and tumor size.
2.3.2. Surgical Data
Surgical data included surgical time, intraoperative bleeding, lymph node dissection, surgical cost, intermediate open heart, postoperative pain, postoperative extubation time, postoperative drainage, postoperative complications, postoperative time out of bed, and postoperative length of stay (the information above was obtained from the patient’s medical and surgical records during hospitalization).
2.3.3. Prognostic Factor
It included survival rate, survival relapse rate, and cumulative incidence (the information above was obtained by regularly following up with patients and reviewing their outpatient records.)
2.4. Statistical Analyses
Statistical analysis of the collected data was conducted using SPSS 26.0 software. Continuous variables were expressed as mean ± standard deviation (x ± s), and intergroup comparisons were performed using the Mann–Whitney U test. Categorical variables were compared between groups using chi-square tests (with Fisher’s exact test used when appropriate). Kaplan–Meier curves were utilized to depict the overall survival, relapse-free survival, and cumulative incidence. Overall survival (OS) was assessed from the date of metastasectomy surgery until death resulting from any cause. Recurrence-free survival (RFS) and cumulative incidence were measured from the date of surgical resection of pulmonary metastases in patients until the detection of recurrence. In cases where no death or relapse occurred, the time of the patient’s last visit was considered as the review time. The log-rank test and gray’s test were employed to assess the association between OS, RFS, and recurrence rate in each study group. A p value of less than 0.05 was considered statistically significant, indicating a noteworthy disparity among the groups.
3. Result
3.1. Patient Baseline Characteristics
All enrolled patients had adenocarcinoma as the pathological type and had undergone prior radical resection for CRC; of these, 38 patients received postoperative chemotherapy. The mean age of patients in the lobectomy group was 60.97 years, with 18 males and 13 females. Two patients were found to have liver metastases preoperatively, and one patient had bone metastases preoperatively. In the segmentectomy group, the mean age of patients was 61.16 years, including 20 males and 11 females. One patient in this group was found to have liver metastases preoperatively. There was a statistically significant difference in tumor diameter between the two groups, with the data derived from postoperative pathological examination reports. Specifically, the tumor diameter in the lobectomy group (1.75 ± 0.33 cm) was larger than that in the segmentectomy group (1.30 ± 0.30 cm). However, no significant statistical differences were observed in other baseline data between the two groups. Detailed information is presented in Table 1.
| Baseline data | All patients | Lobectomy | Segmentectomy | p values |
|---|---|---|---|---|
| Average age (years) | 61.06 ± 8.28 | 60.97 ± 7.99 | 61.16 ± 8.69 | 0.8771 |
| Sex (male/female) | 1.58 | 1.38 | 1.81 | 0.602 |
| DFI (month) | 23 (14.33) | 24 (15.40) | 21 (14.32) | 0.6171 |
| BMI | 22.55 ± 1.65 | 22.54 ± 1.41 | 22.57 ± 1.89 | 0.6571 |
| Complementary therapies (yes/no) | 0.77 | 0.63 | 0.93 | 0.442 |
| Metastasis to other sites (yes/no) | 0.06 | 0.10 | 0.03 | 0.612∗ |
| Tumor diameter (cm) | 1.53 ± 0.38 | 1.75 ± 0.33 | 1.30 ± 0.30 | < 0.0011 |
| Smoking (yes/no) | 0.51 | 0.40 | 0.63 | 0.421 |
| Chemotherapy (yes/no) | 1.69 | 1.38 | 1.82 | 0.602 |
| Comorbidities (yes/no) | 0.79 | 0.55 | 1.06 | 0.20 |
| Diabetes (yes/no) | 0.15 | 0.11 | 0.19 | 0.71∗ |
| Hypertension (yes/no) | 0.38 | 0.29 | 0.48 | 0.39 |
| Coronary artery disease (yes/no) | 0.05 | 0.07 | 0.03 | 0.99∗ |
| COPD | 0.09 | 0.03 | 0.15 | 0.35∗ |
- Note: DFI: The disease-free interval refers to the time between the patient’s initial diagnosis of colorectal cancer and the subsequent diagnosis of colorectal cancer lung metastases.
- Abbreviation: BMI, body mass index.
- ∗Fisher’s exact test.
- 1Mann–Whitney test.
3.2. Short-Term Efficacy and Safety
All 62 patients successfully completed the surgical procedures, including intraoperative lymph node dissection and subsequent testing, with no perioperative mortalities. Among the 31 patients in the lobectomy group, one patient required conversion to open thoracotomy with a blood loss of 1000 mL. Lymph node metastases were detected in four patients, specifically one case in Groups 7, 9, and 10; one case in Group 9; one case in Group 4; and one case in Group 7. Nine patients experienced postoperative complications, including four with persistent low-grade fever, two with fatigue and dizziness, two with sinus tachycardia, and one with hemoptysis. With appropriate treatment, all complications were resolved before discharge. In the segmentectomy group, there were no cases of conversion to open thoracotomy. Lymph node metastases were found in two patients, both in Group 4. Seven patients experienced postoperative complications, including three with persistent fever, two with hemoptysis, and one with fatigue and dizziness. As with the lobectomy group, all complications resolved before discharge following appropriate treatment.
The lobectomy group had significantly higher surgical time, intraoperative blood loss, postoperative Day 1 drainage volume, total postoperative drainage volume, and surgical costs compared to the segmentectomy group. However, there were no significant differences between the two groups in terms of postoperative pain scores, postoperative extubation time, postoperative complications, time to ambulation, postoperative discharge time, lymph node metastasis, local recurrence rates, and disease progression. Detailed information is presented in Table 2.
| Surgical information | All the patients | Lobectomy | Segmentectomy | p values |
|---|---|---|---|---|
| Surgical time (min) | 167.15 ± 62.43 | 205.03 ± 66.65 | 129.26 ± 22.76 | < 0.0011 |
| Intraoperative bleeding (mL) | 86.29 ± 125.02 | 148.55 ± 162.23 | 38.87 ± 27.80 | < 0.0011 |
| Intermediate open chest (yes/no) | 0.02 | 0.03 | 0.00 | / |
| Postoperative pain score | 3.92 ± 1.21 | 3.97 ± 1.11 | 3.87 ± 1.31 | 0.6571 |
| Postoperative Day 1 drainage (mL) | 148.71 ± 100.38 | 196.29 ± 102.80 | 101.13 ± 72.41 | < 0.0011 |
| Total postoperative drainage (mL) | 830.89 ± 430.77 | 1044.84 ± 317.96 | 616.94 ± 426.16 | < 0.0011 |
| Postoperative extubation time (days) | 5.43 ± 1.15 | 5.39 ± 1.05 | 5.48 ± 1.26 | 0.6101 |
| Postoperative complications (yes/no) | 0.26 | 0.40 | 0.29 | 0.562 |
| Postoperative time out of bed (h) | 31.4 ± 8.78 | 31.03 ± 8.28 | 31.77 ± 9.37 | 0.9611 |
| Postoperative discharge time (days) | 7.02 ± 2.29 | 7.06 ± 1.46 | 6.97 ± 2.93 | 0.3401 |
| Cost of surgery (RMB) | 8724.26 ± 1623.31 | 9665.55 ± 1589.75 | 7782.97 ± 999.85 | < 0.0011 |
| Lymph node metastasis (yes/no) | 0.10 | 0.14 | 0.06 | 0.671∗ |
| Local recurrence rates (yes/no) | 0.13 | 0.03 | 0.24 | 0.10∗ |
| Disease progression (yes/no) | 0.82 | 0.63 | 1.07 | 0.31 |
- ∗Fisher’s exact test.
- 1Mann–Whitney test.
3.3. Survival and Recurrence Rates
3.3.1. Survival Rate
In the lobectomy group, the median follow-up duration was 43 (20.66) months, with 9 patient deaths and 7 deaths occurring within 5 years. The Kaplan–Meier curve indicated a median survival time of 92 months. For the segmentectomy group, the median follow-up duration was 36 (20.55) months, with 10 patient deaths and 9 deaths occurring within 5 years. The Kaplan–Meier curve showed a median survival time of 79 months. No significant difference in survival time between the two treatment methods was observed in this study (p = 0.550), suggesting comparable survival rates between the lobectomy and segmentectomy groups as depicted in Figure 1.
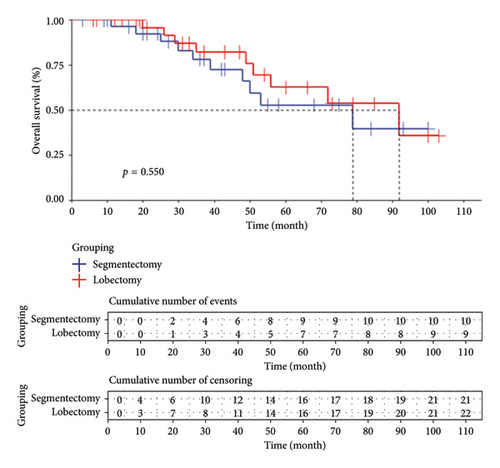
3.3.2. RFS Rate
A total of 15 patients in the lobectomy group achieved RFS, with a median RFS time of 79 months according to the Kaplan–Meier curve. Similarly, in the segmentectomy group, 12 patients survived without recurrence, and the median RFS time was found to be 68 months based on the Kaplan–Meier curve analysis. Please refer to Figure 2.
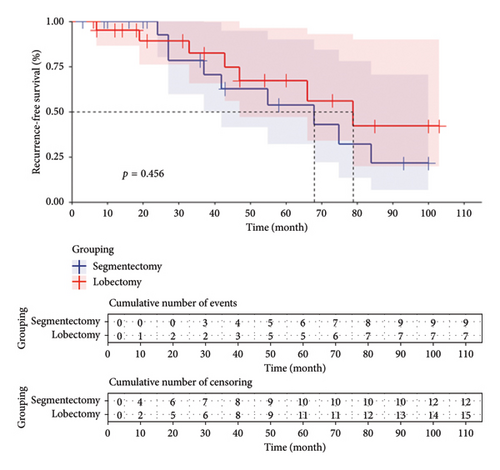
3.3.3. Cumulative Incidence
In the lobectomy group, a total of 12 patients experienced recurrence, with a median recurrence time of 54 months estimated using the Kaplan–Meier curve. Similarly, in the segmentectomy group, a total of 16 patients experienced recurrence, with a median recurrence time of 39 months estimated by employing the Kaplan–Meier curve. Statistical analysis revealed no significant difference in terms of cumulative incidence between the two groups (p = 0.321). Please refer to Figure 3 for representation of these data.
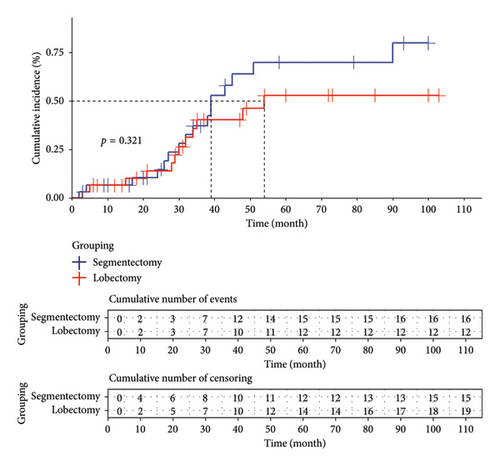
4. Discussion
CRC, the third most common malignancy globally, has a relatively high mortality rate, with a 5-year survival rate of approximately 65.1% [11]. Surgical resection is generally considered for the treatment of isolated pulmonary metastases from CRC [12]. However, there are currently no randomized controlled trials comparing the postoperative outcomes of the three surgical approaches [13]. The postoperative benefits of segmentectomy and lobectomy for the treatment of pulmonary metastases from CRC remain inconclusive. While thoracotomy was once the standard surgical approach for pulmonary metastases from CRC, recent advancements in minimally invasive video-assisted thoracoscopic surgery (VATS) have led to its development as a clinical standard [14], resulting in significant improvements in the prognosis of patients with pulmonary metastases from CRC. Segmentectomy can achieve adequate surgical margins with minimal impairment to lung function but may carry a risk of local recurrence. Lobectomy, on the other hand, can significantly reduce the risk of postoperative recurrence but may affect lung function. To date, it is unclear which surgical approach, segmentectomy or lobectomy, offers a better prognosis after resection of pulmonary metastases. Therefore, we retrospectively analyzed the data of patients who underwent resection of solitary pulmonary metastases from CRC at the department of thoracic surgery, Gansu Provincial People’s Hospital, between September 2015 and September 2024. This study aims to investigate the efficacy and safety of various treatments for solitary pulmonary metastases originating from CRC.
For patients with pulmonary metastasis from CRC, pulmonary wedge resection remains the preferred surgical treatment option [15]. In our center, wedge resection remains the predominant procedure for treating metastatic lesions in most patients. Segmentectomy and lobectomy are also employed in clinical practice; however, their therapeutic efficacy compared to wedge resection requires further clarification [16]. Currently, there is a lack of definitive guidelines to facilitate the selection of surgical procedures. There were significant differences in tumor size between the two groups; however, the overall size was insufficient to influence the selection of surgical methods. Instead, tumor location and extended resection margins served as critical determining factors. First of all, in some patients, imaging reveals lesions confined to a single lobe; however, the possibility of multiple metastatic lesions cannot be excluded. Moreover, in certain cases, the lesions are anatomically challenging to segment, especially when considering specific intraoperative conditions. This makes lobectomy a more appropriate choice. Second, cytologic malignant margins can be categorized into three types: continuous lesions, skipping lesions, and “occult” lesions [17]. Although we ensured that all surgically treated patients achieved R0 resection in the traditional sense, the presence of skipping or “occult” lesions remains uncertain. Even with wedge resections of metastases or segmentectomies performed with sufficiently large margins, recurrence may still occur due to residual cytologic malignancy in the remaining lung tissue [18]. The use of lobectomy effectively addresses this issue by reducing the likelihood of recurrence. The patient’s physical status also influences the selection of surgical methods. For patients with compromised pulmonary function, lobectomy may result in a significant decline in overall physical condition. Even if recurrence is effectively suppressed, the patient’s postoperative quality of life could be adversely affected. Although our results did not demonstrate a statistically significant difference in group assignment, during patient selection, those with poorer lung function were more likely to be chosen for segmentectomy.
Except for tumor diameter, there were no statistically significant differences in baseline characteristics between the two groups of patients. The median DFI was 24 months in the lobectomy group and 21 months in the segmentectomy group, and there was no statistically significant difference in DFI between the two groups. Previous research [19] has identified a DFI of 36 months or longer as an important prognostic factor after resection of pulmonary metastases from CRC, with a statistically significant difference in final survival rates compared to patients with a DFI of less than 36 months who underwent resection of pulmonary metastases.
In our study, 8 patients in the lobectomy group and 7 patients in the segmentectomy group had a DFI of 36 months or longer. We statistically validated the relevant outcome indicators for these 15 patients compared to patients with a DFI of less than 36 months within the same group and did not find any statistically significant differences. In addition, we identified another potential prognostic factor: patients with a DFI significantly longer than 36 months and normal preoperative CEA levels, who have a single metastasis, may be more suitable for resection of colorectal lung metastases [20]. However, our study did not analyze preoperative CEA levels, and the number of patients with a DFI greater than 36 months was too small to validate this conclusion. Further research with a larger sample size is needed to investigate whether DFI affects the resection of pulmonary metastases from CRC.
A randomized controlled trial conducted in 2022 [21] demonstrated that for lung cancer lesions with a diameter of ≤ 2 cm, segmentectomy is noninferior to lobectomy in terms of survival rates. A recent meta-analysis comparing segmentectomy and lobectomy also mentioned that for lung cancer lesions under 3 cm, both procedures can achieve similar outcomes, and there were no statistically significant differences in prognostic indicators such as survival rates [22]. The results of our study showed that there was a statistically significant difference in tumor size between the segmentectomy and lobectomy groups but no difference in outcome indicators. Although the tumor diameter in the lobectomy group was larger than that in the segmentectomy group, the average size was still below 2 cm. Therefore, we can conclude that the difference in tumor diameter between the two groups did not affect our results.
The lobectomy group exhibited significantly higher values in operative time, intraoperative blood loss, postoperative Day 1 drainage volume, total postoperative drainage volume, and surgical costs compared to the segmentectomy group, with statistically significant differences. These findings are consistent with conclusions drawn from multiple studies [23–25]. Since segmentectomy preserves more lung tissue, it results in less postoperative lung function loss, reduced intraoperative bleeding and postoperative drainage, and faster patient recovery with less compromised lung function. However, there were no statistically significant differences between the two groups in terms of postoperative drainage duration, postoperative pain, and discharge time. We believe that the differences in efficacy and safety between different surgical procedures for patients with CRC lung metastases are insufficient to influence surgeons’ choice of surgical approach. In clinical diagnosis and treatment, the selection of the appropriate surgical resection method for lung metastases should be based on the patient’s condition and individual circumstances.
Surgery is the primary treatment for CRC with lung metastases, with a reported 5-year survival rate ranging from 27% to 68%. However, recurrences are still common after surgery, with a high recurrence rate. In particular, wedge resection of the lung has been reported to have a local recurrence rate of 28% [26], while the recurrence rate after segmentectomy has reached 11% [27]. Although there is limited mention of the recurrence rate following lobectomy for solitary lung metastases from CRC, due to its more extensive resection range, we anticipated a lower recurrence rate in the lobectomy group compared to the segmentectomy group prior to our study. The results showed a recurrence rate of 38.71% in the lobectomy group and 51.61% in the segmentectomy group, but statistical testing indicated no significant difference. This may be related to individual differences among patients in our center and the sample size. Although there were no significant differences in baseline characteristics between the two groups, factors such as preoperative physical condition, postoperative quality of life, use of anticancer drugs, and uncertainties during long-term follow-up may have led to bias in some information. In addition, the difficulty in collecting cases for our study was due to the fact that most patients with CRC lung metastases still opt for wedge resection as their treatment, resulting in a limited number of cases that may not have been sufficient to demonstrate a significant difference between the two groups. In the future, multicenter collaborative studies with larger sample sizes or even large-scale randomized trials could be conducted to verify the impact of different resection techniques on patient outcomes.
Local recurrence and disease progression are significant concerns, and even in patients with negative surgical margins, the likelihood of local recurrence remains substantial [28]. In our study, one patient in the lobectomy group experienced a local recurrence of lung cancer, whereas as many as six patients in the segmentectomy group experienced similar recurrences. This may be attributed to the wider resection margin in the lobectomy group. Some scholars argue that for both small and large metastases, clear margins of 3 mm and 8–10 mm, respectively, are required. Ideally, surgical resection margins should aim for 10 or 20 mm [28]. In addition, Young et al. proposed that the presence of floating cancer cell clusters around the lesion, referred to as adjacent scattered free clusters (ASFCs), constitutes one of the critical factors contributing to the recurrence of metastatic tumors [29]. The impact of local recurrence on survival rates has not been fully studied [30]. Since the type of surgical procedure seems to affect the recurrence rate at the resection margin, every effort should be made to prevent recurrence at the resection margin. In cases where obtaining a satisfactory resection margin is not feasible, lobectomy may be an optimal choice. For the discussion of disease progression, no significant difference was observed between various surgical methods used for pulmonary metastasis resection. The progression of CRC is influenced not only by lung metastases but also by metastases in other locations, such as liver metastases [15]. The complex interplay between the level of follow-up treatment and patient individual differences plays a significant role in disease progression, warranting further investigation and exploration.
Our study has several limitations. First, as a comparative study between two surgical techniques, we did not collect detailed preoperative imaging staging or postoperative pathological staging data for lung metastases. Instead, we only included tumor diameter in our analysis, and the impact of metastatic tumor staging on the prognosis of patients in both surgical groups was not discussed. Second, this study is retrospective and involved a relatively small sample size of 62 cases from a single center, potentially introducing a degree of bias in our results. Third, due to issues such as missing data and retrospective bias, we did not collect statistics on the TNM staging of CRC or surgical approach. Finally, follow-up was conducted primarily through telephone, and there may be issues with patient dropout or information bias, which could potentially affect the results of the survival analysis.
5. Conclusion
In conclusion, for patients with solitary pulmonary metastasis from CRC, the two surgical methods exhibit comparable safety and long-term efficacy, while segmentectomy demonstrates relatively superior short-term efficacy. In clinical practice, for patients unsuitable for pulmonary wedge resection, segmentectomy can be considered based on patient preferences and physician evaluation.
Disclosure
A preprint has previously been published [31].
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Qi Wang is the co-first author.
Funding
The production of this article, including editorial support and open-access fees, was funded by the Gansu Provincial Health Commission Project (Grant no. GSWSKY2020-50), the Gansu Provincial Department of Science and Technology Project (Grant no. 23JRRA1293), and the Hospital Project of Gansu Provincial People’s Hospital (Grant no. 21GSSYB-34).
Open Research
Data Availability Statement
The datasets generated during and analyzed during the current study are not publicly available due to privacy and legal restrictions but are available from the corresponding author on reasonable request




