Improving Quality in Surgical Intensive Care: The Critical Impact of Intensivist Presence and the Semiopen Unit Model
Abstract
Background: It is well known that intensivist in the intensive care unit (ICU) is critical for optimal patient care. However, today the majority of surgical intensive care units (SICUs) provide service in an open model. Our aim was to assess the effects of appointing an intensivist to the SICU and transitioning to a semi-open working model on patient outcomes and various quality indicators.
Methods: This retrospective study was conducted in a seven-bed SICU of a university hospital. Two groups were created from patients treated before (preintensivist period) and after (postintensivist period) the change of ICU management. Demographic data of the patients, disease severity scores, surgical interventions performed, and intensive care quality indicators were collected and statistically compared between the two periods. Reporting of this study complied with the “Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0)” standards.
Results: With the introduction of an intensive care specialist during working hours and the adoption of a semiopen working model, the following improvements were observed: the average length of stay decreased from 11 days versus 5 days, the average duration of mechanical ventilation reduced from 5.6 days versus 1 day, and the rate of mechanical ventilation use decreased from 48% versus 21%. A significant reduction in the need for tracheostomy was also noted (p < 0.001). Furthermore, the bed turnover rate increased from 20 versus 24, enhancing bed utilization efficiency. Additionally, the observed mortality rate of 15% was lower than the expected rate of 25%, resulting in a significant reduction in the standardized mortality ratio (from 1.05 vs. 0.6).
Conclusion: This study proves that surgeon-intensivist collaboration and a semiopen model in ICUs play a critical role by reducing mortality, increasing resource utilization efficiency, and improving patient outcomes. Our findings emphasize the need to restructure SICU processes with a multidisciplinary approach.
1. Introduction
The surgical intensive care unit (SICU) is utilized for perioperative monitoring of both elective and emergency surgical cases, as well as for managing critically injured trauma patients. Additionally, SICUs provide hemodynamic monitoring for critically ill patients, regardless of surgical intervention. Patient profiles in SICUs worldwide share similarities. However, there are variations in the intensive care training and experience levels among physicians who care for critically ill patients in these units [1]. Surgical critical care specialization was established as a surgical specialty by the American Board of Surgery (ABS) in 2008 [2]. In the United States, it has been reported that residents from various surgical disciplines may undergo intensive care rotations averaging 3 months, with some variations ranging from 0 to 15 months [3].
However, surgical residents who complete their 4-year basic specialty education can opt for additional training by enrolling in an intensive care certification program of their choice. Upon successfully passing the certification exam, they attain the status of a surgical intensive care specialist [4]. In our country, physicians with a specialization in general surgery have the option to pursue subspecialty training in intensive care, requiring 3 years of dedicated training. However, the number of general surgeons holding this title remains limited in our country.
The follow-up of critically ill patients in SICUs is typically conducted by the operating surgeon through daily rounds. Due to the scarcity of intensive care specialists, SICUs often operate in an open format. While numerous studies have demonstrated the positive impact of having a full-time intensivist on patient outcomes in mixed medical intensive care units (ICUs), limited data exist on this subject in SICUs [5, 6]. Research suggests that the presence of an intensivist in the ICU environment reduces morbidity and mortality, enhances patient safety, and shortens hospital stays, thereby reducing costs [7, 8].
However, more studies specific to SICUs are needed to validate these findings. Encouraging nonsurgeons to work in SICUs increases multidisciplinary collaboration, and collaboration between the surgeon and the intensivist is critical for optimal patient care, regardless of the core expertise of the intensivist in the ICU [8].
The aims of our study were as follows: First, we aimed to identify the quality indicators commonly used to assess the quality of intensive care processes in SICUs. The quality indicators evaluated included the length of stay (LOS), the mechanical ventilator length of stay (VLOS), the standardized mortality ratio (SMR), the bed occupancy rate (BOR), the bed turnover rate (BTR), the brain death diagnosis rate, the readmission rate (RAR), invasive device utilization rates, and invasive device-related infection rates. Our second objective was to evaluate the impact of transitioning to a semiopen working model on these quality indicators following the assignment of an intensivist to the SICU.
2. Materials and Methods
This study included patients treated in the SICU with seven beds (third step) at the Department of General Surgery, Uludag University Faculty of Medicine, between July 1, 2022, and July 1, 2023. Approval for the study was obtained from the Uludag University Faculty of Medicine Scientific Research Ethics Committee under decision number 2011-KAEK-26/418, dated June 15, 2023. As our study was descriptive and retrospective in nature, specific consent from the included patients was not obtained. However, general informed consent was obtained from all patients upon admission to the hospital, and the study adhered to the ethical principles outlined in the Declaration of Helsinki.
The intensive care specialist was assigned to the general surgery ICU to work 5 days a week during daytime hours. The general surgery team, who were the primary physicians of the patients, conducted daily rounds and provided treatment recommendations. Patients who were monitored both before and 6 months after the assignment of the intensivist (January 1, 2023) were divided into two groups. The first group (preintensivist group) consisted of patients admitted in the 6-month period before the intensivist appointment, while the second group (postintensivist group) consisted of patients admitted in the 6-month period after the intensivist started working in the ICU. Initial hospitalization data were collected for patients with multiple hospitalizations. Additionally, the number of patients readmitted within 48 h and 30 days after discharge was documented. Demographic characteristics, reasons for SICU admission, comorbidities, surgical interventions, LOS, VLOS, and treatment outcomes were retrospectively retrieved from the electronic records of the hospital information management system.
Expected mortality rates in ICUs vary considerably depending on patient demographics and the severity of illness. For instance, the expected mortality rate for a young polytrauma patient without comorbidities differs significantly from that of an elderly patient with gastrointestinal perforation, chronic renal failure, and cardiac arrhythmias. Therefore, the crude mortality rate alone is not a reliable indicator of quality. Moreover, in the final stages of illness, when all treatment options have been exhausted, the focus shifts from preventing mortality to providing palliative care aimed at alleviating suffering and ensuring a dignified death. To evaluate mortality data as an indicator of quality, it is essential to calculate the average mortality expectancy for a patient population. In our study, we used the APACHE II severity of the illness scoring system to predict expected mortality for all included patients. The APACHE II scoring system was used to assess the risk of ICU mortality because of its compatibility with the mandatory performance indicators of the Turkish Ministry of Health and the hospital information management system. All parameters used in scoring were obtained from routinely recorded data in electronic patient records. The APACHE II score is calculated based on clinical and laboratory values within the first 24 h after a patient’s admission to the ICU and represents the patient’s expected mortality as a percentage [9]. We calculated SMRs (SMR = observed mortality rate/expected mortality rate) by comparing the mortality expectation based on APACHE II scores with the actual mortality in both study groups.
Furthermore, quality indicators assessing the quality of intensive care processes were calculated using the formulas outlined in Table 1. The Pabon Lasso model (PLM), which integrates BOR, BTR, and LOS criteria, was employed to evaluate the efficiency of our intensive care bed utilization [10]. Quality indicators concerning invasive device utilization rates and infectious complications associated with invasive devices were obtained from Uludag University Faculty of Medicine’s Infection Control Committee reports. Data on invasive device utilization rates, hospital-acquired infections related to invasive devices, and the incidence rates of patients diagnosed with brain death were collected.
| Expected mortality ratio: Following the calculation of the Acute Physiologic and Chronic Health Evaluation II (APACHE II) score (between 0 and 71), the adjusted expected mortality rate is calculated by writing the “y” value corresponding to the current diagnosis in the relevant box from the table prepared for the diagnosis category weight. |
| Observed mortality ratio: In the relevant period, (number of patients who passed away in the intensive care unit/patients transferred from the previous month in the intensive care unit (ICU) + total number of patients hospitalized within 6 months) × 100 |
| Standardized mortality rate (SMR): Observed mortality ratio/expected mortality ratio |
| Number of hospitalization days: The admission date is subtracted from the patient’s discharge date, and the patient’s admission day is counted but not the discharge day; when a patient is admitted and discharged on the same day, the patient’s hospitalization day is considered as one. |
| Length of stay (LOS): Number of days hospitalized/number of patients discharged, transferred to the ward, transferred to another institution, and deceased |
| Bed occupancy ratio (BOR): Number of hospitalized days × 100/180 days × number of patient beds |
| Readmission rate (RAR): Number of patients readmitted to the ICU for the same disease or related complications within 48 h or 30 days after discharge or referral × 100/total number of patients discharged or referred from the ICU. |
| Pressure ulcer ratio: Total number of patients with pressure ulcers/total number of hospitalized patients) × 100 (the number of patients from the previous month is also added, and the number of patients who developed pressure ulcers before the relevant month and those who have ongoing pressure ulcers are included in the number of patients who developed pressure ulcers) |
| CVC utilization ratio: Central venous catheter (CVC) days/patient days |
| CLABSI ratio: Number of central line–associated blood stream infections (CLABSI)/days of central venous catheter use × 1000 |
| Ventilator length of stay (VLOS): Mechanical ventilator (MV) days/patient days |
| Invasive MV utilization ratio (VUR): Total MV days/number of patients monitored with MV |
| VAP ratio: Number of ventilator-associated pneumonia (VAP) infections × 1000/MV day |
| Rates of brain death: Number of patients evaluated for brain death in the relevant period/number of patients hospitalized in intensive care) × 100 |
2.1. Statistical Methods
Quantitative data obtained from two independent study groups were assessed for normality using the Shapiro–Wilk and Kolmogorov–Smirnov tests. Non-normally distributed quantitative data were analyzed using the Mann–Whitney U test. Quantitative data were summarized as mean ± standard deviation. Infections associated with invasive device use were reported as both the number of cases (n) and the proportion (%) of cases. The chi-square test was employed to evaluate qualitative variables in two independent study groups, while the Fischer exact test was used for indicators that did not meet certain assumptions. We first applied a univariate logistic regression model to identify variables correlated with mortality among patients in the SICU. Subsequently, a multivariate regression model was employed to assess the correlated variables and determine the independent factors influencing mortality. Results from qualitative data analyses were presented proportionally. In the analyses, a p value of < 0.05 was considered statistically significant. All analyses were conducted using IBM SPSS Statistics for Windows, Version 28 (IBM Corp., Armonk, NY, USA).
3. Results
This study included a total of 242 patients who were monitored in the SICU and managed by the Department of General Surgery over a one-year period. Of these, 125 patients were followed during the postintensivist period, after an intensivist was appointed to the SICU and a transition to a semiopen working model was implemented. Details regarding comorbid diseases, reasons for hospitalization, and operations performed in the study groups are presented in Table 2. Hypertension, diabetes mellitus, cardiovascular diseases, and malignancies were the most common comorbidities in both groups. The only statistically significant difference between the study groups regarding comorbidities was the higher prevalence of cancer diagnoses in the preintensivist period (23.9% vs. 12%, p = 0.015). Additionally, the number of blunt trauma cases (12.8% vs. 25.6%, p = 0.005) and patients hospitalized for liver mass resection (0.9% vs. 8%, p = 0.008) was significantly higher in the postintensivist period. Forty-three percent of the patients underwent urgent surgery, 26.8% underwent elective surgery, and 30.1% were hemodynamically monitored without surgical intervention. The proportion of patients hospitalized due to trauma (blunt trauma and penetrating injury) was 23.9%.
| Comorbid diseases | Preintensivist period | Postintensivist period | p | Total | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| n | 117 | 48.3 | 125 | 51.7 | 242 | 100 | |
| Hypertension | 31 | 26.5 | 43 | 34.4 | 74 | 30.6 | |
| Diabetes | 19 | 16.2 | 26 | 20.8 | 45 | 18.5 | |
| Cancer (solid/haematological) | 28 | 23.9 | 15 | 12.0 | 43 | 17.7 | |
| Coronary artery disease | 12 | 10.3 | 12 | 9.6 | 24 | 9.9 | |
| Heart failure: Dysrhythmia | 12 | 10.3 | 8 | 6.4 | 20 | 8.2 | |
| COPD: Asthma | 7 | 6.0 | 8 | 6.4 | 15 | 6.2 | |
| Cerebrovascular accident | 4 | 3.4 | 4 | 3.2 | 8 | 3.3 | |
| Dementia | 5 | 4.3 | 1 | 0.8 | 0.111f | 6 | 2.5 |
| Chronic renal failure | 3 | 2.6 | 2 | 1.6 | 5 | 2 | |
| Rheumatic disease | 4 | 3.4 | 1 | 0.8 | 5 | 2 | |
| Inflammatory bowel disease | 4 | 3.4 | 0 | 0.0 | 0.053f | 4 | 1.6 |
| Liver failure | 1 | 0.9 | 2 | 1.6 | 3 | 1.2 | |
| Goiter | 1 | 0.9 | 1 | 0.8 | 2 | 0.8 | |
| Reason for admission and operation performed | |||||||
| Emergency surgery | 50 | 42.7 | 54 | 43.2 | 104 | 42.9 | |
| Nonoperative follow-up | 37 | 31.6 | 36 | 28.8 | 73 | 30.1 | |
| Elective surgery | 30 | 25.6 | 35 | 28.0 | 65 | 26.8 | |
| Blunt trauma | 15 | 12.8 | 32 | 25.6 | 47 | 19.4 | |
| Perforation | 18 | 15.4 | 17 | 13.6 | 35 | 14.4 | |
| Ileus | 21 | 17.9 | 12 | 9.6 | 33 | 13.6 | |
| Sepsis | 9 | 7.7 | 7 | 5.6 | 16 | 6.6 | |
| Mesenteric ischemia | 6 | 5.1 | 8 | 6.4 | 14 | 5.7 | |
| Whipple procedure | 7 | 6.0 | 6 | 4.8 | 13 | 5.3 | |
| Liver mass resection | 1 | 0.9 | 10 | 8.0 | 11 | 4.5 | |
| Fournier’s gangrene debridement | 6 | 5.1 | 5 | 4.0 | 11 | 4.5 | |
| Penetrating injury | 5 | 4.3 | 6 | 4.8 | 11 | 4.5 | |
| Cholecystitis/cholecystectomy | 6 | 5.1 | 4 | 3.2 | 10 | 4.1 | |
| Colon malignancy/colectomy | 3 | 2.6 | 7 | 5.6 | 10 | 4.1 | |
| Others | 17 | 14.5 | 14 | 11.2 | 31 | 12.8 | |
- Note: Bold values indicate statistically significant results (p < 0.05).
- Abbreviation: COPD, chronic obstructive pulmonary disease.
- fFisher’s exact test.
- Chi-square test.
Demographic characteristics, clinical follow-up data, and quality indicators reflecting intensive care processes for patients in both groups are summarized in Table 3, facilitating comparison between the pre- and postintensivist periods. There was no significant difference between the study groups regarding demographic data and APACHE II scores.
| Demographic data and quality indicators | Preintensivist period | Postintensivist period | p |
|---|---|---|---|
| n | (117) | (125) | |
| Age | 59.05 ± 19.17 | 56.53 ± 19.44 | 0.307m |
| Male | (86) − 73% | (84) − 67.20% | |
| APACHE II | 11.21 ± 9.05 | 12.03 ± 9.95 | 0.706m |
| Length of stay (LOS) (days) | 10.97 ± 20.58 | 5.06 ± 10.89 | 0.004m |
| Expected mortality ratio | 21.99% | 25.31% | |
| Observed mortality ratio | (27) − 23.1% | (19) − 15.2% | |
| Standardized mortality ratio (SMR) | 1.05 | 0.6 | |
| Ventilator length of stay (VLOS) (days) | 5.63 ± 17.28 | 1.06 ± 2.82 | <0.001m |
| Ventilator utilization ratio (total VLOS/total LOS) | 47.80% | 20.8% | 0.047m |
| Tracheostomy opening (intubated patient) | (8) − 23.5% | (1) − 3.1% | <0.001f |
| Ventilator-associated pneumonia (VAP) ratio | (2) − 2.52% | (1) − 2.31% | |
| Readmission rate (RAR) within 48 h | (1) − 0.8% | (2) − 1.6% | |
| Readmission rate (RAR) within 30 days | (8) − 6.8% | (6) − 4.8% | |
| Central venous catheter utilization ratio | 74% | 74% | |
| Central line–associated blood stream infections | (9) − 11.8% | (4) − 5.2% | |
| Newly developed pressure ulcer rate | (6) − 5.1% | (4) − 3.2% | |
| Proportion of patients evaluated for brain death | (0) − 0% | (2) − 1.6% | |
| Bed occupancy ratio (BOR) | 100% | 92.4% | |
| Followed by other departments (excluded patient) | (24) | (43) | |
| Bed turnover rate (BTR) | 20 | 24 |
- Note: Values are presented as mean ± SD or (n) − %. APACHE II: Acute Physiologic and Chronic Health Evaluation II score. Bold values indicate statistically significant results (p < 0.05).
- Abbreviation: SD, standard deviation.
- fFisher’s exact test.
- mMann–Whitney U test.
- Chi-square test.
In the postintensivist period, the LOS, ventilator utilization ratio, VLOS, and SMR significantly decreased. Additionally, the bed occupancy ratio decreased, while the BTR increased.
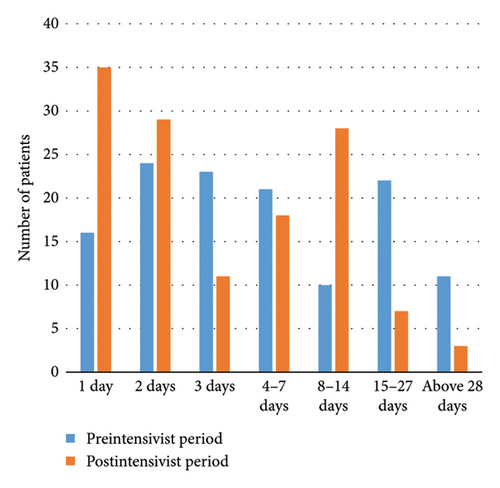
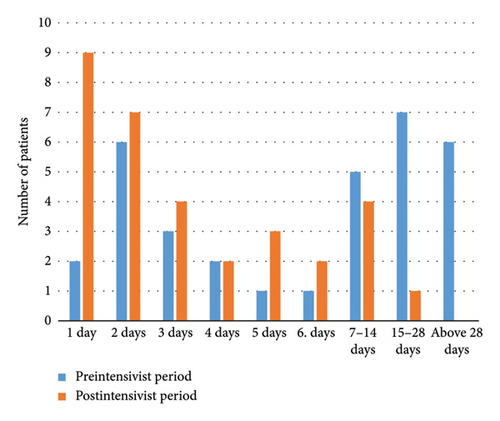
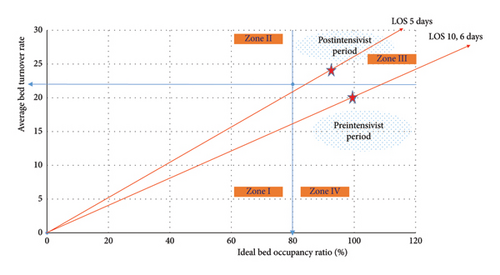
The intensive care follow-up periods of patients in both study groups are depicted in Figure 1(a), while the VLOS for patients in both groups is illustrated in Figure 1(b). Additionally, Figure 2 presents the PLM, allowing for a comparison of intensive care bed usage efficiency between the pre- and postintensivist periods.
The results of logistic regression analyses identifying factors and independent variables associated with mortality are presented in Table 4. Diabetes, VLOS, and APACHE II were found to be independent variables associated with mortality. It appears that shortening the duration of mechanical ventilation reduces the risk of mortality.
| Variable | UnivariateLR OR (95% CI) |
p | MultivariateLR OR (95% CI) |
p |
|---|---|---|---|---|
| VLOS | 0.827 (0.761–0.899) | < 0.001 | 0.841 (0.754–0.939) | 0.002 |
| Diabetes | 0.249 (0.121–0.510) | < 0.001 | 0.131 (0.031–0.555) | 0.006 |
| APACHE II | 0.804 (0.759–0.853) | < 0.001 | 0.842 (0.738–0.863) | < 0.01 |
| Age | 0.964 (0.946–0.983) | < 0.001 | 0.969 (0.944–1.008) | 0.134 |
| LOS | 0.971 (0.953–0.989) | 0.020 | 1.058 (0.990–1.131) | 0.096 |
| Hypertension | 0.280 (0.144–0.544) | < 0.001 | 1.173 (0.353–3.903) | 0.709 |
| Coronary artery disease | 0.341 (0.139–0.837) | 0.019 | 0.461 (0.096–2.211) | 0.333 |
| Cancer diagnosis | 0.616 | |||
| Gender | 0.059 |
- Note: APACHE II, Acute Physiologic and Chronic Health Evaluation II score. Bold values indicate statistically significant results (p < 0.05).
- Abbreviations: CI, confidence interval; LOS, length of stay; OR, odds ratio; VLOS, ventilator length of stay.
- LRLogistic regression.
4. Discussion
SICUs have a different patient profile from mixed ICUs and therefore have a unique operational process. If a general surgeon or intensive care specialist is not permanently assigned to the SICU, these units typically operate in an open-model ICU. Therefore, these units are important candidates for quality improvement efforts. Beyond the quality of surgical skills, the quality of postoperative care contributes significantly to morbidity and mortality [11]. This research focused on the period when an intensivist was appointed to the SICU and the ICU management model changed from open to semiopen.
A 54% reduction in the LOS, a 56% decrease in mechanical ventilation use, and an 82% decline in the VLOS were observed. These improvements in quality indicators are remarkable. Additionally, reductions were noted in VAP rates, the SMR, and the RARs. The significant decrease in tracheostomy requirements was associated with reductions in both the VLOS and the average LOS. Another benefit of reducing the LOS was a lower incidence of new pressure ulcers. Furthermore, ICU bed utilization efficiency improved, and the BOR decreased. These findings highlight the substantial benefits that SICUs can achieve through quality improvement initiatives.
The decrease in the SMR value during the postintensivist period, when the intensive care approach changes, is an important quality indicator in assessing the quality of intensive care processes. Such a significant reduction in the SMR over a short period of 6 months is a positive outcome of the collaboration between surgeons and intensive care specialists. The proportion of patients diagnosed with malignancy was significantly higher in the preintensivist period, which could be expected to result in higher predicted mortality in this group. However, no significant difference was found between the study groups in terms of mortality expectancy calculated using the APACHE II score. Moreover, logistic regression analysis did not identify cancer diagnosis as an independent predictor of mortality. Therefore, we concluded that the difference in malignancy prevalence between the study groups did not constitute a significant confounding factor in the comparison of mortality-related quality indicators.
The marked reduction in both the need for and duration of mechanical ventilation is a key outcome of collaboration with the intensive care specialist. Comparatively, a comprehensive study conducted across 42 ICUs reported a range of the mean VLOS between 2.6 and 7.9 days [12].
In this study, alongside a reduction in the LOS in the postintensivist period group, we noted an increase in the BTR. The BTR signifies how frequently intensive care beds are utilized within a specific timeframe and is a crucial indicator of efficiency. The decrease in the mean LOS and the rise in the BTR contributed to a decrease in the BOR. Ratios exceeding this threshold can lead to staff burnout and an elevated risk of nosocomial infections, whereas ratios falling below this threshold can diminish bed utilization efficiency [13].
In addition to these drawbacks, a BOR of 100% may lead to a lack of space when an intensive care bed is required, an increase in the patient referral rate, and postponement of elective operations. In the second study group, both the number of patients followed up by the general surgery department and the number of patients followed up by other departments increased. The increase in these numbers is evidence of more efficient utilization of beds even without full capacity utilization (92.4%).
In 1986, Lasso developed an analysis method called PLM, which provides an idea about the efficient utilization of beds by taking advantage of the mathematical relationship between three quality indicators [9].
The PLM graph, amalgamating the quality indicators of the BOR, the BTR, and the LOS determined before and after the intensivist period in our clinic, is presented in Figure 2. This method enables comparison of data from at least two periods, revealing an increase in bed utilization efficiency during the postintensivist period.
In the preintensivist period, no brain death evaluations were performed, whereas two patients were evaluated for brain death in the second group. Notably, it has been reported that, between 2012 and 2016, one in every 50 hospital deaths in the United States was attributed to brain death [14]. An intensivist may play a role in the early detection of brain death and in providing high-quality end-of-life care while managing patients in a closed ICU model. Routine practices of intensivists who spend all their shifts in the ICU include assessing the severity of coma and evaluating brainstem reflexes such as pupillary light response, spontaneous respiration, pharyngeal gag reflex, and tracheal cough reflex. Therefore, the presence of an intensivist increases the likelihood of earlier brain death recognition.
Another quality indicator that is relatively frequently evaluated is the RAR within 48 h. Readmission to the ICU after discharge not only hampers the efficient utilization of available beds but also escalates the risk of mortality [15]. It has been proposed as an important quality indicator for ICU efficiency [16]. Data on the RAR in general surgery patients after discharge from the ICU are indeed limited. In a recent study, it was reported that the rate of the RAR or unexpected death within 72 h was 10.9% among patients discharged from the ICU after emergency general surgery operations [17]. It is worth noting that all patients in this study were emergency cases, and their mean APACHE II scores were notably high (27 points), which may have contributed to the elevated RAR.
In the literature, RARs reported between 48 and 72 h in non-SICU, ICUs typically range between 2% and 6.6%. These findings underscore the importance of further research to better understand and address RARs, particularly among general surgery patients following ICU discharge [18–20]. When compared with these referenced studies, the RAR in our clinic falls within acceptable limits. For instance, a Canadian study reported a 6% RAR in the follow-up of a total of 601 patients across eight different general surgery centers. In our study, the 30-day RARs were 6.8% and 4.8%, respectively [21]. However, it is worth noting that two of the readmission cases in the second group were planned hospitalizations for a two-stage liver mass resection procedure (associating liver partition and portal vein ligation = ALPPS).
5. Conclusions
This study proves that surgeon-intensivist collaboration and a semiopen model in ICUs play a critical role by reducing mortality, increasing resource utilization efficiency, and improving patient outcomes. Our findings emphasize the need to restructure SICU processes with a multidisciplinary approach.
Disclosure
In the reporting of this study, “Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0)” standards were complied with. The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding or grants were received for the conduct of this study.
Acknowledgments
The authors acknowledge the Uludag University Infectious Diseases and Clinical Microbiology Department, Infection Control Committee, and the nurse Habibe Imer for her contributions to obtaining our clinic’s invasive device use rates and invasive device-related infection rates.
Open Research
Data Availability Statement
The data associated with this research project are being made available to promote transparency and facilitate further scientific inquiry. For inquiries regarding access to the data or any additional information, do not hesitate to get in touch with the corresponding author.




