The Improvement of General Condition and Immunity Function of CKD After Treatment With Bailing Capsule: A Randomized Clinical Trial
Abstract
Background: The management of chronic kidney disease (CKD) presents significant challenges, with people often exploring new treatment strategies and medications in an attempt to slow disease progression. This study seeks to evaluate the efficacy of Bailing capsules in the treatment of CKD.
Methods: A total of 148 patients with CKD and renal and spleen qi insufficiency were included in this study. The experimental group (n = 87) received Bailing capsules in addition to the standard treatment, while the control group (n = 61) received only the standard treatment. The study used both subjective and objective endpoints, including traditional Chinese medicine (TCM) syndrome scores, routine blood tests, biochemical markers, and lymphocyte counts, to evaluate the efficacy of Bailing capsules.
Results: After statistical analysis, we found that the experimental group showed a significant decrease in TCM syndrome scores and in various biochemical indices, including reduced levels of cystatin C, 24-h urine protein, bilirubin, gamma-glutamyl transferase, and urinary red blood cell count (p < 0.05). Additionally, the experimental group demonstrated an increase in white blood cell counts and regulation of immune function markers, such as CD3, CD4, and CD19 (p < 0.05).
Conclusion: After treatment, the relevant indicators of CKD showed significant improvement, and the patients’ immune function was also partially regulated. Notably, no significant adverse reactions were observed that would necessitate the termination of the study. Further investigation is required to gain a deeper understanding of these findings, particularly regarding the effects of Bailing capsules on immune function regulation in CKD.
Trial Registration: Chinese Registry of Clinical Trials: ChiCTR-IPR-17014157
1. Introduction
Chronic kidney disease (CKD), affecting approximately 700 million people globally, disproportionately impacts socially disadvantaged populations, who face higher incidence rates, greater treatment barriers, and poorer health outcomes [1–5]. It is characterized by a progressive decline in glomerular filtration rate (GFR) and structural abnormalities in the kidneys [6].
The efficacy of the current therapeutic approaches is limited, primarily focusing on blood pressure management and dietary restrictions (protein, salt, and fats). This highlights the urgent need for safer and more effective treatments to halt the progression of renal failure [7]. Renin–angiotensin–aldosterone system (RAAS) inhibitors play a pivotal role in managing hypertension, diabetes, and other conditions that can lead to CKD. Currently, RAAS inhibitors are strongly recommended for CKD patients with comorbidities such as diabetes, hypertension, and albuminuria [8]. These agents provide renal protection and offer cardiovascular benefits [9, 10]. However, RAASi carries the risk of hyperkalemia. To alleviate RAASi-related hyperkalemia, combination therapy with potassium-binding agents, such as patiromer and sodium zirconium cyclosilicate, may be used alongside the treatment. Notably, in patients with diabetic kidney disease, finerenone, a selective oral nonsteroidal mineralocorticoid receptor antagonist, has shown clinical efficacy in reducing the risk of cardiovascular events and slowing CKD progression [7]. Beyond lowering blood sugar, SGLT2 inhibitors are a novel and expanding class of drugs with additional clinical benefits, including heart and kidney protection. Despite the essential role of these medications in treating CKD, there remains a risk of disease progression, highlighting the need for additional therapeutic options.
Both historical and contemporary research studies suggest that traditional Chinese medicine (TCM) can effectively halt the progression of CKD, reduce adverse reactions, and improve patient survival and overall health. Bailing capsules, made by fermenting Cordyceps sinensis strains at low temperatures, are known to promote kidney and lung health, as well as enhance vitality and energy (essence and qi) [11–13]. The research has confirmed that Bailing capsules are rich in amino acids, nucleotides, polysaccharides, and cordyceps acid. These ingredients regulate immune function and have antioxidant properties, making them beneficial for improving lipid and protein metabolism [14, 15]. The aim of this study is to evaluate the therapeutic effect of Bailing capsules on CKD.
2. Materials and Methods
2.1. Participants
The clinical study, conducted from December 2017 to October 2021, included 148 CKD patients who were randomly assigned to either the experimental or control group. Participants were adults aged 18–70 with Stages 3-4 CKD (eGFR: 15–60 mL/min/1.73 m2) and provided informed consent, in accordance with the study’s inclusion criteria. The exclusion criteria for the study were as follows: (1) Diagnosis of acute kidney injury (AKI), defined by an increase in blood creatinine ≥ 0.3 mg/dL within 48 h, a rise to more than 1.5 times the baseline value within 7 days, or urine output < 0.5 mL/kg/h for more than 6 h; (2) life-threatening complications, including severe infections, newly diagnosed malignancies, significant water and electrolyte imbalances, or major organ dysfunction involving the heart, brain, liver, hematopoietic system, or gastrointestinal tract; (3) known hypersensitivity to any study drug; (4) history of psychiatric illness; (5) pregnancy or lactation; and (6) participation in other clinical trials. Before the study began, a comparison of baseline data between the two groups showed no significant differences in sex, age, or disease duration (p > 0.05). The study was approved by the local ethics committee and adhered to the guidelines set forth in the Declaration of Helsinki. All participants were fully informed about the study’s objectives, methods, and potential risks before enrollment. Written informed consent was obtained from each participant to ensure voluntary and well-informed participation. The study was conducted at a national tertiary hospital in China.
2.2. Source of the Experimental Drug
In this study, Bailing capsules were produced and supplied by Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd.
2.3. Trial Design
A randomized pre- and postcontrol design was employed in this multicenter study. The sample size was determined based on statistical power assumptions: (1) maximum degree of variation: 0.5, (2) 90% effectiveness, and (3) error margin: 3%. Using the simple random sampling formula, N = Z2 × (P × (1 − P))/E2, and accounting for the anticipated clinical loss rate, the total number of participants was estimated at approximately 270. A random number table generated using Excel software was used for patient assignment. Enrolled patients were assigned numbers according to the random number table, with odd-numbered patients placed in the control group and even-numbered patients in the experimental group. A total of 148 volunteers were randomized into two groups: the experimental group (n = 87) and the control group (n = 61). The experimental group received Bailing capsules in addition to the standard treatment, while the control group received only the standard treatment. Both groups underwent a 48-week treatment period concurrently (Figure 1). The recruitment coordinator (nonblinded) was responsible for patient allocation and medication management. Both participants and the principal investigator/study doctor were blinded to the allocation throughout the study. Each dose consisted of six Bailing capsules (0.5 g each), taken three times a day, along with other medications as per medical advice. After 48 weeks of treatment, the efficacy of Bailing capsules in slowing the progression of CKD was assessed.

Standard treatment for both the experimental and control groups included the following for CKD management: (1) Dietary Nutrition: According to the Chinese “Expert Consensus on Protein Nutrition Therapy for Chronic Kidney Disease,” the recommended daily protein intake is 0.6–0.8 g/kg·d, with more than 50% of the protein coming from high biological value sources. In addition, a low-protein diet should be supplemented with a caloric intake of 30–35 kcal/kg·d. (2) Blood Pressure Control: For patients with hypertension, the JNC VII and K/DOQI guidelines recommend targeting blood pressure levels of 130/80 mmHg or lower (1 mmHg = 0.133 kPa). For those with significant proteinuria, even stricter control is advised, with a target below 125/75 mmHg. Angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin receptor blockers (ARBs) should be considered first-line antihypertensive agents. If the target blood pressure is not achieved, additional medications, such as calcium channel blockers or other antihypertensive drugs, may be required. (3) Lipid Control: In patients with hyperlipidemia, the 1997 Chinese Lipid Prevention Guidelines and the U.S. National Cholesterol Education Program’s Third Report (NCEP ATP III) (May 2001) recommend the following target levels for lipid markers: total cholesterol < 5.72 mmol/L (< 220 mg/dL), LDL cholesterol < 3.64 mmol/L (< 140 mg/dL), and triglycerides < 2.26 mmol/L (< 200 mg/dL). Lipid-lowering agents, such as atorvastatin (20 mg/day), should be used. (4) Blood Sugar Control: For patients with diabetes, the 2013 Chinese Guidelines for the Management of Type 2 Diabetes recommend the following targets: fasting blood glucose < 7.0 mmol/L, postprandial blood glucose < 10.0 mmol/L, and glycated hemoglobin < 7.0%.
Effective cases were followed up for 6 months after the completion of the treatment. The discontinuation criteria included death, progression to end-stage renal disease (ESRD) with eGFR less than 15 mL/min/1.73 m2 or the need for maintenance renal replacement therapy (hemodialysis, peritoneal dialysis, or kidney transplant), and a 40% decrease in eGFR indicating progressive renal failure (eGFR [mL/min/1.73 m2] = 186 × [Scr−1.154] × [Age−0.203] × [0.742, if female]). The secondary endpoints included cardiovascular events, cerebrovascular events, and peripheral artery disease.
2.4. Observation Indicators
In this study, the evaluation indicators include both subjective and objective measures, such as TCM syndrome scores, complete blood count, urinalysis, biochemical profiles, lymphocyte counts, and others. These indices provide a comprehensive foundation for assessing the effects of Bailing capsules on CKD progression. In the event of adverse reactions, the investigator may implement appropriate measures, such as dose adjustments, temporary interruption of medication, or other interventions, and decide whether to terminate the trial.
2.4.1. Comparison of the Subjective Indicators Between Two Groups
Subjective indicators, also referred to as sensory indices, are measurements based on the patient’s perceptions and clinical assessments, rather than being directly quantified. One key subjective indicator in this study is the TCM symptom rating, which reflects a negative correlation between the scores and the general condition of CKD patients. An improvement in the patient’s condition typically corresponds to a decrease in the relevant TCM symptom scores.
In the assessment of kidney yin deficiency syndrome (TCM syndrome scores), a symptom scoring system based on established guidelines was employed, covering symptoms such as soreness and weakness in the waist and knees, restlessness and heat in the palms, soles, and chest; dry mouth and throat; dizziness; tinnitus; hearing loss; night sweats; emaciation; insomnia; and tongue appearance and pulse condition [16]. Each symptom was categorized into mild, moderate, and severe levels, with corresponding scores of one, two, and three points, respectively.
2.4.2. Detection and Analysis of the Related Objective Indicators Between Two Groups
In this study, the researchers aimed to analyze the objective indicators in both groups before and after treatment with Bailing capsules. These objective indicators included routine blood tests, urinalysis, biochemical profiles, and lymphocyte counts. Specifically, they measured white blood cell (WBC) and lymphocyte counts, 24-h urinary protein, urinary red blood cell counts, and levels of cystatin C, total cholesterol, triglycerides, total bilirubin, and conjugated bilirubin (CB). Immune function parameters, including CD3, CD4, and CD19, as well as their respective percentages, were also analyzed [17]. The number of lymphocytes and WBCs was obtained through routine blood tests, while the percentages of CD3, CD4, and CD19 were determined using flow cytometry. Additionally, the number of CD4 cells was calculated using the formula: Number of CD4 cells = (CD4% × Total number of lymphocytes).
2.5. Statistical Methods
Statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) to compare differences between the experimental and control groups. Pearson’s Chi-square or Fisher’s exact test was used as appropriate. One-way analysis of variance (ANOVA) was employed to assess differences in relevant indicators between the two groups, followed by post hoc analysis. The classification of variables was performed using the Chi-square test. A p value of less than 0.05 was considered statistically significant. All statistical analyses were conducted in strict accordance with research standards and guidelines.
3. Results
3.1. Demographic Characteristics
In this study, a total of 148 participants were included and randomly assigned to the control group (n = 61) and the experimental group (n = 87). As shown in Table 1, no significant differences were observed in sex, age, or disease duration between the two groups (p > 0.05).
| Control group (n = 61) | Experimental group (n = 87) | p | |
|---|---|---|---|
| Gender (male/female) | |||
| Male (%) | 24 (40%) | 37 (43%) | 0.828 |
| Female (%) | 37 (60%) | 50 (57%) | |
| Age (years) | 55.73 ± 11.33 | 54.37 ± 10.96 | 0.467 |
| Marital status (married) | |||
| Married (%) | 59 (97%) | 81 (93%) | 0.556 |
| Unmarried (%) | 2 (3%) | 6 (7%) | |
| Duration of disease (years) | 5.07 ± 4.80 | 5.85 ± 4.51 | 0.320 |
| Blood urea nitrogen (mmol/L) | 11.29 ± 3.88 | 12.53 ± 4.58 | 0.078 |
| Uric acid (μmol/L) | 388.74 ± 95.47 | 413.99 ± 90.92 | 0.109 |
| Creatinine (μmol/L) | 175.09 ± 66.21 | 187.86 ± 57.89 | 0.227 |
| eGFR (ml/min/1.73 m2) | 42.68 ± 17.05 | 39.58 ± 13.02 | 0.234 |
| FPG (mmol/L) | 6.78 ± 3.1 | 5.98 ± 2.72 | 0.107 |
| Comorbid conditions (n) | |||
| Diabetes mellitus | 27 (44%) | 28 (32%) | 0.186 |
| Hypertension | 31 (51%) | 22 (25%) | 0.003 |
| Chronic glomerulonephritis | 27 (44%) | 16 (12%) | 0.001 |
| Gouty nephropathy | 4 (6%) | 6 (7%) | 0.948 |
3.2. Comparison of TCM Syndrome Points Before and After Treatment
The results showed a significant reduction in TCM syndrome scores in the experimental group after treatment, indicating improvement compared to pretreatment scores. Statistical analysis revealed significant symptom improvement following Bailing capsule treatment (p < 0.05), as shown in Table 2.
| Groups | n | Pretreatment | Post-therapy | ||
|---|---|---|---|---|---|
| Mean value | SD | Mean value | SD | ||
| Experimental group | 87 | 15.65 | 8.67 | 8.15△ | 5.94 |
- △p < 0.05 for intragroup comparison.
3.3. Comparison of Cystatin C Before and After Treatment
The primary clinical significance of cystatin C is its ability to reflect the GFR, making it a diagnostic marker for evaluating renal function, with higher specificity and sensitivity than creatinine. Table 3 presents the results of post-treatment analysis, which showed a substantial decrease in cystatin C levels in the experimental group compared to both pretreatment levels and the control group, with a statistically significant difference (p < 0.05).
| Groups | n | Cystatin C (mg/L) | |||
|---|---|---|---|---|---|
| Pretreatment | Post-therapy | ||||
| Mean value | SD | Mean value | SD | ||
| Control group | 61 | 2.32 | 0.78 | 3.14∗ | 1.15 |
| Experimental group | 87 | 2.82 | 4.11 | 2.59△∗ | 0.88 |
- ∗p < 0.05 for comparisons across groups.
- Δp < 0.05 for comparisons within groups.
3.4. Evaluation of 24-h Urine Protein Levels Before and After Therapy
The results of the investigation showed that 24-h urine protein levels in the experimental group significantly decreased after treatment with Bailing capsules (p < 0.05). As shown in Table 4, however, the control group exhibited a significant increase in urine protein levels compared to pretreatment values (p < 0.05).
| Groups | n | 24-h urine protein quantification (mg/24 h) | |||
|---|---|---|---|---|---|
| Pretreatment | Post-therapy | ||||
| Mean value | SD | Mean value | SD | ||
| Control group | 61 | 1482.47 | 1824.85 | 1765.93△ | 2028.43 |
| Experimental group | 87 | 1793.18 | 1597.62 | 1623.06△ | 1390.99 |
- △p < 0.05 for comparisons within groups.
3.5. Evaluation of the Amount of Red Blood Cells in the Urine Before and After Therapy
The data revealed a significant decrease in urinary red blood cell counts in the experimental group compared to both the control group and pretreatment values, with a statistically significant difference (p < 0.05), as shown in Table 5.
| Groups | n | Urinary red blood cell count (/uL) | |||
|---|---|---|---|---|---|
| Pretreatment | Post-therapy | ||||
| Mean value | SD | Mean value | SD | ||
| Control group | 61 | 59.21 | 141.07 | 46.45 | 146.29 |
| Experimental group | 87 | 27.08 | 72.02 | 19.3△∗ | 33.2 |
- △p < 0.05 for comparisons within groups.
- ∗p < 0.05 for comparisons across groups.
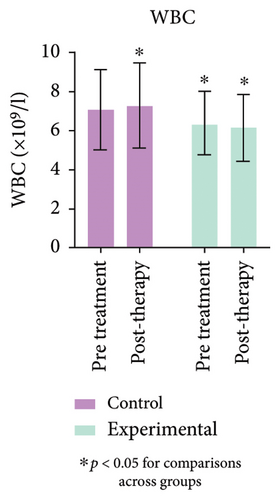
3.6. Comparison of Blood WBCs and Lymphocytes Before and After Treatment
The blood routine results demonstrated a significant decrease in WBC and lymphocytes (L) in the experimental group compared to the control group (p < 0.05). Similarly, Figure 2 (Supporting Table 1) shows that lymphocyte counts in the experimental group were significantly lower after treatment than before (p < 0.05).

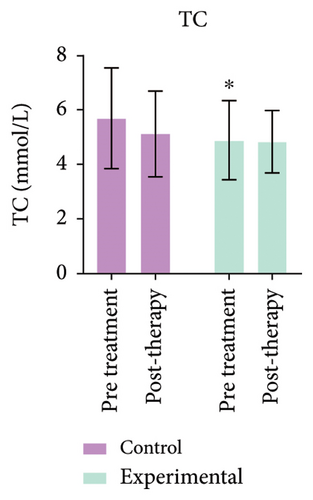
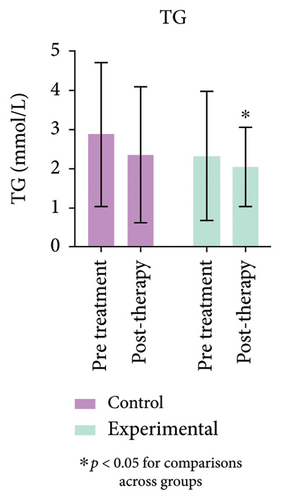
3.7. Comparison of Total Cholesterol and Triglycerides Before and After Treatment
Triglyceride levels in the experimental group were significantly reduced compared to the control group, with a statistically significant difference (p < 0.05). As shown in Figure 3 (Supporting Table 2), total cholesterol levels in the experimental group showed no change before or after treatment, indicating no statistical significance (p > 0.05).
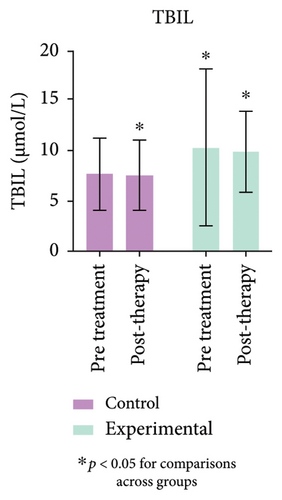
3.8. Comparison of Total Bilirubin, CB, and Gamma-Glutamyl Transferase (GGT)
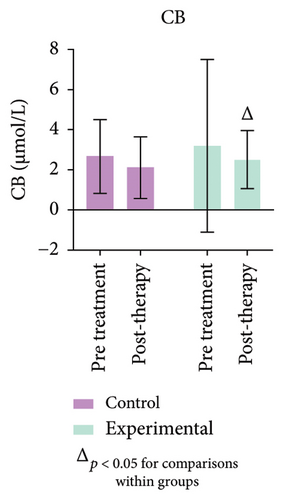
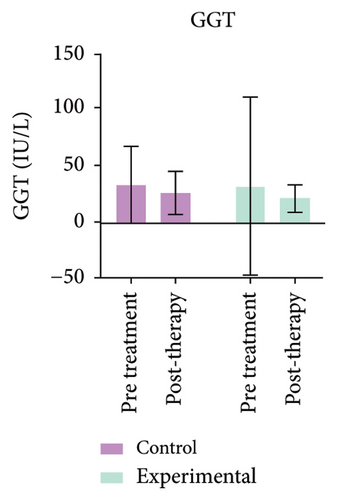
The study’s findings indicate a significant difference (p < 0.05) between the experimental and control groups. Figure 4 (Supporting Table 3) further demonstrates a substantial decrease (p < 0.05) in CB levels in the experimental group compared to pretreatment values. No variation in GGT levels was observed in either group, or between pre- and post-treatment values.
3.9. Comparison of All Immune-Related Indicators Including Absolute Count of CD3, CD4, CD19, and CD4% Before and After Treatment
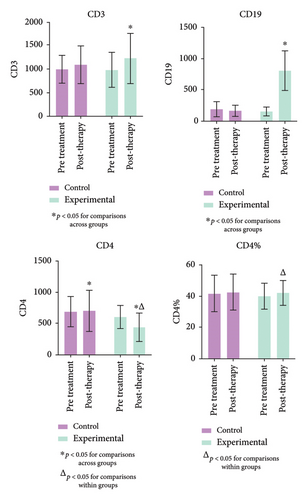
An overview of immune-related indicators is provided, with particular focus on those that showed statistically significant changes, as shown in Figure 5 (Supporting Table 4). Immunological activity, as reflected by CD3 and CD4 levels, depends on lymphocytes. After treatment, CD4 levels in the experimental group were significantly lower than those in the control and pretreatment groups (p < 0.05). Significant changes were also observed in CD3 and CD19 levels. Furthermore, there was a substantial increase in CD4% (p < 0.05) in the experimental group after treatment.
4. Discussion
This randomized clinical trial conducted a comprehensive analysis of the efficacy of Bailing capsules in treating CKD. Our findings indicate that Bailing capsules, when combined with standard treatment, were effective. The results align with those of other studies, further demonstrating the efficacy of Bailing capsules. Significant improvements were observed in TCM syndrome scores, serum cystatin C levels, 24-h urine protein, urine red blood cell count, and various biochemical markers.
CKD represents a significant global public health challenge due to its adverse outcomes and substantial economic burden. Given the heavy burden of CKD and the high costs associated with ESRD, strategies aimed at slowing its progression and reducing comorbidities are critical components of CKD management [18]. Emerging evidence suggests that early intervention, particularly with ACE inhibitors and ARBs, can prevent or delay adverse outcomes. These therapies, which are cornerstones of the current CKD management, work by reducing proteinuria and blood pressure, thereby slowing disease progression [19–22]. TCM, a key component of complementary and alternative medicine, has been recognized for its role in promoting health for thousands of years. Its therapeutic efficacy is widely accepted, and its clinical application is becoming increasingly common. Emerging preclinical studies and clinical trials suggest that TCM holds promise in treating CKD, particularly in reducing proteinuria, mitigating the adverse effects of Western medications, and potentially lowering the risk of progression to ESRD by 60% [23–25], which aligns with our results of reducing the TCM syndrome scores. Meanwhile, patients with CKD Stage III may present with different TCM syndrome patterns, and TCM treatment has shown to significantly improve GFRs and reduce proteinuria [26].
Further studies on CKD pathogenesis have identified inflammation, oxidative stress, apoptosis, and fibrosis as key contributors to disease progression [11–13]. Clinical and laboratory evidence demonstrate that Cordyceps sinensis effectively reduces proteinuria, lowers serum cholesterol levels, enhances immune function, and mitigates renal fibrosis [27, 28]. The research consistently highlights the diverse therapeutic effects of Bailing capsules in CKD, underscoring their potential as an effective adjunct therapy [29]. Additionally, their role in enhancing cell-mediated immunity has been confirmed in several animal models [30, 31]. In an immunosuppressed model, cordyceps-derived compounds significantly increased the mRNA expression of TNF-α and IL-10, along with phosphorylation of Lyn, Syk, and MAPK [32]. Bailing capsules may help restore the balance between proinflammatory and anti-inflammatory cytokines released by macrophages, thereby supporting immune function and triggering intracellular signaling pathways [33, 34]. Our study also demonstrated reductions in serum cystatin C, 24-h urine protein, urine red blood cells, WBCs, and lymphocytes, alongside increased CD3, CD19, and CD4% levels, further validating the efficacy of Bailing capsules in CKD management. Extensive research underscores the multifaceted effects of Bailing capsules’ active ingredients, contributing to their therapeutic potential. These findings suggest that cordyceps-based drug complexes may enhance immunostimulatory activity, presenting a promising therapeutic avenue for immune-related disorders. Given their complex immunomodulatory effects, further investigation is warranted to elucidate their precise role in CKD treatment. Our findings provide additional support for these diverse therapeutic mechanisms.
A study suggests that lipid accumulation in the renal parenchyma adversely affects renal function [35]. CKD is often associated with lipid metabolism disorders, where alterations in cholesterol and fatty acid metabolism can lead to glomerular and tubular cell injury, accelerating CKD progression [36]. Patients with advanced CKD or ESRD typically exhibit a characteristic lipid profile, including hypertriglyceridemia and low HDL cholesterol levels, while LDL cholesterol levels often remain normal [37]. Evidence indicates that impaired renal function directly influences lipoprotein metabolism, composition, and functionality, potentially affecting their response to pharmacological interventions. Improving lipid metabolism is crucial for controlling CKD progression [38]. Notably, lipid-lowering therapy with statins has shown to reduce cardiovascular risk in the early stages of CKD [39]. In our study, triglyceride levels in the experimental group were significantly reduced with statistical significance, suggesting that Bailing capsules contribute to lipid metabolism improvement in CKD patients and may help enhance clinical outcomes.
Limitations of this study include certain biomarkers, such as creatinine, urea nitrogen levels, and eGFR, not meeting initial expectations, with less favorable outcomes observed in the experimental group compared to the control group. This discrepancy may be due to the relatively small sample size, and future studies with larger cohorts could provide more definitive conclusions. Although cystatin C is a sensitive indicator of GFR and can detect kidney damage earlier than blood creatinine and urea nitrogen, future studies should still employ more accurate formulas for estimating eGFR to achieve a more precise and comprehensive evaluation of renal function. Additionally, the follow-up period was limited, restricting the ability to assess long-term efficacy and safety. Despite these limitations, the study’s strengths include rigorous data collection, strict inclusion criteria, and the use of electronic hospital records, ensuring data accuracy and completeness. These findings offer valuable insights into the potential clinical application of Bailing capsules.
5. Conclusion
This study demonstrates that Bailing capsules provide clinical benefits in CKD treatment, with significant improvements in various objective indicators. However, further research is needed to elucidate their role in immune function regulation. Despite this, Bailing capsules hold promise as a therapeutic option, contributing to enhanced kidney function and overall health in CKD patients, with the potential for even better outcomes with further optimization.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (2017LCSY053). Informed consent was obtained from all subjects involved in the study.
Consent
Please see the Ethics Statement.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
W.C., R.Z., X.C., H.Y., and Y.D. were involved in data acquisition and analysis and wrote the manuscript; W.C. was involved in designing and supervising the study, reviewing the manuscript, and contributing critical intellectual content to the manuscript; Y.D. substantively revised it. All authors read and approved the final manuscript.
Funding
The research was supported by Shanghai Clinical Key Speciality Fund ∗ shslczdzk04201.
Acknowledgments
The authors have nothing to report.
Supporting Information
Supporting Information: Supporting Table 1: Comparison of blood routine parameters in CKD3-4 patients with spleen and kidney qi deficiency syndrome before and after treatment. Supporting Table 2: Comparison of total cholesterol and triglycerides in CKD3-4 patients with spleen and kidney qi deficiency syndrome before and after treatment. Supporting Table 3: Liver function comparison in CKD3-4 patients with spleen and kidney qi deficiency syndrome before and after treatment. Supporting Table 4: Immune function–related indicators pre- and post-treatment (statistical analysis included).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




