Effect of Everolimus-Coated Ureteral Stent on Ureteral Anastomosis and Renal Ischemia-Reperfusion Damage: A Rabbit Experimental Model
Abstract
Objective: Ureteral stricture is defined as the narrowing of the ureter, which leads to functional impairment. This study aims to assess the fibrosis-reducing impact of everolimus, an mTOR inhibitor, on ureteral anastomoses and their potential to mitigate ischemia-reperfusion injury (IRI) in the ipsilateral kidney.
Materials-Methods: Sixteen New Zealand rabbits were randomized into four groups. Group 1: it was determined as the control group. Right-sided kidneys were used in all groups. The renal pedicle was clamped with a noncrushing clamp for 30 min to create IRI. Subsequently, the ureter was transected from the midline. End-to-end anastomosis was performed without the use of a ureteral stent. Group 2: similar procedures were performed as Group 1. Differently, everolimus (Certican, 0.75 mg tablet, Novartis) tablet at a dose of 1 mg/kg was administered orally as once daily for 3 days following the surgical procedure. Group 3: following IRI similar to the other groups, ureter end-to-end anastomosis was performed using a bare stent. Group 4: after similar procedures, end-to-end anastomosis was performed using double J stents coated with everolimus. All rabbits were followed for 21 days after the initial surgeries and sacrificed at the end of this period. Both kidneys and ureters of the subjects were resected en bloc for histopathological and biochemical analysis.
Results: There was less renal inflammatory cell infiltration, interstitial edema, vacuolar degeneration, and fibrosis in both oral everolimus and everolimus-coated stent groups than those in the other groups.
Conclusion: We conclude that everolimus has the potential to prevent ureteral strictures.
1. Introduction
Ureteral strictures, defined as pathology leading to functional obstruction, remain a significant concern despite various open and endoscopic management techniques [1]. In transplantation, these strictures contribute substantially to graft loss in both short- and long-term scenarios.
Experimental models have demonstrated heightened hydrostatic forces postureteral obstruction, accompanied by changes in the affected kidney attributed to oxidative stress. Notably, tissue growth factor-β1 (TGF-β1) production exhibits a 20-fold increase in the presence of hydronephrosis. After this elevation, a cascade of inflammatory responses, uncontrolled fibrous tissue overproduction, atrophy, and nephron loss ensues [2]. Recent studies suggest rapamycin can mitigate urethral stenosis and alleviate renal fibrosis by suppressing TGFβ1 expression in rats [3–5].
Another mTOR inhibitor, similar to rapamycin, is everolimus. Everolimus also aims to create an antifibrotic effect by decreasing TGF-β1 levels and increasing MMP-1 enzyme levels. MMP-1 enzymes can degrade type-1 and type-3 collagen, thus having an antifibrotic effect [4]. Recent studies have highlighted the discovery of epithelial–mesenchymal transition (EMT) and its relationship with everolimus. EMT is a cellular program known to be crucial for wound healing and malignant progression, as well as in the progression of renal allograft tubulointerstitial fibrosis. Everolimus reduces ureteral fibrosis by inhibiting EMT [6].
Ureteral strictures pose a risk of kidney failure due to renal damage, nephron loss, and increased infection susceptibility [5]. The most common intervention to prevent such strictures involves temporary or chronic stenting [7]. Consequently, exploring stents with diverse features has emerged as a strategy to mitigate morbidity associated with ureteral stents. The concept of drug-eluting stents, initially implemented in cardiovascular applications, has proven beneficial for vascular healing [8]. The coating of antiproliferative drugs on biocompatible stents, specifically addressing to solve proliferative issues, represents a promising avenue.
The m-TOR inhibitors, such as sirolimus and everolimus, are potent immunosuppressive and antiproliferative agents [9]. This study aims to assess the fibrosis-reducing impact of everolimus, an mTOR inhibitor, on ureteral anastomoses and their potential to mitigate ischemia-reperfusion injury (IRI) in the ipsilateral kidney.
2. Materials and Methods
2.1. Ethical Approval
The commencement of this study received explicit approval from the ethics committee and adhered strictly to the “European Convention for the Protection of Vertebrate Animals for Experimental and Other Scientific Purposes (ETS 123).” The methodological design followed the ARRIVE 2.0 guideline [10].
2.2. Experimental Subjects
A cohort of 16 + 2 male New Zealand rabbits weighing between 2000 and 2600 g constituted the experimental group. Randomization of the rabbits into four groups, each comprising four individuals (n = 4), was executed for the study, which was prospectively planned.
2.3. Experimental Protocol
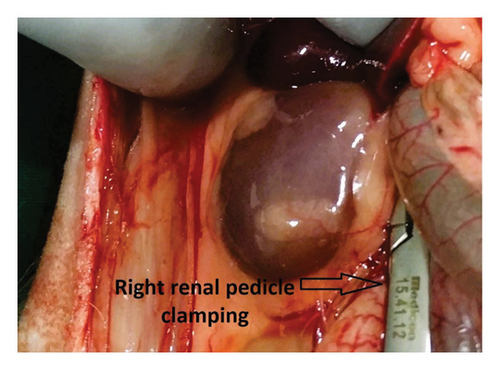
Before the surgical procedure, rabbits underwent a 1-week acclimatization period to mitigate potential stress factors. Subcutaneous anesthesia was administered using 0.35 mL/kg xylazine and 1.25 mL/kg ketamine. A midline laparotomy incision was performed. Subsequently, the right renal pedicle was clamped for 30 min, inducing ischemia via a noncrushing clamp (Figure 1). Following ischemia, reperfusion was initiated by opening the clamp. Subsequently, the right ureter was meticulously dissected from the midline. The ureteral reconstruction involved an end-to-end anastomosis. Subcutaneous administration of 0.05 mg/kg of 0.5% bupivacaine was undertaken twice daily for analgesia. Only one group received everolimus (1 mg/kg) orally for 3 days following the surgical procedure. The oral formulation of everolimus was prepared by dissolving 0.75 mg tablets of everolimus (Certican, Novartis) in water (0.01 mg/mL solution). A 3F-12 cm polyurethane double J stent (Actomed, Biorad Medisys, India) was used for stenting. For optimal stent duration, the study by Yuksel et al. was used as a reference. While there is no definitive optimal stenting time, the study emphasized a range of 14–21 days as the ideal stenting period following after renal transplantation [11].
2.4. Stent Coating With Everolimus
The coating procedure for everolimus on the ureteral stent followed the method outlined by Kram et al. for paclitaxel coating [12]. A solution comprising 10 mg of polylactic acid (PLA, M = 800,000 g/mol, RESOMER LR 708, Evonik Industries AG, Essen, Germany) and 30 mg of polyethylene glycol (PEG, M = 8000 g/mol, Sigma-Aldrich GmbH, Steinheim, Germany) dissolved in 1.775 mL of chloroform (CHCl3) was used as the coating solution. Simultaneously, everolimus (6 mg, SML2282-10 MG, Sigma-Aldrich GmbH, Steinheim, Germany) was dissolved in ethanol (6 mg/mL). The coating and everolimus solutions were mixed in a 0.6 to 0.5 mL ratio. The stents underwent immersion in the solution for 2 s, followed by a drying period of 5 min. This dipping and drying process was iterated five times, with each step meticulously timed using a chronometer. Postcoating, the everolimus-coated stents were autoclaved at 121°C for 1 atm and 15 min.
2.5. Experimental Groups
-
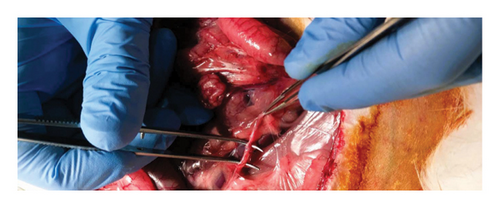
Group 1 (control group): this group was the control group, utilizing unilateral same-side kidneys. IRI was induced in the right kidney, with subsequent transection and end-to-end anastomosis of the ureter (Figure 2). No ureteral stent was employed in this group, and 0.9% isotonic NaCl was applied to the anastomosis line.
-
Group 2: similar to Group 1, the ureter was reconstructed by end-to-end anastomosis and everolimus (Certican, 0.75 mg tablets, Novartis) was administered orally (1 mg/kg) following dissolution in water once daily for three postoperative days.
-
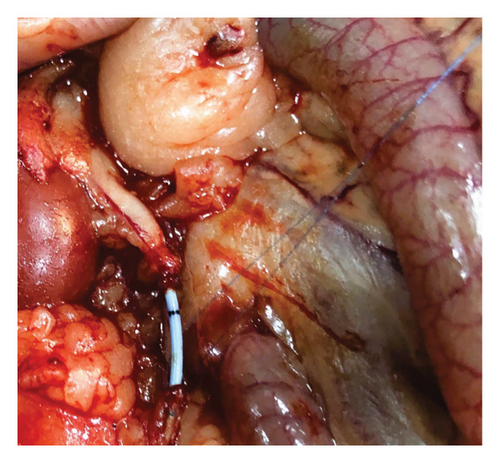
Group 3: following the same surgical procedure as in Group 1, the ureter was transected in the midline, and a 3F polyurethane ureteral double J stent (plain, uncoated with drug) was placed (Figure 3). Then, end-to-end anastomosis was performed.
-
Group 4: after a similar surgical procedure as in Group 1, ureteral anastomosis was performed using a 3F polyurethane ureteral double J stent. However, double J stents used in this group were coated with everolimus before the procedure. End-to-end anastomosis was performed following stent placement.
2.6. Experiment Parameters and Inclusion and Exclusion Criteria
The study commenced with 18 rabbits, but due to per operative problems after anesthesia induction, the study was completed with 16 male New Zealand rabbits. The weights of all rabbits were recorded both preoperatively and postoperatively. On the 1st and 21st postoperative days, serum creatinine and blood urea nitrogen (BUN) levels were measured (before the preop period and prior to sacrification, blood samples were taken into yellow-capped biochemistry tubes and analyzed in our biochemistry laboratory). On the seventh postoperative day, renal ultrasonography was performed to evaluate hydronephrosis (ultrasonography was performed by a specialist veterinarian in the animal research laboratory under subcutaneous anesthesia). For the groups with 3F double J ureteral stents, the position of the stent was evaluated on the seventh postoperative day using direct radiography (the imaging was performed using the x-ray machine in the animal research laboratory under subcutaneous anesthesia). Sacrification of all animals was planned on the 21st postoperative day. Since 3 of the 4 rabbits in the bare stent group died, the data on the remaining rabbit could not be used for statistical analysis, and this subject was excluded from the intergroup comparisons due to the risk of selection bias. However, the data of this subject were included in the comparative analysis of the right and left kidney data and also in the comparison of the control group with the data of the remaining 7 rabbits.
The right kidney and ureter, as well as the left kidney and left ureter, were dissected and removed for comparison. Histopathological assessment for fibrosis was conducted on specimens from the anastomosis line and kidney, utilizing Masson’s trichrome stain. Tissue samples containing 2 cm distal and proximal to the anastomosis line were stored in Eppendorf tubes with saline at −80°. Hydroxyproline levels were studied from these tissue samples.
For the right kidney, IRI was evaluated through hematoxylin and eosin and Masson trichrome stains. Subjective scoring for renal interstitial and tubular lesions, forming the renal injury index parameters, was conducted by two unbiased pathologists.
2.7. Biochemical Analysis
The ureteral tissue from each rabbit underwent washing with phosphate-buffered saline (PBS) and subsequent grinding into powder using liquid nitrogen. Subsequently, 100 mg of powdered ureteral tissue was placed into an Eppendorf tube, and 1 mL of PBS was added. The Eppendorf mixture was homogenized using Tissue Lyser II (QIAGEN, Germany) at 30 Hz for 3 min. For mobile phase A, an aqueous solution of 0.05 M ammonium formate was prepared, to which 3% formic acid was added. Mobile phase B consisted of acetonitrile. Before sample preparation, a precipitation reagent was prepared using 50 mL of acetonitrile and 10 mL of the mobile phase A solution. The sample was then prepared by combining 50 μL of calibrator, control, and sample solutions, 20 μL internal standard solution, and 400 μL precipitation reagent. Analysis was conducted using the Thermo Fisher Scientific Access Max LC-MS/MS spectrophotometer device with positive polarity. Hydroxyproline levels were measured as μmol/mg protein after 16 min of analysis.
2.8. Histopathological Evaluation
Ureteral samples were examined under a light microscope (Olympus BX53) after staining with hematoxylin and eosin. Inflammation within the ureteral samples was assessed. Masson trichrome, a histochemical stain, was employed to determine the presence and severity of fibrosis.
Renal samples were evaluated for various parameters, including inflammatory cell infiltration, interstitial edema, vacuolar degeneration, tubular atrophy, and tubular dilatation. Semiquantitative scoring was applied to all parameters, where Score 0 indicated the absence of specified parameters, Score 1 represented mild, Score 2 indicated moderate, and Score 3 denoted severe fibrosis.
2.9. Statistical Analysis
Descriptive data were presented as percentages using means ± standard deviations (SDs), medians, and ranges (minimum–maximum) for continuous variables. The conformity of the variables to a normal distribution was evaluated using visual tools such as histograms and probability plots, as well as analytical methods, including the Shapiro–Wilk test. For data with a normal distribution, paired group comparisons were conducted using the paired t-test. The Wilcoxon test was used to compare paired groups with non-normally distributed data. For normally distributed data, repeated measures of independent variables (three or more) were analyzed using a repeated ANOVA test. For the comparison of binary independent variables, the independent Student’s t-test was used. In the comparison of three or more non-normally distributed independent variables, the Kruskal–Wallis test was used. In the comparison of two independent non-normally distributed variables, the Mann–Whitney U test was used. In the evaluation of the relationship between dependent variables, Pearson correlation analysis was used for normally distributed data, and Spearman correlation analysis was used for non-normally distributed data. Correlation coefficients (r) were interpreted as follows: 0.0–0.19, “very weak”; 0.20–0.39, “weak”; 0.40–0.59, “moderate”; 0.60–0.79, “strong”; and 0.80–1.00, “very strong.” The statistical significance level was determined to be p < 0.05. All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) Version 25.0 for Windows (IBM SPSS Inc., Chicago, IL).
3. Results
Our study was designed to include 16 rabbits. However, two rabbits died in the first stage of the subcutaneous anesthetic procedure. We excluded these rabbits. The study was extended by including two additional rabbits. Therefore, the study was finalized with a total of 18 rabbits. During the study, six rabbits (excluding the two preoperative animals) died. The study was done using 10 rabbits. We designed our study to include 16 rabbits. Nevertheless, two rabbits died during the subcutaneous anesthesia stage. We excluded these animals. We added two rabbits to replace these subjects. We used a total of 18 rabbits. Six rabbits died during the experiments. Therefore, we completed the study with 10 rabbits. One rabbit from the control group died on the first postoperative day, possibly due to surgical stress. One rabbit from the oral everolimus group also died on the first postoperative day. Among the four rabbits that underwent bare stent placement, three died between the eighth and tenth postoperative days. The autopsy revealed that the right kidneys of the three rabbits with stents revealed acute purulent inflammation and pus. These deaths were associated with infections. One rabbit died in the drug-coated stent group. Within the same group, a rabbit exhibited wound dehiscence on the 17th postoperative day. The wound defect was promptly corrected. This rabbit reached PO on Day 21. The remaining subjects in the drug-eluting stent group reached postoperative Day 21 (PO21) without complications.
3.1. Laboratory Results
There were no statistically significant differences between groups in postoperative weight (Control vs. Oral Everolimus and Control vs. Drug-Coated Stents) (p = 0.101 and p = 0.619). Creatinine values increased in the control and everolimus-coated stent groups during the postoperative period.
Although these values exhibited a positive correlation with coefficients of 0.938 and 0.904, respectively, these correlations were not statistically significant in either group (p = 0.225 and p = 0.281). Postoperative BUN values did not significantly differ between the control and everolimus groups (p = 0.075 and p = 0.366).
3.2. Histopathological Findings
In the control group, intense inflammation was observed along the right ureter anastomosis line. Masson trichrome staining revealed severe fibrosis surrounding the right ureter. A reference image of normal ureteral tissue (left ureter), stained with Masson’s trichrome, was used to distinguish increased fibrosis (Figure 4).
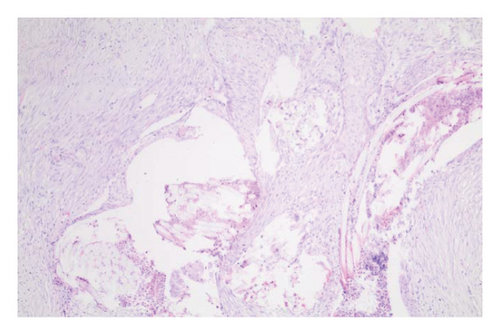
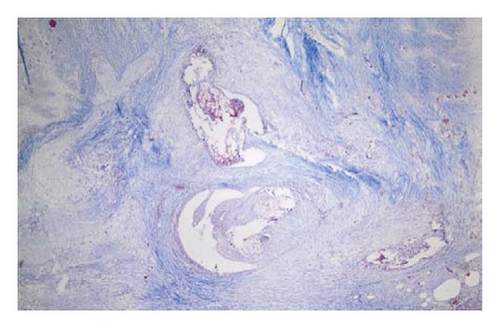
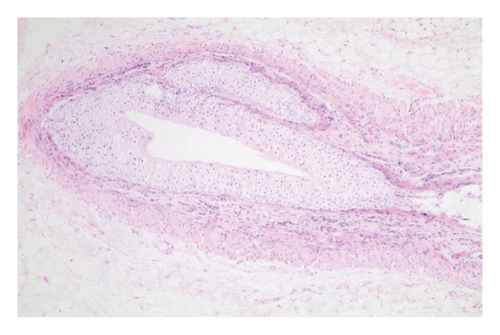
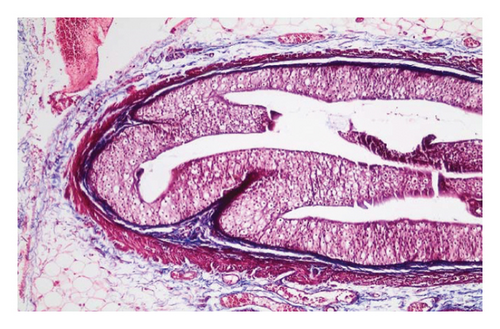
In the everolimus-coated stent group, minimal inflammation along the right ureter anastomosis line and minimal fibrosis around the ureter was observed, attributed to the antiproliferative effect of everolimus. Comparatively, fibrosis and inflammation were less pronounced than in the control and bare stent groups. Despite the systemic antiproliferative effect, histopathological evaluation did not reveal a decrease in fibrosis and inflammation after oral administration of everolimus. Intense inflammation and fibrosis persisted in this group (Figure 5). Tubular dilatation and tubular vacuolar degeneration areas observed in the control group’s right kidney specimen evaluations with HE staining (Figure 6) were reduced in the everolimus-coated stent group due to the antiproliferative effect (Figure 7).
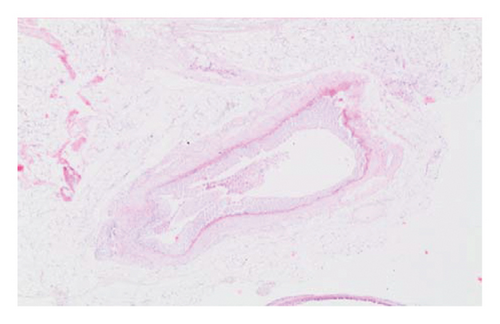
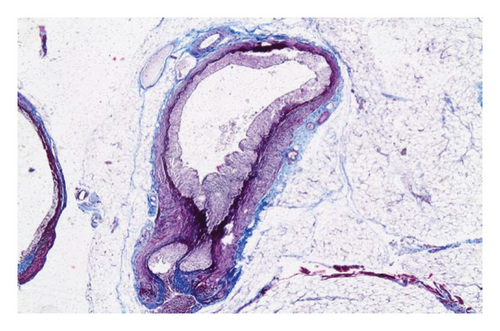
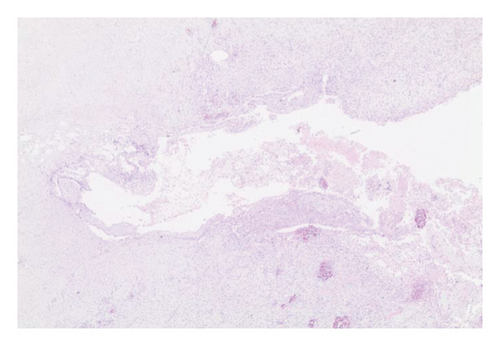
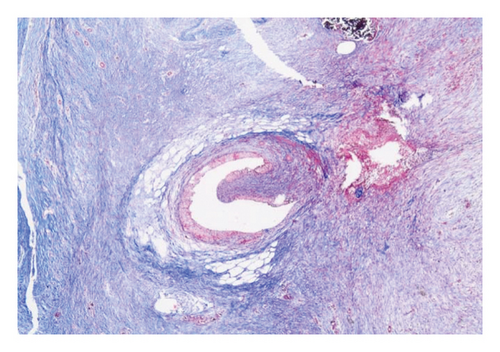
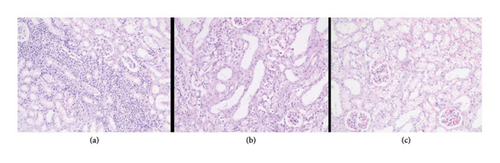
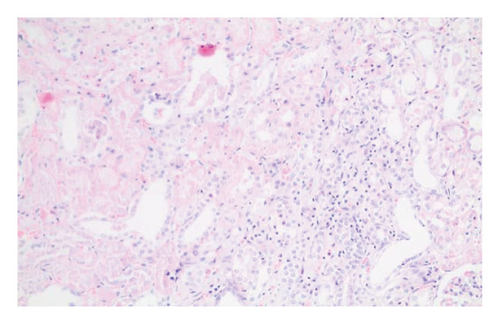
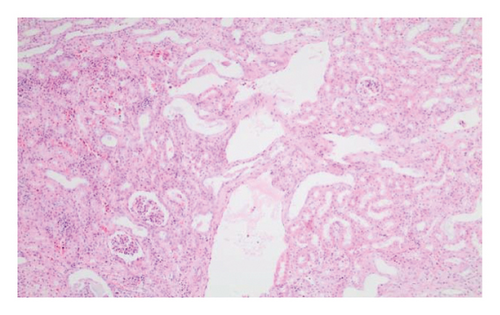
3.3. Histopathological Parameters
The histological evaluation of the kidney specimens included subjective grading for renal cell infiltration, interstitial edema, cellular vacuolar degeneration, degree of tubular atrophy, and tubular dilation. The degree of ureteral fibrosis at the ureteral anastomosis line was evaluated subjectively; a score of 0 indicated the absence of the specified features whereas scores of 1, 2, and 3 represented mild, moderate, and severe situations, respectively. A statistical difference was noted only in the degree of ureteral fibrosis when comparing the control group to the other groups (p = 0.010) (Table 1).
| Histopathological parameters | Control (n = 3) | Other groups (n = 7) | p < 0.05 |
|---|---|---|---|
| Renal cell infiltration | 2 ± 1 | 1.14 ± 0.37 | 0.071 |
| Interstitial edema | 0.66 ± 0.57 | 0 | 0.184 |
| Vacuolar degeneration of cells | 1.33 ± 1.15 | 0.28 ± 0.48 | 0.253 |
| Tubular atrophy degree | 0.33 ± 0.57 | 0 | 0.423 |
| Tubular dilatation | 0.66 ± 1.15 | 1.14 ± 0.37 | 0.517 |
| Degree of ureteral fibrosis | 2.33 ± 0.57 | 0.28 ± 0.48 | 0.010 |
The comparison of mean histopathological subjective scores for the right kidney and ureter between the groups did not demonstrate a statistically significant difference. Although there was no statistical significance, a reduction was noted in the scores of renal cell infiltration, interstitial edema, vacuolar degeneration of cells, tubular atrophy, and tubular dilation in the group administered oral everolimus in comparison with the control group. Similarly, a comparison between the control group and the drug-coated stent group revealed a reduction in the scores for renal cell infiltration, interstitial edema, vacuolar degeneration of cells, and ureteral fibrosis (Table 2). The ischemia-reperfusion applied to the right kidney and the anastomosis performed on the right ureter revealed different histological parameters in comparison to the left kidney. The study of histological data, irrespective of group classification, indicated that the right kidney demonstrated greater renal cell infiltration, cellular vacuolar degeneration, and ureteral fibrosis than the left kidney. The results were statistically significant (Table 3).
| Histopathological parameters | Control (n = 3) | Drug (n = 3) | Drug-eluting stent (n = 3) | P1 | P2 | P3 |
|---|---|---|---|---|---|---|
| Renal cell infiltration | 2 ± 1 | 1.66 ± 0.57 | 1.33 ± 0.57 | 0.643 | 0.374 | 0.519 |
| Interstitial edema | 0.66 ± 0.57 | 0 | 0 | 0.184 | 0.184 | 0.423 |
| Vacuolar degeneration of cells | 1.33 ± 1.15 | 0.66 ± 0.57 | 1 | 0.422 | 0.667 | 0.423 |
| Tubular atrophy degree | 0.33 ± 0.57 | 0 | 0.33 ± 0.57 | 0.374 | 1 | 0.423 |
| Tubular dilatation | 0.66 ± 1.15 | 0.33 ± 0.57 | 0.66 ± 0.57 | 0.678 | 1 | 0.519 |
| Degree of ureteral fibrosis | 2.33 ± 0.57 | 2.33 ± 0.57 | 1.66 ± 0.57 | 1 | 0.233 | 0.230 |
- Note: P1: comparison of the control group and drug group mean scores, P2: comparison of the control group and drug-eluting stent group, P3: comparison of the drug group and drug-eluting stent group (p < 0.05).
| Right (n = 10) | Left (n = 10) | p | |
|---|---|---|---|
| Renal cell infiltration | 1.60 ± 0.69 | 0.9 ± 0.56 | 0.024 |
| Interstitial edema | 0.2 ± 0.42 | 0 | 0.168 |
| Cell vacuolar degeneration | 1 ± 0.66 | 0.2 ± 0.42 | 0.005 |
| Tubular atrophy degree | 0.2 ± 0.42 | 0 | 0.168 |
| Tubular dilatation | 0.6 ± 0.69 | 0.1 ± 0.31 | 0.061 |
| Degree of ureteral fibrosis | 2.2 ± 0.63 | 0.5 ± 0.52 | 0.001 |
3.4. Hydroxyproline Levels
Hydroxyproline levels increased in the drug and drug-eluting stent groups compared with the control group. However, no statistically significant difference was found in the comparison between the two groups (Table 4).
| Control (n = 3) | Drug (n = 3) | Drug-eluting stent (n = 3) | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|
| Hydroxyproline | 0.05 ± 0.01 | 0.37 ± 0.55 | 0.17 ± 0.25 | 0.423 | 0.480 | 0.612 |
4. Discussion
The known antiproliferative, antifibrotic, and immunosuppressive effects of m-TOR inhibitors such as sirolimus (rapamycin) and everolimus have been well established. Previous studies, such as the one by Tie Chong et al., demonstrated that rapamycin inhibited urethral fibroblast formation in rabbits, preventing urethral strictures by reducing collagen deposition [13]. In addition, investigations regarding the effects of paclitaxel-coated stents on ureteral tissue suggested that urothelial cell proliferation and fibrosis in ureteral anastomoses could be significantly reduced, and hyperplastic urothelial reactions could be prevented [12]. Liourdi et al. evaluated the tissue distribution of paclitaxel in the porcine ureter using immunohistochemistry and nuclear magnetic resonance spectroscopy after dilatation with a paclitaxel-eluting balloon. Despite stating that the ureter is a tight and impermeable barrier, they demonstrated that paclitaxel was present in the urothelium, submucosal area, and smooth muscle layer. Furthermore, inflammation was reduced [14]. Kallidonis et al. evaluated the long-term clinical efficacy and safety of paclitaxel-eluting balloon dilatators in nonmalignant ureteral strictures. At the 1-year follow-up, they observed an 88% radiologic success rate. Their study demonstrated that this treatment is both safe and effective [15]. In addition, Barbalias et al. evaluated the distribution of paclitaxel in the rabbit urethra following application of a paclitaxel-eluting balloon. They observed that after dilatation, paclitaxel was distributed in the urothelial, submucosal, and smooth muscle layers of the rabbit urethra. At 24 and 48 h postapplication, paclitaxel and mild inflammation were still present in the region [16]. Similarly, our study revealed less inflammation and fibrosis in ureter sections with everolimus-coated stents, though this difference did not reach statistical significance.
Urothelial dysfunction resulting from hyperplasia can increase solute permeability. Reducing hyperplasia may contribute to maintaining urothelial barrier function by minimizing permeability. Consistent with this idea, Kram et al. investigated paclitaxel’s potential to reduce urothelial dysfunction by decreasing urothelial hyperplasia [12]. Both paclitaxel and everolimus are high molecular weight and lipophilic substances, enabling them to penetrate the urothelial barrier. In our study, urothelial hyperplasia was subjectively observed less frequently in the everolimus group than in the control group in histopathological sections.
Various studies have explored different methods of inducing ischemia by clamping the renal artery or the renal pedicle. It was suggested that arterial clamping alone might cause less renal damage due to preserved venous flow. However, other studies stated that clamping the renal pedicle resulted in less renal damage [17]. The duration of ischemia is a critical factor, with studies reporting that 30 min of ischemia is sufficient for TNF-α mRNA production, leading to peak TNF-α protein expression and bioactivity after 1 h of ischemia followed by 2 h of reperfusion. In our study, we opted for 30 min of renal pedicle clamping, given the small vascular structures of rabbit kidneys.
The choice of unilateral ischemia-reperfusion injury (uIRI) in our study, without contralateral nephrectomy, aligns with preferences for animal models requiring longer ischemia durations. Ischemia exceeding 60 min can lead to acute tubular necrosis (ATN) and renal failure. In comparison, less than 30 min of ischemia may prompt rapid proliferation of tubular epithelial cells and recurrent tubular damage [18].
In their assessment of IRI with everolimus pretreatment (1 mg/kg), Sagiroglu et al. observed that everolimus protected against IRI [19]. In our study, BUN and creatinine values increased in all groups at PO21 compared with the preoperative period. While the correlation coefficients suggested that the increase in creatinine and BUN with everolimus was minimally lower than in the control group, it did not reach statistical significance. Therefore, the observed increase in BUN and creatinine levels after everolimus administration might be associated with its potential nephrotoxic effects. Suyani et al. reported similar increases in BUN and creatinine due to the toxic effects of everolimus on tubular cells [20]. In our study, IRI and ureteral anastomosis were performed on the right kidneys across all groups. In comparison to the control group, the everolimus-coated stent group demonstrated a reduction in renal histological parameters, including renal cell infiltration, interstitial edema, cellular vacuolar degeneration, and ureteral fibrosis. Despite the absence of statistical significance, this decrease suggests that everolimus could offer a protective benefit. However, unlike our study, Suyani et al. observed similar histological findings related to tubular dilation, tubular epithelial separation, and inflammatory cell infiltration in the IRI and IRI + everolimus groups [20].
Reduced renal function is often linked to renal fibrosis, increased interstitial fibrosis, tubular apoptosis, and cell infiltration. In a study aiming to mitigate renal tubular apoptosis and tubulointerstitial fibrosis with sirolimus in animal models with unilateral ureteral obstruction (UUO), Yang et al. found that obstructive nephropathy resulted from cellular destruction due to apoptosis in renal tubular cells. Sirolimus can reduce tubular proliferation and apoptosis, protecting against renal fibrosis, tubular dilatation, and atrophy [21].
Kahn et al.’s study concerning the impact of rapamycin on ureteral anastomosis and abdominal wound healing in pigs revealed decreased hydroxyproline levels compared with the control group [22]. This decrease was particularly notable in the high-dose everolimus group (3.0 vs. 1.0 mg/kg), along with reduced tensile strength and hydroxyproline levels in the fascia. Significant histopathological differences were observed in the anastomosis region in the everolimus group. However, Willems et al. demonstrated that urgent postoperative application of everolimus did not affect wound strength by Day 3 but strongly impacted it by Day 7, reducing wound strength by almost half [23].
In contrast to these findings, our study showed increased hydroxyproline levels in the ureteral anastomoses of the oral everolimus and everolimus-coated stent groups compared with the control group. However, no statistical difference was found in the comparisons. Interestingly, the mean hydroxyproline levels in the oral everolimus group were higher than those in the other groups. Willems et al. suggested that normal tissues in the anastomotic line might impair measurement quality, potentially explaining our conflicting findings [23]. The sampling technique, involving tissue segments 2 cm distal and proximal to the anastomosis line, might have resulted in a high ratio of normal tissue and affected measurement quality, considering that these findings conflict with the expected tissue hydroxyproline level-lowering effects of everolimus.
5. Limitations
There are some limitations of this study that should be taken into consideration when analyzing the findings. First, a power analysis was not performed before the study groups were designed. As all experimental animals were sacrificed after the completion of the 3-week study period, the long-term outcomes of the interventions tested in this study could not be reported. Third, data on potential side effects of everolimus use, such as urinary tract infections, were not included.
In addition to these limitations, some analyses that might have been useful for evaluation could not be performed. In the study, the effect of everolimus on cells could not be directly analyzed through urothelial cell culture. Furthermore, the effect of IRI on lipid peroxidation could not be evaluated by malondialdehyde (MDA) assay. The effect of free oxygen radicals on superoxide dismutase (SOD) and IRI was not investigated in the study. Myeloperoxidase (MPO) activity, which is a marker of neutrophil and mononuclear cell infiltration to evaluate inflammation, was not measured due to cost limitations. Evaluation of MPO activity could have provided an alternative perspective to demonstrate the antiproliferative effect of everolimus. In addition, the blood level of everolimus could not be measured after its oral administration. These limitations were due to financial constraints encountered during the study.
6. Conclusions
Despite the above-described limitations, we declare that everolimus-coated stents demonstrate potential for therapeutic applications, especially in the efficient treatment of ureteral strictures. Everolimus reduced renal histological parameter scores in kidneys exposed to IRI, demonstrating a protective effect at this stage. Our study provided favorable results indicating that stenosis can be prevented. However, additional animal tests or clinical trials with larger sample sizes and more extensive parameters would significantly improve the field.
Ethics Statement
The KOBAY DHL AŞ Ethics Committee approved the study ethics. Ethics declarations date and number: 26.06.2019/389.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
F.S., S.C.G., S.C., and M.A.I.: research conception and designing. F.S. and S.C.G.: provided the creation of animal experiment models and the realization of animal experiments. F.S. and A.K.: data acquisition and contributed to the creation of the article. T.T.T.: performed histopathological evaluations. F.N.A.: performed biochemical analysis. F.S. and S.C.: critical revision of the manuscript. S.C., M.A.I., and F.S.: provided auditing and statistical analysis of the manuscript. S.C. and M.A.I.: supervision of the research. All authors approved of the final version of the manuscript.
Funding
This research received funds from the Scientific Research Community in the University of Health Sciences, Ankara Yıldırım Beyazıt Dışkapı Education and Research Hospital (29/08/2019-73/02).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




