Cognitive Fusion Versus Software Fusion for Subcentimeter Lesions in Transperineal mpMRI/TRUS Fusion-Guided Prostate Biopsy Under Local Anesthesia
Abstract
Background: The accuracy debate between cognitive and software fusion for small lesions in multiparametric magnetic resonance imaging (mpMRI)/transrectal ultrasound (TRUS) fusion-guided prostate biopsy remains inconclusive. This study compares the diagnostic accuracy of these fusion methods for prostate cancer, specifically for lesions < 1 cm under local anesthesia.
Methods: Retrospective analysis of prostate biopsies performed at The Affiliated Yantai Yuhuangding Hospital of Qingdao University (Feb 16, 2019–Feb 16, 2023). The Cognitive Fusion Group used free-hand technique for mentally integrating mpMRI and TRUS fusion, while the Software Fusion Group used MIM software with a fusion guidance grid. Lesions were defined as < 1 cm of maximum diameter on coronal using mpMRI. Each patient underwent 2–4 targeted biopsies for the identified lesion, followed by 12 systematic biopsies. All biopsies were transperineal and performed under local anesthesia.
Results: Histology showed clinically significant prostate cancer (International Society of Urological Pathology ≥ 2) in 55 cases (42.6%) in the software fusion group and 66 cases (40.2%) in the cognitive fusion group within the targeted prostate cancer category. However, cognitive fusion had a shorter median operative time of 11 min compared to 22 min in the software fusion group. Complication rates did not significantly differ between the two fusion strategies.
Conclusion: This study suggests that experienced operators can achieve comparable diagnostic accuracy for sub-centimeter lesions in mpMRI/TRUS fusion-guided prostate biopsy using cognitive fusion. Furthermore, cognitive fusion offers simplicity and shorter procedural time. These findings contribute to the discourse on fusion methods in prostate cancer diagnosis, and further validation studies are warranted.
1. Introduction
Prostate cancer (PCa) is a prevalent neoplasm affecting men, necessitating precise diagnosis for timely intervention and optimal patient survival. Within the realm of diagnostic and therapeutic decision-making, prostatic biopsy assumes a paramount role, where utmost accuracy becomes indispensable [1, 2].
In the past decade, the emergence of multiparametric magnetic resonance imaging (mpMRI)/transrectal ultrasound (TRUS) fusion-guided biopsy has revolutionized the diagnostic landscape of PCa [3]. This technique has significantly enhanced the precision of tumor identification, reduced unnecessary biopsies, and provided invaluable insights for treatment planning [3, 4]. As a result, there has been an increasing interest in optimizing the biopsy procedure itself.
Performing a transperineal fusion biopsy entails a crucial consideration: the imperative of puncture precision, which predominantly achieved through two commonly employed methods–cognitive fusion and software fusion [5]. The ongoing debate regarding the accuracy of software fusion versus cognitive fusion in medical procedures has attracted attention. Although software fusion is generally regarded as more precise, cognitive fusion is sometimes criticized for its perceived subjectivity and potential lack of accuracy [6, 7].
Due to the meticulous nature of the task, often requiring pinpoint accuracy, these techniques typically necessitate general or lumbar anesthesia. However, advancements in anesthesia techniques have led to an increasing exploration of the feasibility of performing the procedure under local anesthesia. Such a development not only enhances patient comfort but also has the potential to alleviate the overall financial burden associated with the procedure [8, 9].
From a technical perspective, the efficacy of a biopsy lies in its ability to precisely target and extract a minute lesion. Despite the improving effectiveness of local anesthetics, even subtle patient responses to painful stimuli can exert a significant influence, resulting in notable displacement of the needle trajectory and fusion site [10]. Numerous studies have attempted to compare cognitive fusion and software fusion, primarily focusing on patients with Prostate Imaging Reporting and Data System (PI-RADS) scores of 4-5, where many lesions exceed a diameter of 1.5 cm [11, 12]. However, these circumstances fail to provide a comprehensive evaluation of the accuracy exhibited by distinct biopsy methodologies. Furthermore, reported findings have been inconsistent, hindering meaningful comparisons.
Multiple research studies have demonstrated that mpMRI exhibits remarkable accuracy, surpassing 85%–90%, in detecting cancerous lesions measuring 0.2 cc or 0.5 cc. Consequently, this pivotal threshold of 0.5 cc is frequently employed to distinguish clinically significant prostatic cancer (csPCa) [13–15]. Based on mpMRI measurements, a lesion of 0.5 cc spans a diameter of approximately 1 cm, we have selected a maximum lesion diameter of 1 cm to examine the comparative accuracy of the two puncture methods performed under local anesthesia.
The diagnostic uncertainty of small mpMRI lesions poses a significant challenge in prostate biopsy decision-making. Recent studies highlight the critical role of targeted biopsy in this population, with csPCa detection rates ranging from 11.2% to 50% for PI-RADS 3 lesions, underscoring the need for optimized strategies [16, 17]. However, existing literature predominantly focuses on larger lesions (> 1 cm) [18, 19], leaving a gap in understanding the performance of cognitive versus software fusion for small, indeterminate targets, particularly under local anesthesia.
By intentionally restricting the lesion size and conducting a comprehensive analysis within this specific patient population, we anticipate providing invaluable insights that can effectively inform clinical decision-making and advance the accuracy of PCa diagnosis.
2. Materials and Methods
2.1. Patient Selection
A retrospective analysis was conducted on a cohort of individuals who underwent prostate biopsy at The Affiliated Yantai Yuhuangding Hospital of Qingdao University between February 16, 2019, and February 16, 2023. The study protocol was approved by the Committees for Ethical Review of Research Involving Human Subjects at the Affiliated Yantai Yuhuangding Hospital of Qingdao University.
2.2. Inclusion Criteria
The inclusion criteria encompassed patients who met the following indications: prostate-specific antigen (PSA) levels equaling or exceeding 4 ng/mL, PSA density ≥ 0.15 ng/mL/mL or PSA Velocity ≥ 0.75 ng/mL/year [20], abnormalities detected upon digital rectal examination, or identification of at least one suspicious lesion measuring a maximum diameter of 1 cm on coronal mpMRI and assigned a PI-RADS score of 3 or 4.
2.3. Exclusion Criteria
Exclusion criteria included individuals with a prior history of prostate puncture or PCa diagnosis, use of medications impacting serum PSA levels, and incomplete clinical information. Please refer to Figure 1 for a representation of the patient filtration process.
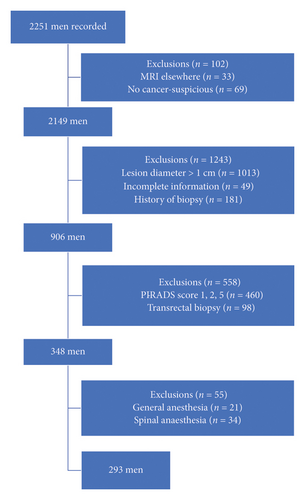
2.4. Patient Groups
The participants were divided into two groups: the cognitive fusion group (n = 164) and the software fusion group (n = 129). The assignment to each group was based on the availability of the respective biopsy techniques during the study period. Patients who underwent biopsy during a period when only cognitive fusion was available were assigned to the cognitive fusion group, while those who underwent biopsy during a period when software fusion was available were assigned to the software fusion group.
2.5. Cognitive Fusion Group
In the cognitive fusion group, the fusion entailed integrating preoperative mpMRI findings with intraoperative TRUS guidance. Prior to the biopsy, highly skilled radiologists meticulously reviewed the mpMRI images to identify suspicious lesions. During the biopsy procedure, the urologist employed real-time TRUS to precisely locate and target the suspicious lesions identified on the mpMRI. Systematic biopsy samples were obtained from the prostate gland using a free-hand technique.
2.6. Software Fusion Group
In the software fusion group, the preoperative mpMRI images were imported into MIM software (MIM Software Inc., Cleveland, USA). The MIM software utilized rigid registration to align mpMRI and intraoperative TRUS images. The outline of the prostate gland and the location of the suspicious lesions were marked on the software. During the biopsy, a grid template based on the fusion of the mpMRI and TRUS images was utilized as a guide. The urologist performed systematic biopsy sampling using a brachytherapy stepper and BK biplane ultrasound (BK medical, Herlev, Denmark).
2.7. Biopsy Procedure
In both groups, each identified lesion of interest underwent 2–4 targeted biopsies to balance diagnostic accuracy and procedural risk [20]. The number of targeted biopsy cores per lesion was stratified based on imaging clarity and anatomical accessibility: 3-4 cores were obtained for lesions with poor visualization or those located in anatomically challenging regions; 2 cores were reserved for lesions with well-defined margins and accessible locations [5]. Additionally, 12 systematic biopsies were conducted to provide a thorough evaluation. All biopsy procedures were transperineal and utilized the fusion of mpMRI/TRUS techniques, with the administration of localized anesthesia.
2.8. Data Collection and Scoring
Clinical data, including age, PSA level, lesion diameter, lesion location, PI-RADS score, Gleason score, operation duration, postoperative complications, and other pertinent information, were collected from electronic medical records. The mpMRI scans were independently reviewed and scored by two experienced radiologists according to the PI-RADS criteria. In cases where discrepancies arose, a consensus was reached through consultation with a third radiologist. To minimize bias, the imaging specialists remained unaware of the pathological results while scoring the mpMRIs.
The biopsy samples were sent for histopathological analysis, and the results were reviewed by two skilled pathologists. A consensus diagnosis was reached in cases of any disagreements. The presence and grade of PCa were determined based on the Gleason scoring system.
In our investigation, targeted PCa (tarPCa) denotes instances where malignancy was exclusively identified through targeted biopsy or cases where lesions were detected in both targeted and systematic samples. Conversely, sysPCa exclusively refers to cases where cancer was solely identified through systematic biopsy. By excluding sysPCa from the calculation of the biopsy positivity rate, we aim to provide a precise evaluation of the accuracy of targeted biopsy methods.
Definition of csPCa: International Society of Urological Pathology score ≥ 2; clinically nonsignificant PCa (nsPCa): International Society of Urological Pathology score = 1.
2.9. Statistical Analysis
To compare the differences between the cognitive fusion group and the software fusion group, the Mann–Whitney U test was employed for continuous variables, and chi-square tests or Fisher’s exact tests were used for categorical variables. A p value less than 0.05 was considered statistically significant. All analyses were performed using SPSS version 23 (IBM Corporation, Armonk, NY, USA).
3. Results
3.1. Patient Demographics and Clinical Characteristics
A total of 293 patients underwent prostate biopsy, with 129 individuals enrolled in the software fusion group and 164 individuals in the cognitive fusion group. There were no statistically significant differences observed in terms of age, PSA level, prostate volume, and PSA density between the two groups (Table 1). The distribution of PI-RADS scores, encompassing both PI-RADS 3 and PI-RADS 4, displayed similar patterns within both cohorts. Moreover, there was no statistically significant difference found in the median diameter of the mpMRI lesion between the software fusion group (7.8 mm) and the cognitive fusion group (7.7 mm) (p = 0.743). Furthermore, the Gleason scores exhibited no substantial variations across the two groups. These findings underscore the comparable baseline characteristics of the patients included in both biopsy strategies.
| Biopsy strategy | p value | ||
|---|---|---|---|
| Software fusion | Cognitive fusion | ||
| Patients, n | 129 | 164 | |
| Age (yr), median (IQR) | 72 (65–79) | 71 (63–78) | 0.45 |
| PSA level (ng/mL), median (IQR) | 8.4 (6.6–8.4) | 8.3 (6.8–9.1) | 0.76 |
| Prostate volume (mL), median (IQR) | 50.1 (46.8–50.1) | 51.75 (46.3–55.1) | 0.29 |
| PSAD (ng/mL/mL), median (IQR) | 0.17 (0.15–0.19) | 0.16 (0.14–0.19) | 0.54 |
| PI-RADS score, n (%) | |||
| PI-RADS 3 | 62 (48.1) | 85 (51.8) | 0.57 |
| PI-RADS 4 | 67 (51.9) | 79 (48.2) | 0.65 |
| Diameter of mpMRI lesion (mm), median (IQR) | 7.8 (6.9–8.5) | 7.7 (6.9–8.5) | 0.74 |
| Gleason score, median (IQR) | 7 (6–9) | 7 (6–9) | 0.84 |
- Note: Values are presented as median (IQR: interquartile range) or n (%) unless otherwise specified.
- Abbreviations: IQR = interquartile range, mpMRI = multiparametric magnetic resonance imaging, PI-RADS = Prostate Imaging Reporting and Data System, PSA = prostate-specific antigen, PSAD = prostate-specific antigen density.
3.2. Histology Findings in Targeted and Systematic Biopsies
Of the 293 patients who underwent prostate biopsy, 51.9% (152) were diagnosed with PCa. Among these, 81.6% (124) had csPCa (Supporting Table 1). Moving forward, the analysis was further stratified into two groups: the software fusion and cognitive fusion groups. Among the 129 patients in the software fusion group, 68 (52.7%) were diagnosed with PCa. In the cognitive fusion group, 84 out of 164 patients (51.2%) received the same diagnosis (Table 2).
| Histology | Biopsy strategy | p value | |
|---|---|---|---|
| Software fusion | Cognitive fusion | ||
| Patients, n | 129 | 164 | |
| All PCa, n (%) | 68 (52.7) | 84 (51.2) | |
| tarPCa† | 0.86 | ||
| csPCa | 55 (42.6) | 66 (40.2) | |
| nsPCa | 10 (7.8) | 13 (7.9) | |
| sysPCa†† | 0.85 | ||
| csPCa | 1 (0.8) | 2 (1.2) | |
| nsPCa | 2 (1.6) | 3 (1.8) | |
| No PCa, n (%) | 61 (47.3) | 80 (48.8) | |
- Note: nsPCa = clinical nonsignificant prostate cancer.
- Abbreviations: csPCa = clinical significant prostate cancer, PCa = prostate cancer.
- †tarPCa include cases where cancer was solely detected through targeted biopsy or combined biopsies.
- ††sysPCa only denotes cases where cancer was solely detected through systematic biopsy.
Further analysis within the tarPCa category revealed that 55 cases (42.6%) in the software fusion group and 66 cases (40.2%) in the cognitive fusion group represented csPCa. Additionally, 10 cases (7.8%) in the software fusion group and 13 cases (7.9%) in the cognitive fusion group were categorized as nsPCa (Table 2). These findings indicate that both software fusion and cognitive fusion biopsy strategies yield comparable histology findings in the diagnosis of PCa. There were no statistically significant differences in the rates of csPCa and nsPCa between the two approaches. Moreover, this study identified eight cases of PCa and three cases of csPCa that were exclusively detected through systematic biopsies but not via targeted biopsies, highlighting the utility of this approach in comprehensive PCa screening (Table 2).
To further assess the performance of two fusion techniques in the localization of csPCa lesions, we present Supporting Table 2. Specifically, among patients who underwent software fusion, 87.5% (49 of 56) of the lesions were identified via combined biopsy, whereas in the cognitive fusion group, this proportion was 83.8% (57 of 68). Both biopsy strategies demonstrated comparable performance in lesion localization (p = 0.83). Furthermore, it is notable that in the cognitive fusion cohort, 6 cases (10.7%) of csPCa were diagnosed exclusively through targeted biopsy, with systemic biopsies yielding negative results. Similarly, in the software fusion cohort, 9 cases (13.2%) of csPCa were identified solely through targeted biopsy, with systemic biopsies also returning negative results.
3.3. Comparison of Operative Time, Visual Analog Scale Score, and Complications Between Software Fusion and Cognitive Fusion Biopsy Strategies
The cognitive fusion strategy achieved a significantly shorter median operative time (11 min, p < 0.001) compared to the software fusion strategy (22 min). This outcome highlights the superior efficiency and effectiveness of the cognitive fusion approach in performing prostate biopsies.
A post-procedural pain assessment using the Visual Analog Scale showed that the cognitive fusion technique yielded a median score of 2 (interquartile range [IQR] 2-3), which was slightly lower than the median score of 3 (IQR 2–4) observed with the software fusion technique. These findings suggest that both approaches effectively manage post-procedural pain, with the cognitive fusion technique demonstrating a slight advantage.
The incidence of Clavien-Dindo classification grade II and III complications, including urinary tract infections (UTI), hematuria, and acute urinary retention (AUR), did not significantly differ between the cognitive fusion and software fusion groups. However, it is worth noting that a grade III complication, scrotal hematoma, was exclusive to the software fusion technique. This isolated occurrence might be attributed to the trajectory of the biopsy needle relative to the lesion’s location above the apex of the prostate, which potentially led to injury to the dorsal venous complex (DVC) (Table 3).
| Biopsy strategy | p value | ||
|---|---|---|---|
| Software fusion | Cognitive fusion | ||
| Patients, n | 129 | 164 | |
| Operative time (min), median (IQR) | 22 (18–27) | 11 (9–15) | < 0.001 |
| VAS score, median (IQR) | 3 (2–4) | 2 (2-3) | 0.12 |
| Complications (clavien-dindo classification), n (%) | |||
| UTI (II) | 4 (1.4) | 6 (2.0) | |
| Hematuria (II) | 2 (0.7) | 3 (1.0) | |
| Subcutaneous hematoma (II) | 0 (0.0) | 1 (0.6) | |
| Vasovagal events (II) | 1 (0.3) | 2 (0.7) | |
| AUR and catheterization (III) | 1 (0.3) | 1 (0.6) | |
| Scrotal hematoma (III) | 1 (0.3) | 0 (0.0) | |
| Urosepsis (IV) | 0 (0.0) | 0 (0.0) | |
- Abbreviations: AUR = acute urinary retention, IQR = interquartile range, UTI = urinary tract infections, VAS = visual analog scale.
3.4. PCa Detection Rates in Various PI-RADS Scores
For PI-RADS 3 lesions, the software fusion approach yielded a csPCa detection rate of 25.8%, while the cognitive fusion approach achieved a rate of 23.5%. The nsPCa detection rate was 4.8% for the software fusion approach and 7.1% for the cognitive fusion approach. Although there is a slightly higher csPCa detection rate and a slightly lower nsPCa detection rate with the software fusion approach, the differences were not statistically significant.
In the case of PI-RADS 4 lesions, both fusion approaches exhibited a commendable and equivalent csPCa detection rate of 58.2%. The nsPCa detection rate was 10.4% for the software fusion technique and 8.9% for the cognitive fusion technique. These results demonstrate comparable efficacy in identifying clinically significant PCa between the two methods (Figure 2).
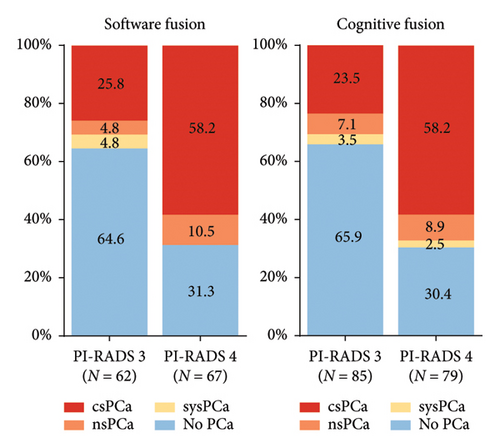
4. Discussion
Our examination reveals no statistically significant disparity in the identification rates of csPCa and nsPCa when comparing the fusion software approach to the cognitive fusion approach in the realm of PCa diagnosis, after excluding the influence of sysPCa. The cognitive fusion technique offers benefits such as reduced operative time and potential alleviation of post-procedural discomfort.
Our results showed that targeted biopsy exhibits a distinct advantage by effectively identifying suspicious lesions when systematic biopsy results are negative, thereby reinforcing its unique role in detecting clinically significant lesions. This capability not only addresses the limitations of systematic biopsies but also significantly enhances the detection rate of csPCa, highlighting its pivotal role in improving diagnostic accuracy.
Additionally, this study identified eight PCa and three csPCa cases detected exclusively through systematic biopsies but not via targeted biopsies. The failure of targeted biopsies to detect these lesions may be due to either mpMRI’s inability to identify them or their location in regions challenging to sample with targeted approaches. Although both fusion strategies provide similar diagnostic outcomes, the integration of systematic and targeted biopsies offers a more comprehensive approach to PCa detection, ensuring that lesions overlooked by one method are identified by the other.
The debate between rigid and elastic registration in prostate biopsy is ongoing. Rigid registration maintains prostate architecture but does not address local deformations, which can be problematic for small lesions. Elastic registration, by adjusting for prostate shape changes, has the potential to improve alignment and accuracy for small lesions [21]. However, it can compromise data integrity due to image distortion [22]. Additionally, lesions near the phantom’s edge exhibited increased registration errors with elastic registration.
In this study, we employed rigid registration for software fusion using MIM Software. Our results demonstrate that both fusion strategies are equally effective in discerning PCa lesions smaller than 1 cm. This suggests that operator expertise in cognitive fusion can mitigate registration errors caused by prostate deformation under local anesthesia. Our findings align with recent studies emphasizing that cognitive targeting remains a reasonable approach for lesions that are small or in difficult locations [23, 24].
The debate between cognitive and software fusion persists, particularly for small lesions. Certain investigations have suggested that software fusion may provide a greater level of precision in contrast to cognitive fusion techniques [25]. One plausible explanation for this finding is the difficulty of preventing muscle contractions or slight movements during a biopsy, notwithstanding improvements in local anesthesia [26, 27]. In such instances, patient reactions to stimuli may cause the needle to deviate from its intended target area, particularly when dealing with small lesions [28]. Software fusion may necessitate frequent realignment in the event of changes in the patient’s position. Conversely, cognitive fusion occurs mentally within the operator and is less susceptible to such alterations, potentially affording an advantage in maintaining accuracy during the procedure [29].
The process of software fusion follows a predetermined grid that dictates the distribution of puncture points. Nevertheless, this fixed arrangement can pose challenges in achieving optimal outcomes for small lesions located amidst these puncture points. In certain scenarios, slight bending of the needle may be necessary to obtain a satisfactory result [30]. Furthermore, when addressing larger prostates, the grid may not provide adequate coverage, further complicating the procedure. Additionally, obstructions such as the pubic bone and rectum can impede grid-based biopsies, particularly when the lesion is situated anterior to the prostate or beneath the peripheral zone [31]. In contrast, cognitive fusion employing freehand biopsy allows operators to mentally align the needle without relying on a fixed grid. By offering greater flexibility in insertion angles, freehand biopsy empowers surgeons to overcome obstacles such as the pubic bone and rectum, thereby optimizing the procedure [32].
When compared to fixed template-guided biopsies, freehand biopsies without template guidance offer more flexibility in adjusting needle insertion angles, ultimately enhancing the precision of biopsy samples [33]. Additionally, Marra et al. emphasized in a 2019 study [34] that in cases where fixed templates posed challenges in covering larger prostates or encountered anatomical obstacles, freehand biopsies without template guidance could be a preferable option. This flexibility and adaptability hold the potential to augment the accuracy and overall success of the biopsy procedure.
Furthermore, the cognitive fusion strategy demonstrated a significantly shorter median operative time compared to the software fusion strategy when the software fusion step was omitted. This highlights the efficiency and effectiveness of the cognitive fusion approach in conducting prostate biopsies. The reduced operative time can potentially enhance patient comfort and workflow in clinical settings.
It is essential to acknowledge several limitations of this study. Firstly, the retrospective nature of the study may introduce selection and information biases. Secondly, the study was conducted at a single center, which may limit the external validity of the results. Additionally, our study specifically focused on lesions smaller than 1 cm, and the findings may not be applicable to larger lesions or different populations.
5. Conclusion
Our research suggests that cognitive fusion can be just as accurate as software fusion for experienced operators, while offering additional advantages such as simplicity of use and shorter procedural time. With further investigation and implementation, cognitive fusion has the potential to enhance procedural accuracy, operator performance, and patient satisfaction.
Ethics Statement
The study was approved by the Committees for Ethical Review of Research Involving Human Subjects at the Affiliated Yantai Yuhuangding Hospital of Qingdao University (approval number: 2019-YHD-047).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Hejia Yuan: data curation, writing – original draft preparation; Peng Peng: data curation, writing – original draft preparation; Fan Feng: data curation, investigation; Yining Zhao: methodology, software; Yantao Lou: methodology, writing – reviewing and editing; Jipeng Wang: validation, writing – reviewing and editing; Hongwei Zhao: visualization, investigation; Jitao Wu: conceptualization, supervision.
These authors contributed equally to this work: Hejia Yuan, Peng Peng, Fan Feng.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81870525] and the Natural Science Foundation of Shandong Province [grant number ZR2020QH186].
Acknowledgments
The authors have nothing to report.
Supporting Information
Additional data supporting this study are available in the Supporting Information.
Open Research
Data Availability Statement
The data supporting the findings of this study are available upon request from the corresponding author.




