Current Clinical Practices and Future Perspectives for Primary Healthcare Use of Point-of-Care Devices: A Scoping Review
Abstract
Background: Expanding access to screening through primary care is essential to address changes in disease patterns, patients’ needs, and demographics. Point-of-care test (POCT) devices play a crucial role in providing primary care and have positive operational and economic impacts compared to central laboratories. Despite their importance, the implementation of POCT devices in primary care remains low. This scoping review aims to map the current evidence on POCT types and their current uses in primary care.
Methods: A scoping review was conducted using the framework proposed by Arksey and O’Malley, and further refined by Levac et al. and the Joanna Briggs Institute. Our process included five stages: identifying the research question, identifying relevant studies, study selection, charting the data, and collating, summarizing, and reporting the results. We searched MEDLINE (Ovid), CINAHL, Embase, Web of Science, Cochrane Library, and Google Scholar for studies published between 2018 and 2023 on the use of POCTs in primary care settings for adult populations. Our findings were summarized using descriptive statistics and thematic analysis.
Results: The search yielded 43,913 publications, of which, 167 met the inclusion criteria. From these studies, biomarker detection tests were used in 33.5%, antibody tests in 21.6%, other POCTs in 17.4%, POC technologies in 11.4%, antigen tests in 8.4%, and POC molecular tests in 7.8%. Across studies, 46.7% targeted communicable diseases, 39.5% targeted noncommunicable diseases, and 13.8% for other diseases. POC devices are mainly used for communicable diseases in urban healthcare settings, particularly in low- and middle-income countries (LMIC). POC devices are used primarily for noncommunicable diseases in urban healthcare settings, particularly in high-income countries.
Conclusion: This scoping review has mapped the evidence and highlighted gaps regarding POCTs used in primary care. The findings can be used to design and effectively implement sustainable patients’ healthcare journeys to accommodate the changes in patients’ demographics and healthcare needs.
1. Introduction
The increase in the geriatric population, patients with noncommunicable diseases (NCDs), and multimorbidity has subsequently increased the demand for the primary healthcare sector [1, 2]. It was reported by the World Health Organization (WHO) [1] that early diagnosis of NCDs (e.g., chronic cardiovascular and respiratory diseases and cancer) and expanding access to screening in primary care are associated with a decrease in mortality rate. This indicates that primary healthcare needs to accommodate the fluctuation in patients’ needs and growing demand by being more sustainable and patient-centric, particularly in the post-COVID-19 era [1, 3, 4].
Point-of-care tests (POCTs) are essential in providing primary healthcare. POCT is a test conducted by a qualified staff member that can support clinical decision-making, and it is performed near the patient’s side and on any part of the patient’s body, either during or shortly after the consultation [5]. They could be categorized into two groups: physical POCTs (e.g., portable monitor, pulse oximeter, and portable X-ray or electrocardiogram machines) and body fluid POCTs (i.e., including urine, blood, and nasopharyngeal specimen) [6]. Using POCT will provide valuable information to support clinical decision-making and improve patients’ healthcare outcomes [7].
The benefits of utilizing POCTs are not limited to the clinical aspect but also to the economic and operational aspects of the healthcare service compared to the standard laboratory system [8, 9]. They are used in primary care to avoid unnecessary referrals, exclude an illness, or guide treatment decisions [10]. Furthermore, evidence suggests that assessing intermediate markers for chronic diseases (e.g., albumin–creatinine ratios, glycated hemoglobin, and lipid profiles) by POCTs improves workflow and satisfaction of patients, healthcare staff, and clinicians [11, 12]. However, although the evidence shows that POCTs are cost-effective in areas with limited medical infrastructure where they are typically used to diagnose conditions with high prevalence, the adoption of POCT in primary care is still low, particularly for low- and middle-income countries (LMICs) and in resource-limited settings [13]. In addition, the evidence shows that POCTs’ design is unsuitable for the end users. Some of the reported barriers are lack of space to place the POCT device, complexity in the preparation, and difficulties in transporting the device [13]. The slow adoption of POCT was also attributed to several reasons, mainly related to the potential increase in workload and costs [14].
A systematic review conducted by Lingervelder et al. examined the literature on the evaluation and usage of POCTs in primary care in high-income countries from 2007 to 2017 [10]. Their study excluded POC technologies that diagnose patients, use decision support systems, or electronic health records. There is a global objective to use advanced technologies in the provision and promotion of healthcare to maximize the quality, safety, and efficiency within the healthcare system [15, 16]. POC devices utilizing information technologies (e.g., decision support systems or electronic health records) are necessary for creating electronic healthcare systems [17]. Hence, understanding the current clinical practices related to POC devices and technologies in primary care with the changes in patients’ needs and demographics might provide valuable evidence for guiding the development of the new model of care. This scoping review aims to map the current evidence regarding POC device usage in primary care. Specifically, we aim to identify the current POC devices used in primary care. Second, we want to identify the clinical conditions for which healthcare providers in primary care found POC devices useful in supporting the diagnostic decision.
2. Methods
We conducted a scoping review following the methodological framework proposed by Arksey and O’Malley [18] and further refined by Levac et al. [19] and the Joanna Briggs Institute (JBI) [20]. The review process consisted of five steps: (1) identifying the research question, (2) identifying relevant studies, (3) selecting studies to be included in the review, (4) charting the data, and (5) collating, summarizing, and reporting the results. The protocol was registered in the Open Research Framework (OSF) (https://osf.io/f3hqr).
2.1. Step 1: Identifying the Research Question
- 1.
What are the point-of-care devices used in the primary care setting for adult patients?
- 2.
What are the medical conditions associated with using each point-of-care device for adult patients?
These questions were deemed sufficiently broad to capture the current state of knowledge while maintaining a manageable scope for the review. A preliminary search of the literature confirmed that a scoping review on this topic had not been previously conducted and that there was sufficient literature to warrant the review.
2.2. Step 2: Identifying Relevant Studies
We collaborated with a medical librarian to develop a comprehensive search strategy, including keywords, Medical Subject Headings (MeSH), and databases. The search strategy was designed to balance breadth and specificity, ensuring that all relevant studies were captured while minimizing the inclusion of irrelevant papers. The following databases were searched in March 2023: MEDLINE (Ovid), CINAHL, Embase, Web of Science, Cochrane Library, and Google Scholar. The search terms included variations of “point of care testing,” “primary care,” “adult patients,” and general types of POC devices (e.g., rapid tests, biomarker indicators, and bedside tests). The complete search strategy is available in the (Supporting Information, eTable 1).
Inclusion criteria were defined as primary, secondary, and gray literature written in English, available in full text, involving adult human participants, POC devices, and specified patient medical conditions. In addition, as previously mentioned, to build on Lingervelder et al.’s systematic review [10] and to ensure the currency of results particularly which relate to the POC devices and technologies, the literature was limited to papers published between 2018 and 2023. Exclusion criteria were studies not aligned with the conceptual framework, using laboratory or in-hospital POC devices, using self-test POC devices, providing general summaries of POC devices, or lacking actual application of the POC device. Editorials, protocols, and letters were also excluded. For studies that included both adult and pediatric populations, only data pertaining to adults were extracted when possible.
2.3. Step 3: Selecting Studies to be Included in the Review
We used EndNote Version 20 [22] for reference management and duplicate removal. To identify potentially relevant studies from the initial search results, we constructed and validated a high-performance machine learning classifier based on the bidirectional encoder representations from transformers’ (BERTs) architecture following the guidance of Marshall and Wallace [23]. The training process involved two reviewers (AA and RF) independently screening a random sample of 10% of the papers for inclusion, discussing discrepancies until a high level of agreement (≥ 90%) was achieved, and annotating titles and abstract records from the database. We assessed the model’s performance against our classification using precision, recall, specificity, and accuracy metrics. Following the validation, we employed the algorithm to analyze the titles and abstracts of the remaining publications, generating predictions for inclusion or exclusion. The inclusion criteria were then applied to the remaining papers, with one reviewer (AA) conducting the screening and the second reviewer (RF) verifying a random sample of 20% of the papers to ensure consistency. Disagreements were resolved through discussion; if not, disagreements were reconciled by a third reviewer (OU). The screening process involved reading both the title and abstract of each paper. If an abstract was not available, a full-text review was conducted. Screening papers by title alone was deemed insufficient, as the contents of a paper are not always well reflected in the title. The reasons for excluded articles will be highlighted in a PRISMA flowchart [24].
2.4. Step 4: Charting the Data
A data extraction form was collaboratively developed by the team, including items such as study type, clinical setting, study location, name and type of POC device, and disease type (Supporting Information, eTable 2). Additional categories were included to capture data specific to the research questions, such as the diagnostic accuracy of the POC devices and the impact on patient outcomes. An overview of the extracted data items is included in the Supporting Information. Data extraction for the included full-text articles was conducted using a bespoke online form on the Airtable website [25]. The extraction form was tested on 10% of the included articles. As the primary focus of this review is to map the evidence regarding POC devices used in primary care and following the JBI recommendations, we did not conduct a critical appraisal.
2.5. Step 5: Collating, Summarizing, and Reporting the Results
We conducted both numerical and thematic analyses of the extracted data. Numerical findings, such as the frequency of different types of POC devices and diseases, were presented in tables and charts to highlight the most salient aspects of the review. Descriptive statistics were used to summarize the characteristics of the included studies, such as study design, sample size, and geographical location. Thematic analysis was conducted using an inductive approach, following the six-phase process described by Braun and Clarke [26]. This involved familiarization with the data, generating initial codes, searching for themes, reviewing themes, defining and naming themes, and producing the report. Two reviewers independently (AA and OU) coded a subset of the extracted data, meeting regularly to discuss and refine the codebook. The entire dataset was then coded using the agreed-upon codebook, with the reviewers meeting to discuss any new codes or themes that emerged.
In this study, POC devices will be classified based on two primary criteria: their technological function and the specific disease conditions they were used to diagnose or monitor. This classification approach was designed to provide a comprehensive understanding of the diverse applications and roles of these devices in various clinical settings in primary care. The results of the scoping review were reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA–ScR) checklist [27] (Supporting Information, eTable 3). This included a flow diagram depicting the study selection process, a summary of the characteristics of the included studies, and a narrative synthesis of the key findings organized by themes.
3. Results
The keywords used to search the databases yielded 23,569 references after duplicates were removed, from which, 22,311 were removed after titles and abstracts’ screening and applying the automation tool, and 1057 were excluded based on a full-text review. A further 14 references were added to the final review. The literature search process and the reasons for exclusion are shown in Figure 1.
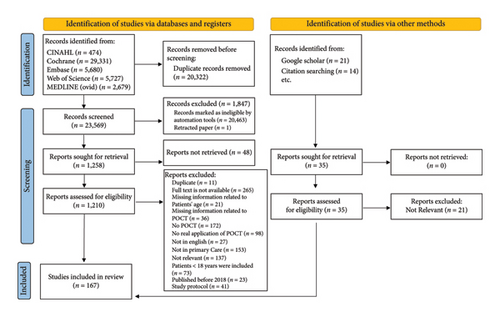
3.1. Overview of the Included Studies
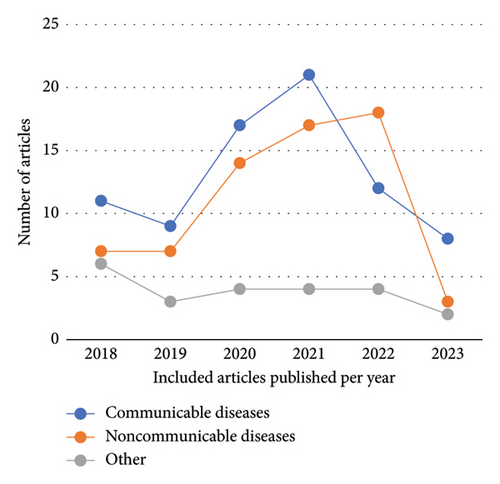
The number of published articles increased in 2020 and 2021, followed by a decline in the following years (Figure 2). The methodologies used in the articles were quantitative for 155 (92.6%) articles, qualitative for 7 (3.8%) articles, and 5 (3.6%) were mixed method studies. The top five countries with the highest publications were the United States (n = 17, 10.18%), the United Kingdom (n = 17, 10.18%), South Africa (n = 13, 7.78%), Spain (n = 12, 7.19%), and Australia (n = 9, 5.39%). The publications from high-income countries were (n = 108, 65.01%), compared to (n = 29, 18.93%) in upper-middle-income countries, (n = 20, 11.20%) in LMICs, and (n = 10, 4.86%) in low-income countries. Most of the studies were conducted in urban settings (n = 128, 79.7%), followed by rural settings (n = 18, 10.9%), both rural and urban settings (n = 20, 9.3%), and not mentioned (n = 1, 0.1%). A descriptive summary of the articles’ characteristics is mentioned in Table 1.
| Authors | Country | Date | Sample size (age/gender) | Method | Name of the POC device | Disease/condition |
|---|---|---|---|---|---|---|
| Sullivan et al. | United States of America–Brazilian Hospital | 2021 | 814 patients (mean age was 24, 88% females, 12% males) | Quant | Sonosite, POCUS |
|
| Yadav et al. | India | 2020 | 102 females (23.3 +-3.4/female only) | Quant | HemoCue 201+ and HemoCue 301 | Anemia in pregnant women |
| Zhenqiang Wu | New Zealand | 2022 | 4102 participants (50–84 years/NA) | Quant |
|
Cardiovascular diseases |
| Florian Wolf et al. | Germany | 2022 | 11 physicians (NA/general doctors and pediatricians) and 22 medical assistants (doctors/10 female, and 1 male. Assistants were all female) | Quant | ID NOW COVID-19 rapid test | SARS-CoV-2 |
| Wintergerst et al. | Germany | 2021 | 75 patients (mean ages are 69/37 male and 38 female) | Quant | EyeArt Version 2.1.0 | Diabetic retinopathy |
| Williams et al. | United Kingdom | 2019 | 100 patients (72–92 years/61 female) | Quant | Vscan, POCUS | Valvular diseases |
| Williams et al. | Australia | 2019 | 174 participants (35–48 years/68% were male) | Quant |
|
Hepatitis C virus |
| Willemsen et al. | Netherlands and Belgium | 2019 | 303 patients. (NA/48.8% male) | Quant | Clinical decision support system and POCT | Acute coronary syndrome |
| Webster et al. | Australia | 2021 | 4477 (mean age for the two arms was inter 50.4|cont 50.7/the gender for inter 58.7% female|contr 59.3%) | Quant | HealthTracer decision support system | Cardiovascular disease |
| Caroline Ward | United Kingdom | 2018 | 141 patients (NA/NA) | Quant | Afinion, C-reactive protein | Lower respiratory tract infection |
| Wake et al. | South Africa | 2019 | 493 patients (> 18 years, NA) | Quant | Cryptococcal antigen (CrAg) lateral flow assay (LFA) | Cryptococcal meningitis |
| Vohra et al. | India | 2022 | 1057 pregnant women (mean age 24.4 years/all females) | Quant |
|
Anemia in pregnant women |
| Virdee et al. | United Kingdom | 2022 | 1,325,996 eligible patients but model validation used 250,000 patients (NA/NA) | Quant | Prediction model based on full blood count | Colorectal cancer |
| Verbakel et al. | United Kingdom | 2020 | 462 adult patients (median age 81/59.3% female) | Quant |
|
Agreement level with lab test results |
| Velu et al. | Malaysia | 2022 | 30,691 patients’ records (NA/NA) | Quant | Naïve Bayes and C4.5 decision tree models | Liver disease |
| Vaes et al. | Belgium and the Netherlands | 2020 | 567 patients (mean age 84 years with CVD, 85 years without CVD/70 males with CVD, 122 males without CVD) | Quant | Risk model using biomarkers | Cardiovascular diseases |
| Tollanes et al. | Norway | 2020 | 22,778 (median age was 67 years/43.7%) | Quant |
|
Diabetes |
| Thell et al. | Austria | 2021 | 541 included adults (49.1 -+19.7 years/54.7% females) | Quant | SARS-CoV-2 Rapid Antigen Test | SARS-CoV-2 |
| Tegenaw et al. | Ethiopia | 2023 | 4 (NA/all are females) | Quant | CDSS | Pregnant patients, antenatal and postnatal care |
| Tadesse et al. | Ethiopia | 2020 | 199 females (26.4 years mean/all females) | Quant | Illumigene | Malaria in pregnancy |
| Sundararajan et al. | Uganda | 2021 | 17 healers with 500 clients (intervention 40 years mean, control 47 years/44% female intervention group, 25% female control group) | Quant | OraQuick HIV testing kits | HIV |
| Stafylis et al. | United States of America | 2018 | 274 patients (> 18 years/244 male, 30 female) | Quant |
|
|
| Spyratos et al. | Greece | 2021 | 5226 patients (58% +- 12.7 years/61.5% male) | Quant | SpiroLab II | COPD |
| Spooner et al. | South Africa | 2022 | 578 patients (31 median age/430 years female) | Quant | PIMA POC CD4 | HIV |
| Spaeth et al. | Australia | 2018 | 200 patients (NA/NA) | Quant | i-STAT POCT | Chest pain, chronic renal failure due to missed dialysis session(s), and acute diarrhea |
| Sow et al. | Senegal | 2022 | 3665 participants (median age 49 in community, 43 in workplace/75.3% is female in communities, 78% are male in workplaces) | Quant | Determine®, Hepatitis B Surface Antigen (Hbsag) Screening Test | Hepatitis B virus |
| Sossen et al. | South Africa | 2021 | 115 adults prospectively and 77 retrospectively | Quant | Determine lipoarabinomannan Ag assay | Tuberculosis |
| Solomon et al. | India | 2020 | 11,993 at baseline and 11,721 at evaluation (the median age of evaluation survey was 26–36/493 female) | Quant |
|
Hepatitis C virus |
| Smits et al. | Netherlands | 2022 | 84 patients, 29 nurses and 11 GPs (mean age 64.6 years for HbA1c survey, 59.5 years for professional glucose/53% male for HbA1c, 42% male for professional glucose) | Quant |
|
Diabetes |
| Shiha et al. | Egypt | 2020 | 1475 participants (for the village clinic positive patients: median age was 55 years, and for the 2nd site: their median age was 47 years/for the 1st site: 60.5% male, for the 2nd site: 89.5% male) | Quant | InTec RDT | Hepatitis B and C infections |
| Baker et al. | Uganda | 2021 | 144 patients (NA/NA) | Quant | Lumify®, POCUS | Cardiac and obstetric examinations. Also, pelvic, biliary, pleural, renal/bladder, Msk/soft tissue, aorta, abdomen, thyroid, testicular, Fash |
| Dalmacion et al. | Philippines | 2018 | 460 females (25 +-6.2 for rural, 26 +- 6.4 for urban/all females) | Quant | Vscan, POCUS | Obstetrics |
| Funston et al. | United Kingdom | 2021 | 29,962 females were included. (mean age was 55 years/all females) | Quant | Prediction model based on age + biomarker (CA125) prediction model (2) based on (age, ethnicity, BMI, height, abdominal/pelvic pain, distension, CA125 level, platelet level, and albumin level) | Ovarian cancer |
| Andersen et al. | Denmark | 2021 | 30 GPs (> 18 years/50% female) | Quant |
|
Obstetrics, abdominal and musculoskeletal, diagnose shoulder or knee pathology, postvoid residual urine, hydronephrosis, cholecystolithiasis and intrauterine pregnancy or to locate an intrauterine device |
| Berbudi et al. | Indonesia | 2020 | 61 patients (NA/NA) | Quant | HemoCue® HbA1c 501 system | Diabetes |
| Brookes-howell et al. | Wales, England, Spain, and Netherlands | 2019 | 35 clinicians (NA/27 females) | Qual | Flexicult | Urinary tract infection |
| Boere et al. | Netherlands | 2022 | 241 participants (84.3 years for the intervention group, 84.5 for the control group/104 females in intervention, 49 females in control) | Quant | QuikRead go, CRP POCT | Lower respiratory tract infection |
| Butler et al. | England, Netherlands, Spain, Wales | 2018 | 329 participants (mean 47.6 years/all females) | Quant | Flexicult | Urinary tract infection |
| DeGennaro et al. | Haiti | 2018 | 705 from rural, 1419 from urban (mean 40.8 years/females 61.1%) | Quant |
|
|
| Gomez et al. | United States of America | 2021 | 32 students. (18–25 years/NA) | Quant | BD Veritor SARS-CoV-2 | SARS-CoV-2 |
| Frank et al. | Australia | 2020 | 14 GPs, and 4 nurses (NA/NA) | Quant | Hemocue WBC DIFF system | Antimicrobials prescription |
| Ginting et al. | Indonesia | 2018 | 616 participants (median 42 years/76.3% females) | Quant | Combur 10 urinary dipstick test | Urinary tract infection |
| Howell et al. | Australia | 2022 | 70 participants (42.3 mean/64.3% males) | Quant |
|
Hepatitis C virus |
| Jedrzejek et al. | Poland | 2022 | 150 participants, 51.3% in primary care (≤ 50 years = 51 participants, > 50 years = 26 participants/86% female) | Quant | Flu SensDx device | Influenza virus |
| Markby et al. | Malaysia | 2021 | 15,366 adults (median age was 38 years/59.4% male) | Quant | SD BIOLINE Anti-HCV POCT | Hepatitis C virus |
| Ochom et al. | Uganda | 2018 | 15 community health workers (34 median age, 80% females) | Mixed methods | Determine HIV-1/2 | HIV |
| Petroff et al. | Germany | 2021 | 622 patients (mean 45 years/44.5% male) | Quant | Xpert HCV VL FS assay cartridge | Hepatitis C virus |
| Phillips et al. | Australia | 2023 | 10 GPs (NA/NA) | Quant | TE7 portable ultrasound machine. POCUS | Obstetric scans especially for evaluating fetal heart rate and intrauterine viability, followed by procedural, and abdominal scans |
| Puschel et al. | Chile | 2021 | 522 participants (mean 42.5, 32.7, 33 years for three different groups/217 were females) | Quant | COVID-19 IgM/IgG test | COVID-19 |
| Tajes et al. | Spain | 2020 | 3328 participants (median 56.3 years/43.5% male) | Quant |
|
|
| Saludes et al. | Spain | 2018 | 580 individuals (mean MSM 34.2 years, MSW 27.7, TWSW 30.8/male or trans women) | Quant |
|
|
| Schuijt et al. | Netherlands | 2018 | 1756 patients (18–97 years/NA) | Quant | Afinion AS100 Analyzer | Diverticulitis, acute cough |
| Schermer et al. | Netherlands | 2018 | 5450 patients for the intervention group, 6389 patients for the control group (mean 64 years for intervention, 62.7 for control/40% males in intervention, 45% males in control) | Quant | COPD-6®, model no. 4000, microspirometry device | COPD |
| Rosa et al. | Brazil | 2020 | 1390 patients (mean age was 61.9 years/63% female) | Quant | Cobas b 101®POC device | Diabetes |
| Rohde et al. | Germany | 2022 | 1450 patients (median 43 years positive, median 40 years/male 54.3% positive, male 53.7%) | Quant | Roche SARS-CoV-2 Rapid Antigen Test | SARS-CoV-2 |
| Reynolds et al. | New Zealand | 2020 | 2156 participants (mean 52 years/78% were female) | Quant | CareSens N, POC blood glucose meters and test strips | Diabetes |
| Reddy et al. | South Africa | 2020 | 642 patients, 13 healthcare providers (provider’s median age is 31 years in experienced clinics and 45.5 years for nonexperienced clinics/80% female for both clinics) | Qual | PIMA POC CD4 (now Abbot) | HIV |
| Kumar et al. | India | 2019 | 198 participants (45–64 years, ≥ 65 years/male 124 participants) | Quant | Medi-Test Combi 9 | Chronic kidney disease |
| Prince-Guerra et al. | United States of America | 2021 | 3419 patients with three age groups (1885 patients represent 55.1% of the sample aged 18–49 years, 743 represent 21.7% of sample aged 50–64 years, 555 represent 16.2% of sample aged ≥ 65 years/female 49.2%, 13.1% undisclosed) | Quant | BinaxNOW COVID-19 Ag card | COVID-19 |
| Pratt et al. | United States of America | 2022 | 11 clinicians (NA/NA) | Qual | Clinical decision support system | Diabetes and prediebetes |
| Pooran et al. | South Africa, Zambia, Zimbabwe, and Tanzania | 2019 | 1502 adult patients (> 18 years/NA) | Quant | Xpert MTB/RIF performed at the point of care | Tuberculosis |
| Pernille et al. | Denmark | 2019 | 117 women (< 30 is 50%, 31 years - 60 is 39%, > 61 years is 11%/all females) | Quant |
|
Urinary tract infection |
| Papadima et al. | France | 2018 | 981 patients (mean 34.5 years/54.5% women) | Quant |
|
HIV |
| Palomino-Padilla et al. | United Kingdom and Peru | 2023 | 456 patients in Peru and 610 patients in the United Kingdom (Peru: mean age was 37.8 for Genedia, and 39.1 years for Active Xpress. United Kingdom: 42.6 for Genedia, and 40.9 for ActiveXpress/Peru: Genedia 48.1% female, ActiveXpress 67.7% female. United Kingdom: Genedia 56.7% female, ActiveXpress 61.8% female) | Quant |
|
COVID-19 |
| O′Callaghan | Ireland | 2021 | 172 staff, 799 patients (mean 53 years/60% female) | Quant |
|
COVID-19 |
| Norman et al. | New Zealand | 2022 | 180 patients (low-risk mean age is 52, nonlow-risk mean 69, nonlow-risk referred to hospital is 69, nonlow-risk managed at home is 69 years/the percentage of female is 60.4% in low-risk, 30.4% in nonlow-risk, 32.1% in nonlow-risk referred to hospital, 23.1 in nonlow-risk managed at home) | Quant | i- STAT cTnI assay.Troponin | Ischemic myocardial infarction |
| Nemlander et al. | Sweden | 2023 | 542 case patients, 2139 control patients (mean 71.2 years/50.2% males) | Quant | Stochastic gradient boosting (SGB) predictive model | Nonmetastatic colorectal cancer |
| Naruse et al. | Japan | 2020 | 590 patients (median 65 years/34.1% female) | Quant |
|
Chronic kidney disease |
| Musabaev et al. | Uzbekistan | 2023 | 60,769 participants (≥ 18 years/16,761 male) | Quant |
|
|
| Munyemana et al. | Rwanda | 2021 | 417 patients (> 21 years/58.7% female) | Quant | SD BIOLINE HCV rapid test | Hepatitis C virus |
| Bennett et al. | United Kingdom | 2022 | 1453 patients (median 59 years/38.6 female) | Quant |
|
Chronic liver disease |
| Mclnziba et al. | South Africa | 2023 | 25 patients with HIV, and 5 health workers (18–55 years for patients/15 out of 25 patients were females) | Qual | Urine Tenofovir Rapid Assay (UTRA) | HIV |
| O’Grady et al. | United States of America | 2019 | 12 healthcare coaches (mean 36 years/75% females) | Mixed methods | Provider focused app called “the SBIRT.” Screening, brief intervention, and referral to treatment | Substance use |
| Natarajan et al. | India | 2022 | 125 patients (mean 37.6 years/all are female) | Quant | Pocket Colposcope | Precancerous cervical lesions |
| Santos et al. | Portugal | 2019 | 85 years old female | Quant | Lumify POCUS | Heart failure |
| Bobb-Semple et al. | United States of America | 2018 | 40 pregnant women, and 20 healthcare practitioners (mean 26 years/females). No information about clinicians’ age or gender | Qual | NicAlert test strip system | Smoke detection |
| Selmouni et al. | Morocco | 2022 | 9763 patients (mean 60 years female 61 years male/73.3% female) | Quant | DIAQUICK FOB test | Colorectal cancer |
| Igloi et al. | Netherlands | 2020 | 970 participants (mean 42 years/54.7% females) | Quant | SD Biosensor Rapid Diagnostic Test | SARS-CoV-2 |
| Linares et al. | Spain | 2020 | 105 patients attending the two clinics only (mean 39 years/53.3% females) | Quant | Panbio COVID-19 Ag Rapid Test Device | COVID-19 |
| Masia et al. | Spain | 2021 | 913 patients (mean age 40.6 years/46.3% men) | Quant | Panbio COVID-19 Ag Rapid Diagnostic Test | COVID-19 |
| Loots et al. | Netherlands | 2022 | 336 patients (median age is 80 years/58% males) | Quant | StatStrip Xpress lactate | Infection |
| McGonigle et al. | United States of America | 2018 | 791 patients (mean age in homeless center is 42.7 years, abuse center is 36.9 years/homeless center is 91.2% males, abuse center is 78.1% males) | Quant | OraQuick HCV Rapid Antibody Tests | Hepatitis C virus |
| Mubanga et al. | Zambia and Tanzania | 2021 | 29 end users (mean age is 39 years/17 men) | Quant | Taenia solium POC test | Taenia solium infection |
| Sayre et al. | France | 2018 | 20 patients (mean age is 49 years/11 men) | Quant | TROD INSTI Multiplex VIH 1 &2/syphilis test | HIV |
| Mukora et al. | South Africa | 2018 | 1307 tests were performed (adults/NA) | Quant |
|
|
| Mirzazadeh et al. | Iran | 2022 | 171 participants (median age is 39 years/89.5% male) | Quant |
|
|
| Miller et al. | New Zealand | 2022 | 1073 patients (mean age is 63 years/48% female) | Quant |
|
Acute myocardial infarction |
| Kahn et al. | Malawi | 2020 | 181 participants (median 40 years/55.2% female unlikely to have TB, 48.2% female probable/confirmed TB) | Quant | ClearVue 650. POCUS | Tuberculosis present in HIV patients |
| Aklilu et al. | Peru | 2021 | 171 (56% were between 30 and 49 years/all female) | Quant |
|
Breast cancer |
| Esteve et al. | Spain | 2018 | 350 participants (mean age is 42.1/266 females) | Quant | Symtomax test kit | Celiac disease |
| Holmes et al. | United Kingdom | 2018 | NA (median age 58.5 years/55% female) | Quant | Alere Afinion AS100 Analyzer | Infection/antibiotic prescriptions |
| Cooper et al. | United Kingdom | 2018 | 27,066 participants (60–74 years/to many age groups) | Quant | Risk prediction model | Colorectal cancer |
| Bindiya et al. | Mozambique | 2021 | 692 patients (median age is 29 years/all female) | Quant | POC m-PIMA HIV-1/2 VL test | HIV |
| Lasmanovich et al. | Isreal | 2022 | 53 participants (media age 41 years/79.3% male) | Quant |
|
|
| Kerkhoff et al. | United States of America | 2022 | 6631 participants (median 39.3 years/52.3% female) | Quant | A1cNow®+, HbA1c test | Diabetes and prediabetes |
| Joseph et al. | Malawi, Nigeria, Senegal, Uganda, and Zimbabwe | 2023 | 15,766 participants (median age is 38 years/All females) | Quant | Cepheid GeneXpert | Human papillomavirus |
| Faridah et al. | Indonesia | 2019 | 237 patients (NA/NA) | Quant | DENV NS1 | Dengue virus |
| Dickens et al. | United Kingdom | 2020 | 544 participants (mean age was 69.6%/64.2% were male) | Quant | Lung monitor | COPD |
| Bulilete et al. | Spain | 2021 | 1369 participants (mean age is 42.5 years, 54.3% females) | Quant | Panbio rapid antigen test kit for SARS-CoV-2 | SARS-CoV-2 |
| Barroso et al. | Spain | 2020 | 895 participants (mean age is 50 years/53.3% females) | Quant | Cobas b101 POC device | Diabetes |
| Shah et al. | United States of America | 2021 | Pre 49,053 patients, post 49,980 patients (40–75 years/all men) | Quant | Risk stratify algorithm | Prostate cancer |
| Pan et al. | China | 2021 | 2445 participants (mean age is 59.8/39.1% male) | Quant | COPD-6 screen test | COPD |
| Mutabhatsindi et al. | South Africa, Uganda, Malawi, Namibia, Ethiopia, Gambia | 2021 | 1403 patients (mean age is 35.4 years/49.3% males) | Quant | Determine™ HIV-1/2 test | HIV |
| May et al. | United States of America | 2022 | 10,081 patients (≥ 18 is 63.2%/female in the control period is 62.3%, female in the intervention period is 61.40%) | Quant | Cobas Liat Strep A assay | Strep A pharyngitis/antibiotic prescriptions |
| Martinez-Sanz et al. | Spain | 2020 | 7991 participants (median age is 43 years/65.9% female) | Quant |
|
|
| Martinez-Sanz et al. | Spain | 2020 | 7991 participants (median age was 43/65.9% female) | Quant | Anti-HCV test WB/s/p | Hepatitis C virus |
| Mamulwar et al. | India | 2021 | 2585 patients tested by POCT (mean age is 30.7 years/62.9% male) | Quant |
|
HIV |
| Magel and Conway | Canada | 2020 | 451 participants (mean age is 51 years/70% male) | Quant |
|
|
| Lubitz et al. | United States of America | 2022 | 30,715 patients (mean age control 74 years, screening 73.9 years/control 58.1% female, screening 59.7% female) | Quant | AliveCor KardiaMobile ECG device | Atrial flutter |
| Londono-Martinez et al. | Colombia | 2023 | 783 participants (patients ≥ 18 years old are 54.3% negative, 45.7% positive/all female) | Quant | Toxoplasma ICT-IgG-IgM” rapid test | Toxoplasma |
| Liu et al. | United States of America | 2021 | 180 participants (mean age is 57.4 years/46.1% male) | Quant | Automated Retinal Imaging Analysis Systems (ARIAS) | Diabetic retinopathy |
| Lim et al. | Malaysia | 2022 | 10,914 participants (mean age is 49 years/40% male) | Quant |
|
|
| Lhopitallier et al. | Switzerland | 2021 | 469 patients (median age is 53/59% female) | Quant |
|
Lower respiratory tract infection/antibiotic prescription |
| Lee et al. | United States of America | 2020 | 4293 participants (baby boomer 31% in year 1, 48% in year 2/59% male in year 1, 57% in year 2) | Quant | OraQuick HCV test | Hepatitis C virus |
| Latham et al. | Australia | 2019 | 19 participants (median age is 44 years/74% male) | Qual | OraQuick® HCV test | Hepatitis C virus |
| Lafferty et al. | Australia | 2021 | 15 healthcare workers, and 5 managers (NA/NA) | Qual | GeneXpert CT/NG assay | Sexually transmitted diseases |
| Kubiak et al. | South Africa | 2021 | 1369 patients (mean age is 31.3 years/44.4% male) | Quant | A1cNow®+, HbA1c test | Diabetes |
| Khadanga et al. | India | 2021 | 114 patients (mean age is 53.4 years/39.5% female) | Quant |
|
Diabetes |
| Kasaro et al. | Zambia | 2019 | 3213 patients (median age is 25 years/all female) | Quant |
|
|
| Holm et al. | Denmark | 2020 | 1545 patients (30–59 years is 33% of the whole sample/83% female) | Quant | Flexicult, urinary kit | Urinary tract infection |
| Ho et al. | Belgium | 2020 | 571 patients (all patients were > 18 years (no age provided)/NA) | Quant | Vikia HBsAg tests | Hepatitis B virus |
| Hirst et al. | South Africa | 2021 | 185 patients (mean age is 56.3 years/65% female) | Mixed methods | Afinion HbA1c assay POC Analyzer | Diabetes |
| Hex et al. | Belgium | 2018 | 94 patients, included in the analysis are 92 only (mean age is 72 years/56.4% female) | Quant | Roche Cardiac proBNP test strip | Heart failure |
| Heselmans et al. | Belgium | 2020 | 3815 patients (control group: mean age is 64.60 years, intervention group: mean age is 67.24 years/51.38% female in the control group, 47.59% female in the intervention group) | Quant | EBMeDs system | Diabetes |
| Hayward et al. | United Kingdom | 2020 | 47 patients (NA/NA) | Mixed methods | i- STAT | Respiratory tract infection, urinary tract infection, respiratory condition, gastroenteritis/abdominal pain, dizziness, endocrine, acute renal failure, lab test abnormal, dehydration, sepsis, heart failure, malaise/fatigue, generalized pain, chest pain, cellulitis, musculoskeletal disease, iron deficiency anemia, edema, multiple superficial injuries, terminal illness |
| Harasemiw et al. | Canada | 2021 | 1353 participants (mean age is 45.9 years/60.8% female) | Quant |
|
|
| Gomez-Ayerbe et al. | Spain | 2019 | 5329 participants (median age was 37 years/50.4% female) | Quant | INSTI Rapid HIV Test | HIV |
| Generaal et al. | Netherlands | 2021 | 152 participants (median age is 47 years/87% male) | Quant | Xpert HCV Viral Load assay, HCV RNA testing | Hepatitis C virus |
| Luna et al. | Colombia | 2023 | 244 patients (median age was 35 years/72.6% male) | Quant |
|
Syphilis |
| Gadalla et al. | England, Netherlands, Spain, Wales | 2022 | 333 patients (mean age was 48.4 years/all female) | Quant | Flexicult™ | Urinary tract infection |
| Francis et al. | United Kingdom | 2020 | 653 patients (mean age was 68.1 years/51.6% men) | Quant | Alere Afinion AS100 Analyzer, C-reactive protein (CRP) POCT | COPD/antibiotic prescribing |
| Fernandez-Lopez et al. | Spain | 2020 | 125,876 participants (multiple age groups with not exact number, mean, or median. 25–34 years is the largest group./77.0% men) | Quant |
|
HIV |
| Scognamiglio et al. | Italy | 2018 | 2949 patients (median age was 31 years/70% male) | Quant | OraQuick Advance® Rapid HIV-1/2 Antibody Test | HIV |
| Evans et al. | Malawi | 2020 | 710 patients of which 371 patients in the community healthcare centers (median age is 38 years/56% female) | Quant |
|
Kidney disease |
| Escribano et al. | Spain | 2022 | 600 patients (median age was 44 years/63% female) | Quant |
|
SARS-CoV-2 |
| Eley et al. | United Kingdom | 2020 | 268 CRP tests used (18–64 years no mean or median/NA) | Quant | Afinion CRP POCT | Acute cough/antibiotic prescribing |
| Draper et al. | Myanmar | 2021 | 633 participants (median age was 42 years/64% male) | Quant |
|
Hepatitis C virus |
| Dixon et al. | United Kingdom | 2022 | 248 tests were recorded (adults/NA) | Mixed methods | Afinion™2 point-of-care analyzer with Afinion™CRP cartridges | Antibiotic prescribing |
| Demorat et al. | France | 2018 | 270 patients (mean age was 44.2 years/51.5% male) | Quant | The INSTI HIV-1/HIV-2 Rapid Antibody Test FSB | HIV |
| Deerin et al. | United States of America | 2018 | 196 participants (mean age was 56 years/50% were male) | Quant | OraQuick® HCV Rapid Antibody Test | Hepatitis C virus |
| Darraj et al. | Saudi Arabia | 2023 | 246 patients (mean age was 51.6 years/51.2% female) | Quant |
|
Diabetes |
| Currin et al. | South Africa | 2021 | 700 participants (< 30 (i.e., the lab results) 45 years, ≥ 30 43 years, > 300 55 years/NA) | Quant |
|
Chronic kidney disease |
| Currin et al. | South Africa | 2021 | 674 participants (median age was 43 years/31% male) | Quant |
|
Chronic kidney disease |
| Crocker et al. | United States of America | 2021 | 530 intervention (POCT), 377 control (mean age was 66.8 years in control, and 69.9 years in intervention/females were 44% in intervention, 44% in control) | Quant | DCA Vantage Analyzer | Diabetes |
| Chugh et al. | United States of America | 2022 | 175 patients (mean age was 68.2 years/54.6% male) | Quant | Vscan. POCUS | Valvular heart disease, left ventricular function (LVEF), and major extra-cardiac findings |
| Chami et al. | United Kingdom | 2022 | 27 patients (mean age was 77.6 years/63% female) | Quant | Cardiac proBNP test strip analyzed by Cobas h 232 device | Heart failure |
| Camargo et al. | Brazil | 2021 | 913 patients (< 40 years was 6.7%, 40–59 was 39.2%, 60–79 was 46.5, ≥ 80 was 7.6%/female were 66.4%) | Quant | Afinion 2 Analyzer | Diabetes |
| Butler et al. | United Kingdom | 2019 | 653 patients (mean age was 68.1 years/51.6% were male) | Quant | Afinion desktop devices for CRP POC testing | Acute COPD exacerbation/antibiotic prescribing |
| Butaru et al. | Romania | 2020 | 320 patients (median age was 66 years/64.4% female) | Quant | Anti-HCV Test WB/S/P | Hepatitis C virus |
| Bomfim et al. | Brazil | 2021 | 2691 participants (median age was 24 years/50.3% female) | Quant |
|
|
| Blanchard et al. | Canada | 2019 | 1649 patients (mean age was 58.7 years/49% were female) | Quant |
|
Agreement level with lab test results |
| Bhansali et al. | India | 2020 | 136 patients (31–50 years was 48.53%/81.61% female) | Quant |
|
Urinary tract infection |
| Bassett et al. | South Africa | 2019 | 4815 participants (median age was 27 years/51% male) | Quant |
|
|
| Bachtiger et al. | United Kingdom | 2022 | 1050 patients (mean age was 62 years/51% male) | Quant | Single-Lead AI-ECG | Heart failure |
| Agutu et al. | Kenya | 2022 | 1500 participants (median age for men was 26.4 years, for women was 25.1 years/40.9% male) | Quant | GeneXpert® HIV-1 Qual | HIV |
| Chen et al. | Australia | 2022 | 48,569 patients in the whole database. 288 patients’ health records for the experiment (median age was 46 years/female 56%) | Quant | Algorithms for CKD, diabetes, hypertension, and cardiovascular disease | CKD, diabetes, hypertension, and cardiovascular disease |
| Swe et al. | Myanmar | 2023 | 864 patients (median age was 43 years/75.7% were male) | Quant | OraQuick®HCV test | Hepatitis C virus |
| Piekarska et al. | Poland | 2020 | 723,654 participants (NA/NA) | Quant | Rapid anti-HCV kits | Hepatitis C virus |
| Novak et al. | United Kingdom | 2022 | 56 patients (median age was 86 years/35 women) | Quant | I-STAT | Frail patients |
| Ngwira et al. | Malawi | 2019 | 1842 patients (median age was 33 years/41% male) | Quant | Xpert MTB/RIF | Tuberculosis |
| Garrett et al. | South Africa | 2018 | 267 patients (median age was 23 years/all female) | Quant |
|
|
| Ellis et al. | United States of America, United Kingdom, and Germany | 2021 | Out of the 3 studies, the recruited 100 participants in the NOVEL-3 study (mean age was 50 years/64% were female) | Quant | LumiraDx D-Dimer Test | Venous thromboembolism |
| Dhanda et al. | United States of America | 2023 | 472 patients (the range was 18 to > 75 years/86.4% female) | Quant | NoMicro model | Urinary tract infection |
| Drain et al. | South Africa | 2020 | 657 patients (median age was 32 years/60% were female) | Quant |
|
HIV |
| Boere et al. | Netherlands | 2021 | 241 participants (mean age was 84.4 years/64% were females) | Quant | QuikRead go CRP kits | Lower respiratory tract infection |
| Little et al. | United Kingdom, Netherlands, Belgium, Poland, and Spain | 2019 | At baseline: 6771 patients (mean age was 49.6 years/62% female). | Quant | QuikRead go CRP kits | Lower/upper respiratory tract infection |
| Rzepka and Mania | Poland | 2023 | 631 patients (median age was 48 years/367 female) | Quant | Actim Influenza A&B test | Influenza A and B viruses |
3.2. Overall Results
To answer the review questions, the 167 identified studies were categorized into two groups (i.e., types of POC devices and types of diseases) with multiple subcategories. The percentage and count of each subcategory are provided in Table 2. In addition, a list of POCTs’ names and manufacturers was added to the Supporting Information (eTable 4).
| Types | Frequency n = 167, 100% |
|---|---|
| Communicable diseases | 78 (46.7%) |
| Antibody tests | 35 (21.0%) |
| Antigen tests | 14 (8.4%) |
| Biomarker detection tests | 8 (4.8%) |
| Other POCTs | 9 (5.4%) |
| POC molecular tests | 12 (7.2%) |
| Noncommunicable diseases–diabetes | 17 (10.2%) |
| Biomarker detection tests | 13 (7.8%) |
| POC technologies | 4 (2.4%) |
| Noncommunicable diseases–cardiovascular | 14 (8.4%) |
| Biomarker detection tests | 6 (3.6%) |
| Other POCTs | 4 (2.4%) |
| POC technologies | 4 (2.4%) |
| Antibiotic/antimicrobial prescription | 12 (7.2%) |
| Biomarker detection tests | 11 (6.6%) |
| POC molecular tests | 1 (0.6%) |
| Noncommunicable diseases–urinary tract infection | 8 (4.8%) |
| Biomarker detection tests | 2 (1.2%) |
| Other POCTs | 5 (3.0%) |
| POC technologies | 1 (0.6%) |
| Noncommunicable diseases–cancer | 8 (4.8%) |
| Biomarker detection tests | 1 (0.6%) |
| Other POCTs | 2 (1.2%) |
| POC technologies | 5 (3.0%) |
| Noncommunicable diseases–kidney disease | 5 (3.0%) |
| Biomarker detection tests | 5 (3.0%) |
| Noncommunicable diseases–respiratory | 4 (2.4%) |
| Other POCTs | 4 (2.4%) |
| Noncommunicable diseases | 4 (2.4%) |
| Biomarker detection tests | 3 (1.8%) |
| POC technologies | 1 (0.6%) |
| Pregnancy care | 3 (1.8%) |
| Other POCTs | 2 (1.2%) |
| POC technologies | 1 (0.6%) |
| Ultrasound physical examinations | 3 (1.8%) |
| Other POCTs | 3 (1.8%) |
| Noncommunicable diseases–liver disease | 2 (1.2%) |
| POC technologies | 2 (1.2%) |
| Agreement level with lab test results | 2 (1.2%) |
| Biomarker detection tests | 2 (1.2%) |
| Noncommunicable diseases–anemia | 2 (1.2%) |
| Biomarker detection tests | 2 (1.2%) |
| Tobacco screening | 1 (0.6%) |
| Biomarker detection tests | 1 (0.6%) |
| POCT blood tests | 1 (0.6%) |
| Biomarker detection tests | 1 (0.6%) |
| Frail patients | 1 (0.6%) |
| Biomarker detection tests | 1 (0.6%) |
| Autoimmune disease | 1 (0.6%) |
| Antibody tests | 1 (0.6%) |
| Substance use | 1 (0.6%) |
| POC technologies | 1 (0.6%) |
- Note: The bold values represent the expanded types of diseases associated with POCT use (noncommunicable disease in specific).
3.3. Types of POC Devices
3.3.1. Biomarker Detection Tests
Biomarker detection tests identify and measure specific biomarkers in biological samples, such as blood or other body fluids [28]. Biomarker levels can indicate disease presence, progression, or response to treatment [28]. Most of the biomarker detection tests in this review were used for diabetes (n = 13) [29–41], followed by antibiotic/antimicrobial prescription (n = 12) [42–53], communicable diseases (n = 10) [54–63], cardiovascular disease (n = 6) [64–69], multiple NCDs (n = 3) [70–72], kidney disease (n = 2) [73, 74], agreement level with lab tests (n = 2) [75, 76], and anemia (n = 2) [77, 78]. The remaining studies (one reference each) were tobacco screening [79], POC blood tests [80], frail patients [81], and colorectal cancer [82].
The biomarkers mentioned in the articles were as follows: 15 studies used hemoglobin A1c (HbA1c) as a biomarker [29–41, 71, 72]. Ten out of the 11 studies that focused on antimicrobial/antibiotic prescription used c-reactive protein as a biomarker [43–52], while one study used leukocyte and differential count change to evaluate the prescription trends [42]. Biomarkers used in cardiovascular studies were N-terminal pro-B-type natriuretic peptide (NT-proBNP) [64, 67, 68], cardiac troponin I (cTnI) [64–66, 70], cardiac troponin T (cTnT) [64, 66], and D-dimer [69]. Only one paper used high-sensitivity cTnT and cTnI assays [64].
Studies that investigated multiple NCDs used potassium [70], saliva urea nitrogen (SUN) [73], and creatinine [70, 71, 73] to measure chronic or acute renal failure risk. One study used sodium, potassium, and chloride to assess people with potential acute dehydration [70]. One study used cholesterol (total, high-density lipoprotein [HDL], and triglycerides) [71]. Two studies used urine albumin-to-creatinine ratio (UACR) [72, 83]. One study used [72] urine protein-to-creatinine ratio (UPCR) and estimated glomerular filtration rate (eGFR). All of the studies that focused on anemia used hemoglobin as a biomarker [77, 78]; only one study applied a phone application used by the end user (i.e., nurse) to document test results in real time [78]. One study used cotinine biomarkers [79].
3.3.2. Antibody Tests
Antibody tests encompass devices that detect the presence of antibodies in the blood, indicating past or present exposure to pathogens [84]. The majority of POC antibody tests in this review targeted communicable diseases (35 out of 36 papers) [85–119]. One study assessed the cost-effectiveness and usefulness of identifying patients with celiac disease (CD) in primary care using the antibody test [120]. Of all studies that focused on communicable diseases, twenty-four papers focused on hepatitis C virus (HCV) [61, 62, 85, 87–89, 92, 95, 96, 99, 100, 102, 103, 105, 107, 108, 115–119, 121–123], while eighteen papers focused on HIV [61, 86, 90, 93, 98–102, 104, 105, 109, 110, 112–114, 117, 124], five papers focused on syphilis [62, 86, 109, 111, 117], and four studies focused on hepatitis B virus (HBV) [61, 88, 92, 117]. The remaining studies were one reference each, focused on toxoplasmosis [106], taenia solium infection [97], COVID-19 [91] and SARS-CoV-2 [94].
3.3.3. POC Technologies
POC technologies include devices that leverage advanced algorithms, decision support systems, and predictive models to assist diagnostic decision-making [125]. In this review, predictive models were the most common POC technology applied in primary care (n = 8) [126–133], followed by clinical decision support system (CDSS) (n = 6) [134–139], risk prediction model (n = 4) [140–143], and mobile health app (n = 1) [144].
Studies that created predictive models targeted cardiovascular disease [128], heart failure [131], liver disease [127], colorectal cancer [126], nonmetastatic colorectal cancer [130], ovarian cancer [129], and urinary tract infection [133]. Only one study created a predictive model for multiple chronic diseases (i.e., chronic kidney disease, hypertension, diabetes, and cardiovascular disease) [132]. Studies that created a CDSS applied it for cardiovascular disease [135], acute coronary syndrome [134], antenatal/postnatal care [136], diabetes/prediabetes [137, 138], and prostate cancer [139]. Studies that created risk prediction models focused on diabetes retinopathy [140, 143], chronic liver disease [141], colorectal cancer [142], and prostate cancer [139]. Only one study developed a mobile application for healthcare providers for substance use [144].
3.3.4. Antigen Tests
Antigen tests are immunoassays that detect specific antigens related to infectious agents [145]. Most of the identified studies used antigen tests for SARS-CoV-2 infection (n = 10) [146–155] and HBV (n = 2) [122, 156]. The remaining studies, one reference each, were about influenza A and B viruses [157], cryptococcal meningitis [158], dengue virus [159], and tuberculosis [63].
3.3.5. Molecular Tests
POC molecular tests comprise devices that employ molecular techniques to identify genetic material from pathogens [160]. In this review, sexually transmitted infections (STIs) were the main cause for using molecular tests (n = 6) [161–166]. Other causes for using molecular tests were HCV (n = 4) [121, 123, 167, 168], tuberculosis (n = 3) [124, 169, 170], SARS-CoV-2 [171], antibiotic prescription [172], and malaria in pregnancy [173].
3.3.6. Other POC Tests
Other POCTs that are employed for a broad range of diagnostic purposes include point-of-care ultrasound (POCUS) (n = 11) [53, 174–183]; among them, only six papers used handheld POCUS [53, 175–177, 180, 183]. In addition, urine culture was used in five papers [184–188], POC airflow measurements test (n = 4) [189–192], pocket colposcope [193], and handheld electrocardiograph [194].
3.4. Types of Diseases
As shown in Table 2, most of the studies (n = 78, 46.7%) targeted communicable diseases, followed by NCDs (n = 66, 39.5%) and others (n = 23, 13.8%).
3.4.1. Communicable Diseases
The most common diseases that were diagnosed/screened by using POCT were HCV with 16 papers [85, 87, 89, 95, 96, 103, 107, 108, 115, 116, 118, 119, 121, 123, 167, 168], followed by HIV (n = 16) [54, 55, 59, 90, 93, 98, 101, 104, 110, 112–114, 161, 164, 166, 195], then SARS-CoV-19 (n = 7) [146–151, 171], COVID-19 (n = 6) [91, 94, 152–155], tuberculosis (n = 3) [57, 169, 170], and lower respiratory tract infection (n = 3) [46, 47, 49].
3.4.2. NCDs
Most of the studies used POCT to assess for diabetes (n = 17) [29–41, 137, 138, 140, 143], followed by cardiovascular diseases (n = 14) [64–69, 128, 131, 134, 135, 175, 180, 183, 194], cancer (n = 8) [82, 126, 129, 130, 139, 142, 182, 193], urinary tract infections (n = 8) [133, 184–188, 196, 197], respiratory diseases (n = 6) [51, 52, 189–192], kidney disease (n = 5) [73, 74, 83, 198, 199], multiple NCDs (n = 4) [70–72, 132], liver diseases (n = 2) [127, 141], and anemia (n = 2) [77, 78].
3.4.3. Other Types of Disease/Condition
Among the 23 articles that focused on other conditions not directly related to communicable or NCDs, 10 studies examined the prescription of antibiotics/antimicrobials [42–50, 172]. Three studies examined the use of ultrasound in physical examinations [174, 176, 178]. Another three studies used POCT to provide pregnancy care [136, 177, 179]. Two studies analyzed the agreement level of POCT results with laboratory tests [75, 76]. The remaining five studies (one study each) were focused on the general use of POCT for blood tests [80], care for frail patients [81], diagnosis of CD [120], substance use detection [144], and tobacco screening [79].
4. Discussion
4.1. Main Findings
This scoping review provides a comprehensive overview of the current landscape of POCT devices used in primary care settings. Our findings highlight the diverse range of POCT devices available, including biomarker tests, antibody tests, antigen tests, molecular tests, and emerging technologies such as microfluidic devices and biosensors. These devices are used for a wide spectrum of clinical applications, ranging from communicable to NCDs. Based on our review, we identified several key practice and research implications. First, there is a need to expand the use of POCT devices for NCDs in LMICs, where the burden of these diseases is rapidly growing and difficulties in accessing healthcare (e.g., cost and travel) are more prominent. In addition, integrating POCT devices with digital health technologies, such as electronic health records, telemedicine, and mobile health applications, could enhance the efficiency and effectiveness of primary care delivery. Furthermore, research is required to evaluate the clinical and cost-effectiveness of specific POCT devices in real-world primary care settings, as well as to develop and validate novel POCT technologies that are affordable, reliable, and user-friendly. Lastly, implementing POCT in primary care requires addressing various organizational, financial, and regulatory challenges, requiring collaborative efforts among researchers, clinicians, policymakers, and industry partners.
This scoping review highlighted the current practices and mapped the evidence regarding POC devices in primary care. One hundred sixty-seven studies met the inclusion criteria and were synthesized to answer the review questions. The number of publications related to POC devices increased in 2020 and 2021, commensurating with the rapid need to diagnose patients suspected of having coronavirus [200]. In addition, aligned with the global trend [15], many POC devices utilizing information technology were identified in this review (i.e., 9.2% of the included articles). Our findings show that POC devices are mainly used for communicable diseases, particularly for LMICs and mostly in urban healthcare settings. Moreover, unlike LMIC countries, POC devices used in high-income countries are targeting NCDS in mostly urban healthcare settings.
Our findings build on Tenorio-Mucha et al.’s [13] recent scoping review focused on POCT devices for cardiometabolic diseases. They found a substantial gap in evidence regarding POCT device application in LMIC. In their review, they found that more research had examined the application of POCT devices for communicable diseases than cardiometabolic conditions [13]. Moreover, despite the increasing morbidity and mortality of NCDs [201], Tenorio-Mucha et al. argue that POCT utilization for NCDs in limited-resource settings is at an “early stage.” In contrast to Tenorio-Mucha et al.’s review, the scope of our review was broader, and we did not focus on a particular disease. Their findings could be attributed to the increasing prevalence of infectious diseases in LMIC [202] with the poor availability and accessibility of the necessary diagnostics [203].
One of the Sustainable Development Goals (SDGs) targeted by the United Nations is Goal 3.4, which aims to reduce premature death caused by NCDs by one-third through prevention and treatment [204]. Our review findings show that 8.4% (n = 14) of all articles are focused on cardiovascular disease compared with 10% (n = 17) on diabetes. The number of publications is insufficient, considering that cardiovascular diseases are the leading cause of death globally [205]. This is concordant with Sohn et al. [206], Cals et al. [207], and Howick et al.’s [7] studies, and they found that diabetes mellitus was the most diagnosed condition by primary care doctors utilizing POCT. However, this does not reflect the current clinical needs for POCTs that allow primary healthcare providers to take immediate actions (e.g., treatment decisions or urgent referrals) [7, 208]. Studies conducted in the United Kingdom [208], the Netherlands [207], and an international survey on five high-income countries [7] found that primary care providers want to use POCTs to diagnose and reduce unnecessary referrals of patients with acute cardiac diseases. This might be linked to the increasing epidemic of Type 2 diabetes and obesity faced by primary care providers [206] and the fact that there are concerns regarding cardiovascular POCT accuracy [209].
4.2. Implications for Practice and Research
The findings of this scoping review mapped the current evidence and highlighted gaps that should be prioritized in future POC device applications. Despite the increasing burden of NCDs in LMICs [210], our review found that POC devices are primarily used for communicable diseases in these settings. Implementing POC devices for NCDs in LMICs could improve access to care, reduce health disparities, and enhance patient outcomes [211]. Future studies should engage diverse stakeholders, including patients, communities, and healthcare providers, to codesign, deploy, and evaluate POC testing interventions that are culturally appropriate, socially acceptable, and economically viable [212, 213]. In addition, research is needed to build the evidence base on the role of POC devices in advancing health equity and social justice in primary care.
POC devices’ integration with digital health technologies could enhance the efficiency and effectiveness of primary care delivery. Our review identified several studies that used POC technologies, such as CDSSs, risk prediction models, and mobile health applications. Integrating these technologies with POC devices could enable real-time data analysis, facilitate remote monitoring and consultation, and support evidence-based decision-making. Future research should explore the potential of emerging POC technologies, such as microfluidic devices, biosensors, and lab-on-a-chip systems, in primary care. These technologies offer the promise of miniaturization, multiplexing, and automation, which could enable more comprehensive and efficient testing at the point of care [214]. Although their performance, usability, and cost-effectiveness in primary care settings remain largely untested, a proof-of-concept study by Wang et al. demonstrated the potential of a smartphone-based microfluidic device for rapid and accurate detection of multiple infectious diseases [215].
The evaluation of the clinical and cost-effectiveness of POC devices in real-world primary care settings could help inform decision-making and resource allocation. While our review identified several studies that reported on the diagnostic accuracy and operational benefits of POC devices, there was limited evidence of their impact on clinical decision-making, patient management, and long-term health outcomes. Future research should conduct pragmatic clinical trials and observational studies to assess the effectiveness and cost-effectiveness of POC devices in different primary care populations and settings [216]. For example, a cluster-randomized trial by Boere et al. found that using POC C-reactive protein testing to guide antibiotic prescribing for acute respiratory infections in primary care reduced antibiotic use without compromising patient safety [49]. Similar studies are needed for other types of POC devices and clinical conditions to inform evidence-based practice and policy.
The implementation of POC devices in primary care requires addressing various organizational, financial, and regulatory barriers. Several studies in our review reported challenges to implementing POC devices in primary care, related to training and support for healthcare providers, quality control and data management, reimbursement, and regulatory issues [217]. Overcoming these barriers requires collaborative efforts among policymakers, healthcare organizations, industry partners, and researchers [218]. In addition, applying implementation science frameworks and methods in evaluating barriers and enablers of POC device application will systematically facilitate effective and sustainable long-term adoption in different primary care contexts [219].
4.3. Study’s Strengths and Limitations
A key strength of this review is that we conducted a comprehensive search of multiple electronic databases and gray literature sources, using a broad range of search terms and a clear conceptual framework. This approach aimed to capture the breadth and depth of the available evidence on POC devices used in primary care settings. In addition, we followed a rigorous and transparent methodology based on the JBI guidance and the PRISMA–ScR checklist to systematically identify, select, and chart the relevant studies. This enhances the reproducibility and trustworthiness of our findings. Furthermore, we involved a multidisciplinary team of researchers, clinicians, and stakeholders throughout the review process to provide diverse perspectives and expertise on the topic.
This review has some limitations that should be acknowledged. Despite the comprehensiveness of our search strategy, we only included studies published in English, which may have excluded relevant research from non-English speaking countries. Given the global nature of the topic and the importance of POC testing in LMICs, this language restriction may limit the generalizability of our findings [220]. Second, we focused on studies published between 2018 and 2023 to provide an updated overview of the recent literature on POC devices in primary care. While this approach ensures the currency and relevance of our findings, it may have excluded earlier studies that could provide valuable insights into the historical development and adoption of POC testing in primary care. Third, a notable limitation of our review is that we were unable to access full texts for 265 papers (21% of potentially eligible studies) despite extensive efforts through institutional access and interlibrary loans. This relatively high proportion of inaccessible texts could potentially impact the comprehensiveness of our findings, particularly if these papers contained unique or contrasting evidence about POC device implementation in primary care settings. While our included sample of 167 papers still provided rich data and clear thematic patterns, suggesting adequate coverage of the topic, we cannot rule out the possibility that the inaccessible papers might have provided additional insights or alternative perspectives. Fourth, we only included studies that involved adult populations, which may have excluded relevant research on POC testing in pediatric primary care settings. While this population’s focus aligned with our research questions and the high burden of chronic diseases in adult populations, it may limit the applicability of our findings to children and adolescents. Lastly, we did not assess the methodological quality or risk of bias of the included studies, as this is not typically required for scoping reviews [221]. While we provide a descriptive overview of the study designs and methods used, we cannot draw conclusions about the internal validity or reliability of the individual studies. Despite these limitations, this scoping review provides a valuable and timely overview of the current landscape of POC devices used in primary care settings. The findings can inform future research, policy, and practice initiatives to optimize the implementation and impact of POC testing in primary care.
5. Conclusions
In conclusion, this scoping review maps the current evidence on POCT devices in primary care and identifies key research gaps and future directions for the field. While POCT has the potential to transform primary care delivery and improve patient outcomes, realizing this potential will require targeted research, policy, and practice initiatives that prioritize the needs of diverse primary care populations and settings. As the burden of chronic diseases continues to grow and the demand for patient-centered care increases, POCT is likely to play an increasingly important role in primary care. This review provides a foundation for future research and innovation in this rapidly evolving field.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this research.
Acknowledgments
The research team would like to thank Sam Johnson for her assistance in developing the search strategy.
Supporting Information
eTable 1: Search strategy.
eTable 2: Scoping review extraction table fields.
eTable 3: Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA–ScR) Checklist.
eTable 4: List of POCTs’ names and manufacturers.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article.




