Potential Biomarkers in Pleural Effusions: ACE1, ACE2, and KLK12 Levels
Abstract
Aim: This study aims to investigate the potential roles of angiotensin converting enzyme (ACE) 1 and ACE2, along with kallikrein-related peptidase 12 (KLK12) levels in pleural fluids and serum, in distinguishing between transudative and exudative pleural effusions.
Methods: Pleural fluid and serum samples were collected from a total of 46 patients. Levels of ACE1, ACE2, and KLK12 were measured using the ELISA method. Patients were classified as having transudative or exudative pleural effusions based on Light’s criteria. Data were analyzed using statistical methods.
Results: According to the study findings, ACE1 levels in pleural fluid were significantly higher in the exudative group compared with the transudative group. There was no significant difference observed in ACE2 levels between the two groups. Interestingly, KLK12 levels were found to be higher in the transudative group compared with the exudative group. In serum, both ACE1 and ACE2 levels were significantly higher in the exudative group.
Discussion: These results indicate that ACE1 and KLK12 may serve as important biomarkers in the diagnostic evaluation of pleural effusions. In particular, the pleural fluid to serum (PF/S) ratios of ACE1 and KLK12 showed statistically significant differences between transudative and exudative groups, highlighting their potential in differentiating pleural effusion etiologies. While pleural fluid ACE2 levels alone did not show a significant difference, serum ACE2 concentrations and PF/S ACE2 ratios differed significantly, suggesting possible systemic changes. These findings underscore the importance of evaluating serum parameters alongside pleural fluid analysis as such comparisons may enhance diagnostic accuracy. However, the relatively small sample size limits the generalizability of these results, and further studies with larger cohorts are warranted to confirm these findings.
1. Introduction
Under normal physiological conditions, only a small amount of fluid exists in the pleural space. However, this fluid balance can be disrupted by more than 60 pathological conditions, potentially leading to excessive accumulation due to impaired resorption and/or increased production. To guide diagnosis, an essential first step is to classify pleural effusions as either transudates or exudates [1, 2].
Transudative effusions are typically caused by systemic factors that alter hydrostatic or oncotic pressures, resulting in fluid accumulation. Common causes include congestive heart failure, which often leads to bilateral effusions; hepatic cirrhosis, causing hepatic hydrothorax; nephrotic syndrome due to hypoalbuminemia; and occasionally pulmonary embolism, which can also present with an exudative pattern. Less common transudative causes include myxedema and sarcoidosis.
Exudative effusions, in contrast, result from local factors such as inflammation, infection, or malignancy. These include pleural metastases, lung and breast cancer, and mesothelioma. Infectious causes—such as parapneumonic effusions related to community-acquired or nosocomial pneumonia, empyema, and tuberculosis—are also significant. Pulmonary embolism can sometimes lead to an exudative effusion. In addition, gastrointestinal conditions such as pancreatitis, intra-abdominal abscess, and esophageal perforation may contribute. Rheumatologic and vasculitic diseases such as rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, amyloidosis, granulomatosis with polyangiitis (Wegener’s disease), and systemic sclerosis are recognized causes. Other exudative etiologies include lymphangioleiomyomatosis, Langerhans cell granulomatosis, Meigs syndrome, certain medications, radiation-induced injury, and hemothorax. Though not discussed in detail here, chylothorax—marked by the presence of chylous fluid—is another distinct entity [3].
Angiotensin (Ang) peptides are bioactive molecules that play central roles in the renin–Ang–aldosterone system (RAAS), a complex hormonal network that regulates blood pressure, fluid balance, and electrolyte homeostasis [4]. This pathway begins with angiotensinogen, a globular protein produced by the liver. Renin, an aspartyl protease secreted by juxtaglomerular cells of the kidney cleaves angiotensinogen to form the decapeptide angiotensin I (Ang I). Ang I is then converted into the potent vasoconstrictor angiotensin II (Ang II) by Ang-converting enzyme (ACE), a membrane-bound zinc metalloproteinase predominantly found in the pulmonary endothelium [5]. Ang II, the main effector of RAAS, binds to Ang II type 1 receptors (AT1Rs), triggering vasoconstriction, aldosterone release, sodium retention, and sympathetic activation.
The kallikrein–kinin system (KKS) functions as an intrinsic biochemical cascade, influencing several physiological processes, including blood coagulation, kidney function, blood pressure regulation, and innate immune responses [6]. Kallikrein-related peptidase 12 (KLK12), a recently identified member of the human tissue kallikrein family, is a secreted serine protease. It is initially produced in an inactive proenzyme form and becomes active through autoactivation. KLK12 exhibits trypsin-like activity by cleaving peptide bonds following arginine and lysine residues. Once active, it is prone to rapid autodegradation. Its enzymatic activity can be effectively inhibited by zinc ions and α2-antiplasmin, which form stable covalent complexes with the enzyme [7, 8].
2. Materials and Methods
2.1. Collection of Samples
This study involved the measurement of ACE1, ACE2, and KLK12 levels in pleural fluid and serum samples obtained from 46 patients with pleural effusion (23 transudative and 23 exudative). Patients were classified as having transudative or exudative pleural effusions based on Light’s criteria, a clinical guideline used to distinguish between the two types, primarily based on pleural fluid protein and lactate dehydrogenase (LDH) levels. Blood and pleural fluid samples were centrifuged at 3500 RPM for 10 min at 4°C to obtain serum and supernatant, which were then stored at −20°C until analysis.
2.2. Patient Selection and Potential Biases
Patients were consecutively enrolled based on availability of both pleural fluid and serum samples and categorized as having transudative or exudative effusions according to Light’s criteria. To minimize selection bias, no restrictions were applied based on age, sex, or underlying disease etiology beyond the diagnostic classification. All samples were processed under the same preanalytical conditions to ensure comparability.
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) Analyzes
Pleural fluid and serum samples were analyzed using the ELISA method, a widely employed laboratory technique for quantitatively detecting specific proteins in biological samples through antigen–antibody reactions. ELISA kits from BT-LAB (Cat. no: E5532Hu for KLK12, E0760Hu for ACE1, and E3169Hu for ACE2) were used according to the manufacturer’s instructions.
All ELISA analyses were performed in duplicate, and standard curves were generated using the calibrators provided in each kit. The intra-assay and interassay coefficients of variation (CVs) were below 10%, in accordance with the manufacturer’s specifications. Quality control samples were included in each run to ensure assay reliability and consistency. Absorbance values were measured using a microplate reader at 450 nm, and the concentrations were calculated via standard curve interpolation.
2.4. Statistical Analysis
The normality of data distribution within each group was assessed using the D’Agostino and Pearson omnibus test. All data were found to be normally distributed, as supported by QQ plots (see Figure 1). Given the normal distribution and the comparison of two independent groups, unpaired (two-tailed) t-tests were employed to assess differences in biomarker levels between transudate and exudate groups. Homogeneity of variances was verified using the F-test. As no more than two groups were compared at any point, correction for multiple testing was not required. A p value of < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism Version 8.0.1 (GraphPad Software, Inc., CA, USA).
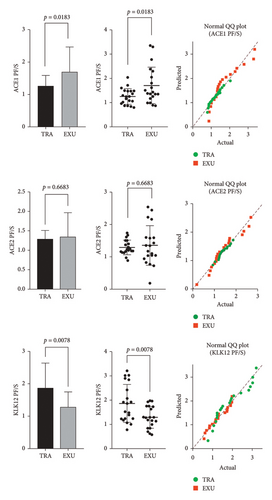
3. Results
The comparison of ACE1, ACE2, and KLK12 levels between transudative and exudative pleural effusions revealed several significant differences. According to pleural fluid/serum (PF/S) ratios, ACE1 levels were significantly higher in the exudative group (1.707 ± 0.7551 ng/mL) compared with the transudative group (1.253 ± 0.3284 ng/mL, p = 0.0183). Conversely, KLK12 PF/S ratios were significantly elevated in the transudative group (1.865 ± 0.7814 ng/mL) relative to the exudative group (1.297 ± 0.4546 ng/mL, p = 0.0078). No significant difference was found in PF/S ratios for ACE2 (p = 0.6683).
In pleural fluid samples, ACE2 levels were significantly higher in the transudative group (4.388 ± 1.132 ng/mL) than those in the exudative group (3.384 ± 1.107 ng/mL, p = 0.0049). However, ACE1 and KLK12 levels in pleural fluid did not show statistically significant differences between the groups (p = 0.3350 and p = 0.0613, respectively).
In serum samples, both ACE1 and ACE2 levels were significantly higher in the transudative group (14.14 ± 5.181 ng/mL and 3.500 ± 0.9898 ng/mL, respectively) than those in the exudative group (9.760 ± 4.754 ng/mL and 2.596 ± 0.8271 ng/mL, respectively), with p values of 0.0083 and 0.0021. There was no significant difference in serum KLK12 levels between the two groups (p = 0.6925).
The findings of this study indicated that ACE1 levels in pleural fluid were significantly higher in the exudative group compared with the transudative group (p = 0.0183). This suggests that ACE1 is an increased enzyme in exudative pleural effusions and may be considered as a potential biomarker. Conversely, there was no significant difference observed in ACE2 levels between the two groups (p = 0.6683), implying limited diagnostic value of ACE2 in pleural fluid analysis (Table 1 and Figure 1).
| Groups | Analytes (ng/mL) | Transudative | Exudative | p value |
|---|---|---|---|---|
| PF/S | ACE1 | 1.253 ± 0.3284 | 1.707 ± 0.7551 | 0.0183 |
| ACE2 | 1.288 ± 0.2261 | 1.351 ± 0.6122 | 0.6683 | |
| KLK12 | 1.865 ± 0.7814 | 1.297 ± 0.4546 | 0.0078 | |
| PF | ACE1 | 17.00 ± 5.486 | 15.21 ± 6.122 | 0.3350 |
| ACE2 | 4.388 ± 1.132 | 3.384 ± 1.107 | 0.0049 | |
| KLK12 | 39.68 ± 10.34 | 32.78 ± 12.19 | 0.0613 | |
| S | ACE1 | 14.14 ± 5.181 | 9.760 ± 4.754 | 0.0083 |
| ACE2 | 3.500 ± 0.9898 | 2.596 ± 0.8271 | 0.0021 | |
| KLK12 | 24.93 ± 12.48 | 26.29 ± 8.961 | 0.6925 | |
- Note: Values are presented as the mean ± standard deviation. Statistical significance was considered at p < 0.05. PF/S: ratio of pleural fluid results to serum results, S: serum.
- Abbreviation: PF, pleural fluid.
In the transudative group, KLK12 levels were found to be significantly higher compared with the exudative group (p = 0.0078). This result indicates that KLK12 is an increased enzyme in transudative effusions and may play an important role in diagnostic evaluation. In serum, both ACE1 (p = 0.0083) and ACE2 (p = 0.0021) levels were significantly higher in the exudative group. These findings suggest that measured ACE1 and ACE2 levels in serum could provide additional information in distinguishing exudative pleural effusions (Table 1 and Figure 1).
4. Discussion
The findings of this study suggest that ACE1 and KLK12 could be significant biomarkers in the diagnostic evaluation of pleural effusions. The significant elevation of ACE1 levels in the exudative group supports its role in inflammatory and malignant processes. Literature supports ACE’s significant role in inflammation and tissue repair processes, particularly with increased levels reported in malignancies [9, 10]. This finding suggests that ACE1 could be considered a potential biomarker in exudative pleural effusions.
The higher levels of KLK12 in the transudative group indicate its association with systemic factors and potential diagnostic value in transudative effusions. Human kallikreins, including KLK12, are members of a diverse family of serine proteases involved in various physiological processes such as inflammation, fibrinolysis, and vascular regulation. KLK12, like other kallikreins, may contribute to the modulation of proteolytic cascades that intersect with the kinin–kallikrein system, which plays a critical role in vascular permeability, blood pressure regulation, and inflammatory responses [11, 12]. Although the specific functions of KLK12 in these pathways are still under investigation, its enzymatic properties, including trypsin-like activity and ability to activate other kallikreins, suggest it could participate in broader proteolytic networks similar to those mediated by plasma kallikrein [13]. The higher presence of KLK12 in transudative effusions supports its interaction with systemic factors and potential diagnostic value.
The lack of significant differences in ACE2 levels between transudative and exudative groups in pleural fluid suggests that ACE2 is unlikely to serve as a reliable biomarker for distinguishing between these effusion types. ACE2, a key regulatory enzyme within the RAAS, plays a crucial counter-regulatory role by converting Ang II into Ang-(1–7), thereby exerting vasodilatory, anti-inflammatory, and antifibrotic effects, particularly in cardiovascular and pulmonary systems [14, 15]. Recent studies have highlighted the centrality of ACE2 in maintaining vascular homeostasis and protecting against RAAS overactivation, especially in the context of cardiovascular pathology and inflammatory conditions [16, 17]. Despite its established systemic role, data on ACE2 expression and regulation in pleural compartments remain limited. The findings of the current study contribute to this knowledge gap by demonstrating that pleural fluid ACE2 levels do not differ significantly between exudative and transudative effusions, further suggesting that local ACE2 activity in the pleural space may not reflect systemic pathophysiological states or offer discriminatory power for effusion classification.
In serum, the elevated levels of both ACE1 (p = 0.0083) and ACE2 (p = 0.0021) in the exudative group indicate that these parameters could provide additional information in diagnostic processes. ACE1 and ACE2 are critical components of the RAAS, and beyond their classical roles in cardiovascular homeostasis, recent studies have highlighted their involvement in inflammatory regulation and tissue remodeling processes [18, 19]. Notably, ACE2 has emerged as a multifaceted molecule, with evidence supporting its diagnostic and prognostic potential in a range of pathological conditions including chronic inflammatory lung diseases, gastrointestinal cancers, and systemic infections [20, 21]. Elevated serum ACE2 levels have also been proposed as potential biomarkers reflecting disease severity and immune status, particularly in the context of pulmonary inflammation [22]. Therefore, the increase of ACE1 and ACE2 in serum observed in this study may not only reflect the underlying inflammatory burden associated with exudative effusions but may also indicate their utility as accessible systemic markers. These findings support the potential of serum ACE measurements to complement routine diagnostic criteria and improve the overall accuracy in distinguishing exudative pleural effusions.
These findings underscore the potential diagnostic utility of ACE1 and KLK12 as promising biomarkers in the assessment of pleural effusions, particularly in differentiating between transudative and exudative types. The lack of significant diagnostic value of ACE2 in pleural fluid may reflect its context-dependent biological activity, which appears more prominent in systemic rather than local compartments. This observation highlights the complexity of the renin–Ang system and suggests the need for further investigation into the compartment-specific roles of ACE2.
Overall, the study provides important preliminary evidence that may guide the development of novel diagnostic strategies in pleural fluid analysis. Incorporating ACE1 and KLK12 into diagnostic workflows may improve accuracy and help inform more individualized clinical decision-making. Future research should aim to validate these findings in larger and more diverse patient populations, as well as explore the integration of additional molecular markers to establish a more comprehensive and reliable diagnostic panel for pleural effusions.
Although our results highlight the potential diagnostic value of ACE1 and KLK12 in differentiating between transudative and exudative pleural effusions, we recognize that certain limitations should be taken into account when interpreting the findings. The number of patients included in the study was relatively limited, which may affect the strength of broader conclusions. In addition, while we aimed to ensure group comparability, some individual clinical variables—such as comorbidities or medication use—could have influenced the biomarker levels to some extent. As for ACE2, although it plays important physiological roles, its diagnostic distinction between groups was not as evident in our data. This might be due to its complex regulation or to factors outside the scope of our current design. We believe that future studies with larger, clinically well-characterized cohorts will be valuable in further clarifying these observations and supporting the diagnostic utility of these biomarkers.
4.1. Limitations of the Study
One significant limitation of this study is its small sample size, involving only 46 patients. This could affect how broadly the findings can be applied or generalized.
5. Conclusion
This study provides evidence that ACE1 and KLK12 could be significant biomarkers in pleural fluid analysis, with potential applications in distinguishing between transudative and exudative pleural effusions. The absence of statistically meaningful variation in ACE2 concentrations between transudative and exudative pleural fluid groups implies that ACE2 may not be a useful parameter for diagnostic differentiation in pleural effusion cases. However, incorporating serum parameters may enhance diagnostic accuracy. These findings provide significant contributions toward developing new approaches in the diagnostic evaluation of pleural effusions. Future studies should include larger sample groups to further clarify and validate the clinical utility of these biomarkers.
Ethics Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Noninvasive Clinical Research Ethics Committee of Afyonkarahisar Health Sciences University on April 7, 2023 (approval number: 2023/4).
Consent
Before taking part in the study, all participants were informed about the study procedures and voluntarily gave their written consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
S.S. and A.D. contributed to the study design, supervision, and material preparation. S.S. conducted data collection and/or processing, performed data analysis and interpretation, and drafted the original manuscript. S.S. initiated the article draft and approved the final version.
Funding
This study was supported by Afyonkarahisar Health Sciences University Scientific Research Projects Coordination Unit project number 23.KARIYER.008.
Acknowledgments
This study was supported by Afyonkarahisar Health Sciences University Scientific Research Projects Coordination Unit project number 23.KARIYER.008.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




