Clinical Characteristics and Drug Susceptibility Profiling of Multidrug-Resistant Pseudomonas aeruginosa Infections
Abstract
Objective: To investigate the epidemiology of Pseudomonas aeruginosa (PA) in hospitalized patients with PA respiratory infections and to analyze the clinical characteristics and antibiotic resistance profiles of drug-resistant PA, providing valuable insights to inform clinical management strategies.
Methods: A retrospective analysis of clinical data from hospitalized patients with PA respiratory infections was collected and analyzed to investigate the clinical features and their correlation with PA drug resistance.
Results: In the study cohort, 34.1% (n = 93) of patients were classified as having multidrug-resistant Pseudomonas aeruginosa (MDR-PA), while 65.9% (n = 180) were non-MDR-PA. Logistic regression identified that male gender, ICU admission, hemiplegia, and mechanical ventilation were independent risk factors for MDR-PA infection (all p < 0.05). Multivariate analysis showed male gender (OR 2.44, 95% CI 1.15–5.17) and hemiplegia (OR 2.99, 95% CI 1.02–8.82) as significant factors for MDR-PA infection. Both analyses also confirmed mechanical ventilation as an independent risk factor for difficult-to-treat Pseudomonas aeruginosa (DTR-PA) infections. MDR-PA exhibited low susceptibility to meropenem (32.3%) and imipenem (18.3%), whereas aminoglycosides showed higher effectiveness, with over 80% susceptibility against MDR-PA and more than 50% susceptibility against DTR-PA.
Conclusion: Male patients with hemiplegia should be closely monitored for MDR-PA infections when PA is detected. Mechanical ventilation necessitates vigilance against DTR-PA. Aminoglycoside-based combination therapy offers an effective empirical option for resistant PA infections. These findings indicate the need for antimicrobial stewardship or infection control measures to address MDR-PA and DTR-PA infections.
1. Introduction
Pseudomonas aeruginosa (PA), a Gram-negative bacterium widely distributed in nature, is a major pathogen in respiratory infections, particularly among immunocompromised and hospitalized patients [1]. As a critical member of the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, PA, and Enterobacter spp.), PA is recognized by the World Health Organization (WHO) as a “priority 1: critical” pathogen due to its multidrug resistance (MDR) and high mortality rates in nosocomial infections [2]. Individuals with pre-existing structural lung abnormalities are notably predisposed to PA infections [3, 4]. It has been reported that PA accounts for up to 67.0% of community-acquired pneumonia (CAP) in patients with conditions such as bronchiectasis, severe chronic obstructive pulmonary disease, or tracheostomy [5].
Notably, the 5-year resistance rates of PA to imipenem (22.4%), aztreonam (21.5%), and meropenem (19.3%) remain high [6]. In recent years, infections caused by multidrug-resistant Pseudomonas aeruginosa (MDR-PA) have posed considerable challenges to the treatment of lower respiratory tract infections (LRTIs). Mechanisms such as biofilm formation and efflux pump systems enable ESKAPE pathogens like PA to “escape” the bactericidal effects of conventional antibiotics, further complicating therapeutic interventions [7].
A retrospective study covering the period from 2007 to 2016 on hospital-acquired pneumonia (HAP) in adults found that PA was the second most common pathogen, following Acinetobacter baumannii, with a higher prevalence of MDR-PA, especially in cases of ventilator-associated pneumonia [8]. MDR-PA was more frequent in those from respiratory infections and from intensive care unit (ICU) than non-ICU locations [9]. These infections are associated with poor clinical outcomes [10], and MDR-PA infections have been linked to higher mortality rates, particularly in elderly individuals with CAP [11]. A previous study revealed significant differences between MDR-PA and non-MDR-PA groups regarding initial inappropriate antibiotic therapy and hospitalization in more than two departments within the previous 30 days [12].
While previous studies have extensively explored risk factors, treatment options, and outcomes associated with MDR pathogens, there remains a paucity of research that delves into the distinct clinical and microbiological characteristics of difficult-to-treat Pseudomonas aeruginosa (DTR-PA). In this study, we meticulously collected and comprehensively analyzed the clinical data of patients infected with MDR-PA and compared it with the data of patients infected with non-MDR-PA. Furthermore, the MDR-PA group was further divided into two subgroups: DTR-PA, which exhibits resistance to nearly all first-line antibiotics including carbapenems, and general-resistant Pseudomonas aeruginosa (GR-PA), which shows resistance to three or more antimicrobial classes but retains susceptibility to at least one first-line agent (e.g., piperacillin–tazobactam or cephalosporins). The purpose is to elucidate the unique risk factors, clinical outcomes, and resistance patterns associated with MDR-PA (particularly DTR-PA) and provide practical guidance for clinicians dealing with MDR-PA infections.
2. Materials and Methods
2.1. Study Subjects
Between December 28, 2018, and September 28, 2021, we retrospectively collected drug susceptibility results of PA from sputum samples at the Second Affiliated Hospital of Fujian Medical University, along with relevant clinical data from the patients. Exclusion criteria included incomplete clinical data, incomplete drug susceptibility results, and age under 14 years. Comorbidities were assessed using the Charlson Comorbidity index [13]. This retrospective study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Fujian Medical University (Ethics Approval No. 2021J01123490). The requirement for informed consent was waived by the Medical Ethics Committee due to the retrospective nature of the study and the use of fully anonymized data. All data were deidentified, stored in a secure, password-protected database, and accessible only to authorized personnel. The waiver adhered to local regulations and ensured minimal risk to participants while respecting patient autonomy. All PA drug susceptibility results were interpreted following the 2021 Clinical and Laboratory Standards Institute (CLSI) guidelines for antimicrobial susceptibility testing [14].
2.2. Definition of Drug-Resistant PA
Drug resistance in PA was defined based on susceptibility testing to various antimicrobial classes, including aminoglycosides, carbapenems, cephalosporins, fluoroquinolones, penicillins with beta-lactamase inhibitors, monobactams, polymyxins, and fosfomycin. MDR-PA was characterized by non-susceptibility to three or more classes of antimicrobial agents, with at least one agent in each class [15]. Notably, susceptibility to polymyxins was classified as intermediate or resistant, acknowledging their potential ineffectiveness against pneumonia [14]. Fosfomycin susceptibility results were not available from our hospital’s sputum cultures and were therefore not included in the definition of MDR-PA in this study.
PA resistant to a group of drugs including piperacillin–tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem, ciprofloxacin, and levofloxacin was categorized as DTR-PA. Carbapenem-resistant PA (CR-PA) was defined as resistance to any carbapenem such as imipenem, meropenem, or doripenem [16]. In this study, doripenem and fosfomycin susceptibility results were excluded from the definitions of CR-PA and MDR-PA, respectively, as these data could not be obtained from our hospital’s sputum cultures. In China, testing for doripenem and fosfomycin susceptibility is not routine in clinical laboratories; commercial AST kits usually focus on more prevalent agents like meropenem and imipenem. This exclusion reflects real-world clinical practice and aligns with standardized laboratory protocols. Statistical analyses were based on available data without imputation, as the missing data were systematically absent and this handling does not significantly affect the assessment of PA resistance patterns.
2.3. Grouping of Cases
Based on drug susceptibility results, patients were categorized into two groups: the MDR-PA group and the non-MDR-PA group. Clinically, DTR-PA is characterized by a lack of effective antimicrobial agents, distinct clinical features, and resistance profiles. Therefore, the MDR-PA group was further subdivided into the DTR-PA group and the GR-PA group.
2.4. Statistical Methods
Statistical analysis was performed using SPSS 25.0 software (IBM Corp., Armonk, NY, USA). Normally distributed data were presented as mean ± standard deviation (SD), while non-normally distributed data were presented as median (interquartile range, IQR). Categorical variables were expressed as counts (%). Depending on the distribution of the data, paired sample t-tests or analysis of variance (ANOVA) was used for comparisons of continuous variables. Non-parametric tests were applied when the data did not follow a normal distribution. Specifically, the Mann–Whitney U test was used to compare differences between two groups, while the Kruskal–Wallis test was used to compare differences among three groups.
Single-factor analysis and multivariate logistic regression analysis were employed to explore factors influencing the clinical characteristics of MDR-PA infections. Variables with a p-value < 0.05 in univariate analysis were included in the multivariate logistic regression model to identify independent risk factors for MDR-PA infections. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Case Screening Process
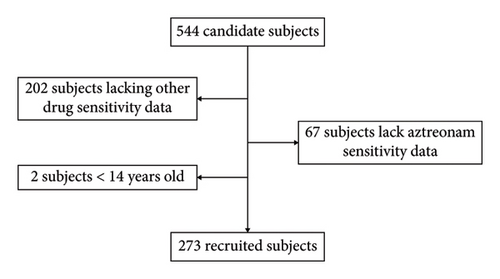
A total of 544 patients with sputum cultures positive for PA were included. Drug susceptibility results were incomplete for 269 cases, including 67 cases with missing aztreonam susceptibility data. Additionally, two patients were under the age of 14. Ultimately, 273 PA patients meeting the study’s inclusion criteria were analyzed (Figure 1). The comparison of the characteristics between the excluded subjects and the recruited subjects can be found in the Supporting Information.
3.2. Demographic and Clinical Characteristics of Patients Infected With PA
In the study cohort, 34.1% (n = 93) of patients were classified as having MDR-PA, while 65.9% (n = 180) were non-MDR-PA. No significant differences were observed between the MDR-PA and non-MDR-PA groups concerning age, Charlson Comorbidity index, length of hospital stay, and total hospitalization costs (all p > 0.05). The mean ages were 61.81 ± 12.76 years for the MDR-PA group and 60.31 ± 13.56 years for the non-MDR-PA group. Charlson Comorbidity index scores were similar, with medians of 2 (1, 3) for both groups. The mean length of hospital stay was 29.0 (15.5, 43.5) days in the MDR-PA group and 28.0 (15.0, 47.0) days in the latter. The mean total hospitalization cost was 84.0 (33.0, 154.0) thousand RMB in the MDR-PA group and 71.0 (28.0, 127.0) thousand RMB in the latter. The proportion of males was significantly higher in the MDR-PA group compared to the latter (89.2% vs. 76.1%, p < 0.05). Univariate logistic regression analysis identified male gender as an independent factor associated with MDR-PA infection (OR 0.38, 95% CI 0.18, 0.81). Overall, the incidence of PA infection varied across different departments. Specifically, the incidence was highest in the ICU (24.9%), respiratory medicine (24.5%), and neurosurgery (20.9%) departments. Moreover, the ICU and respiratory medicine together accounted for 58.0% of all MDR-PA infections, with the ICU contributing 33.3% and the respiratory medicine department 24.7%. Among PA-infected patients, the proportion of those with MDR-PA also differed by department, 45.6% being in the ICU, 34.3% in the respiratory department, and 33.3% in the neurosurgery department. Regarding complications, the incidence of hemiplegia was significantly higher in the MDR-PA group compared to the latter (11.8% vs. 5.0%, p < 0.05). No significant differences were found between the groups for other comorbidities or the Charlson index (p > 0.05). Ventilator use was more prevalent in the MDR-PA group (43.0%) compared to the latter (26.1%, p < 0.05), while other medical interventions, including bronchoscopy, tracheal intubation, surgical procedures, and puncture operations, showed no significant differences between the groups (p > 0.05) (Table 1).
| Factors | MDR-PA, n = 93 | Non-MDR-PA, n = 180 | p |
|---|---|---|---|
| Male, n (%) | 83 (89.2) | 137 (76.1) | 0.01 |
| Department of admission, n (%) | 0.01 | ||
| ICU | 31 (33.3) | 37 (20.6) | |
| Respiratory medicine | 23 (24.7) | 44 (24.4) | |
| Neurosurgery | 19 (20.4) | 38 (21.1) | |
| Other departments | 20 (21.5) | 61 (33.9) | |
| Comorbidities, n (%) | |||
| Hypertension | 28 (30.1) | 58 (32.2) | 0.72 |
| Diabetes | 12 (12.9) | 26 (14.4) | 0.73 |
| Chronic kidney disease | 6 (6.5) | 8 (4.4) | 0.48 |
| Chronic pulmonary disease∗ | 22 (23.7) | 31 (17.22) | 0.20 |
| Heart disease | 23 (25.0) | 37 (21.0) | 0.43 |
| Solid malignant tumor | 26 (28.0) | 59 (32.8) | 0.42 |
| Hemiplegia | 11 (11.8) | 9 (5.0) | 0.01 |
| Cerebrovascular accident | 37 (39.8) | 56 (31.1) | 0.15 |
| Liver dysfunction | 9 (9.7) | 22 (12.2) | 0.53 |
| Charlton index (points) | 2 (1.3) | 2 (1.3) | 0.83 |
| Risk factors for medical exposure, n (%) | 23 (24.7) | 51 (28.3) | 0.41 |
| Surgical interventions ventilator use | 40 (43.0) | 47 (26.1) | 0.01 |
| Tracheoscopy | 39 (41.9) | 68 (37.8) | 0.51 |
| Tracheal intubation or tracheotomy | 38 (40.9) | 59 (21.7) | 0.19 |
| Puncture procedure∗∗ | 17 (26.6) | 44 (24.0) | 0.71 |
- Abbreviations: ICU, intensive care unit; MDR, multidrug-resistant.
- ∗Chronic pulmonary disease includes bronchiectasis, interstitial pneumonia, chronic obstructive pulmonary disease, asthma, pneumoconiosis, and obstructive sleep apnea hypopnea syndrome.
- ∗∗Puncture procedures include thoracentesis, abdominocentesis, bone marrow aspiration, lumbar puncture, lung biopsy, and other similar procedures.
Univariate logistic regression analysis revealed that ICU admission, hemiplegia, and ventilator use were independent factors influencing MDR-PA infection, with odds ratios (ORs) of 1.34 (95% CI 1.07, 1.67), 3.89 (95% CI 1.39, 10.88), and 0.47 (95% CI 0.28, 0.79), respectively (Table 2). Multivariate logistic regression analysis further identified male gender and hemiplegia as significant factors influencing MDR-PA infection, with ORs of 2.44 (95% CI 1.15, 5.17) and 2.99 (95% CI 1.02, 8.82), respectively (p < 0.05) (Table 3).
| Variables | B | SE | Wald | p-value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Department (ICU, others) | 0.29 | 0.11 | 6.59 | 0.01 | 1.34 | 1.07, 1.67 |
| Gender | −0.96 | 0.38 | 6.43 | 0.01 | 0.38 | 0.18, 0.81 |
| Diabetes | −0.13 | 0.36 | 0.12 | 0.73 | 0.88 | 0.42, 1.83 |
| Chronic lung disease | 0.40 | 0.31 | 1.61 | 0.20 | 1.49 | 0.81, 2.76 |
| Chronic kidney disease | 0.39 | 0.56 | 0.50 | 0.48 | 1.48 | 0.50, 4.41 |
| Heart disease | 0.24 | 0.30 | 0.62 | 0.43 | 1.27 | 0.70, 2.30 |
| Solid tumors | −0.23 | 0.28 | 0.66 | 0.42 | 0.80 | 0.46, 1.38 |
| Hemiplegia | 1.36 | 0.53 | 6.70 | 0.01 | 3.89 | 1.39, 10.88 |
| Charlton index | −0.01 | 0.07 | 0.05 | 0.83 | 0.99 | 0.87, 1.12 |
| Ventilator therapy | −0.76 | 0.27 | 7.92 | 0.01 | 0.47 | 0.28, 0.79 |
| Bronchoscopy | −0.18 | 0.26 | 0.48 | 0.49 | 0.83 | 0.50, 1.40 |
| Puncture procedure | 0.14 | 0.32 | 0.20 | 0.66 | 1.15 | 0.62, 2.15 |
- Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
| Variables | B | SE | Wald | p-value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Department (ICU, pulmonology, neurosurgery, others) | −0.50 | 0.43 | 1.36 | 0.24 | 0.61 | 0.26, 1.41 |
| Gender | 0.89 | 0.38 | 5.39 | 0.02 | 2.44 | 1.15, 5.17 |
| Ventilator therapy | 0.45 | 0.33 | 1.85 | 0.17 | 1.56 | 0.82, 2.97 |
| Hemiplegia | 1.10 | 0.55 | 3.95 | 0.05 | 2.99 | 1.02, 8.82 |
- Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
3.3. Antimicrobial Susceptibility Profile of MDR-PA
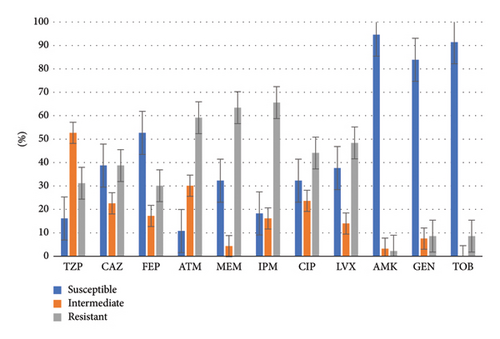
Aminoglycoside antibiotics demonstrated a high susceptibility rate exceeding 80% against MDR-PA. Cefepime exhibited a susceptibility rate of 52.7%, while other antibiotics showed sensitivities below 50%. Aztreonam exhibited the lowest susceptibility rate at 10.8%. Among carbapenems, meropenem showed a higher susceptibility rate compared to imipenem, with rates of 32.3% and 18.3%, respectively. Figure 2 illustrates the drug susceptibility characteristics of MDR-PA.
3.4. Clinical Features and Drug Resistance Characteristics of DTR-PA Patients
In the MDR-PA group, 17 patients were infected with DTR-PA, while 76 patients had non-DTR-PA infections. Of the DTR-PA cases, 10 were from the ICU, 3 from the respiratory department, and 2 each from neurosurgery and other departments. The primary diagnoses included severe pneumonia (six cases), cerebrovascular accidents (five cases), sepsis (two cases), and other conditions such as severe pancreatitis, spinal cord injury, gastric cancer, and chest trauma (one case each).
3.4.1. Clinical Differences Between the DTR-PA Group and the Non-MDR-PA Group
Significant differences were observed in the total hospitalization costs between the DTR-PA group (143 thousand RMB [63.0–167.0]) and the non-MDR-PA group (71.0 thousand RMB [28.0–127.0]) (p = 0.02). Additionally, the DTR-PA group had a higher proportion of patients with comorbid chronic kidney disease (23.5%) compared to the latter (4.4%). The incidence of mechanical ventilation was higher in the DTR-PA group (70.6%) compared to the latter (26.1%). Bronchoscopy was also performed more frequently in the DTR-PA group (64.7%) than in the latter (37.8%) (all p < 0.05).
Univariate logistic regression analysis identified ICU admission, chronic kidney disease, mechanical ventilation, bronchoscopy, and invasive procedures as significant factors influencing DTR-PA infection compared to the latter. The ORs for these factors were 5.52 (95% CI 1.97–15.50), 4.61 (95% CI 1.10–19.4), 6.79 (95% CI 2.27–20.30), and 3.02 (95% CI 1.07–8.54), respectively (all p < 0.05), as shown in Table 4. Multivariable logistic regression identified ventilator therapy as an independent factor influencing DTR-PA infection, with an OR of 4.12 (95% CI 1.07–15.89) (p = 0.04).
| Variables | Groups∗ | B | SE | Wald | p-value | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| Department (ICU, others) | G1 versus G3 | 1.71 | 0.53 | 10.54 | 0.01 | 5.52 | 1.97, 15.49 |
| G2 versus G3 | 0.39 | 0.32 | 1.52 | 0.22 | 1.48 | 0.79, 2.74 | |
| Gender | G1 versus G3 | 0.38 | 0.66 | 0.34 | 0.56 | 1.47 | 0.40, 5.34 |
| G2 versus G3 | 1.13 | 0.43 | 6.79 | 0.01 | 3.09 | 1.32, 7.24 | |
| Chronic kidney disease | G1 versus G3 | 1.53 | 0.73 | 4.36 | 0.04 | 4.61 | 1.10, 19.34 |
| G2 versus G3 | −0.12 | 0.69 | 0.03 | 0.86 | 0.88 | 0.23, 3.43 | |
| Hemiplegia | G1 versus G3 | 1.35 | 0.86 | 2.48 | 0.12 | 3.87 | 0.72, 20.85 |
| G2 versus G3 | 1.36 | 0.55 | 6.20 | 0.01 | 3.90 | 1.34, 11.37 | |
| Ventilator therapy | G1 versus G3 | 1.92 | 0.56 | 11.76 | 0.01 | 6.79 | 2.27, 20.30 |
| G2 versus G3 | 0.51 | 0.29 | 2.94 | 0.09 | 1.65 | 0.93, 2.93 | |
| Bronchoscopy | G1 versus G3 | 1.11 | 0.53 | 4.34 | 0.04 | 3.02 | 1.07, 8.54 |
| G2 versus G3 | −0.04 | 0.28 | 0.02 | 0.89 | 0.96 | 0.55, 1.67 | |
| Puncture procedure | G1 versus G3 | −0.06 | 0.53 | 0.01 | 0.91 | 0.94 | 0.33, 2.67 |
| G2 versus G3 | −0.11 | 0.29 | 0.14 | 0.71 | 0.90 | 0.51, 1.58 | |
- Abbreviations: CI, confidence interval; DTR-PA, difficult-to-treat Pseudomonas aeruginosa; GR-PA, general-resistant Pseudomonas aeruginosa; OR, odds ratio.
- ∗G1: DTR-PA group, G2: GR-PA group, and G3: non-MDR-PA group.
3.4.2. Clinical Differences Between the GR-PA Group and the Non-MDR-PA Group
Univariate logistic regression analysis indicated that male gender and hemiplegia were independent factors influencing the GR-PA group compared to those in the non-MDR-PA group. Multivariate logistic regression analysis confirmed these findings, with OR values of 3.02 (95% CI 1.28–7.10) for male gender and 3.73 (95% CI 1.26–11.0) for hemiplegia, as shown in Table 5.
| Groups∗ | Variables | B | SE | Wald | p-value | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| G1 versus G3 | Department (ICU, others) | 1.16 | 0.64 | 3.27 | 0.07 | 3.18 | 0.91, 11.14 |
| Chronic kidney disease | 0.93 | 0.84 | 1.22 | 0.27 | 2.53 | 0.49, 13.12 | |
| Ventilator therapy | 1.42 | 0.69 | 4.22 | 0.04 | 4.12 | 1.07, 15.89 | |
| Bronchoscopy | 0.40 | 0.60 | 0.44 | 0.51 | 1.49 | 0.46, 4.78 | |
| Puncture procedure | −0.98 | 0.61 | 2.59 | 0.11 | 0.38 | 0.12, 1.24 | |
| G2 versus G3 | Gender | 1.10 | 0.44 | 6.40 | 0.01 | 3.02 | 1.28, 7.10 |
| Hemiplegia | 1.32 | 0.55 | 5.64 | 0.02 | 3.73 | 1.26, 11.03 | |
- Abbreviations: CI, confidence interval; DTR-PA, difficult-to-treat Pseudomonas aeruginosa; GR-PA, general-resistant Pseudomonas aeruginosa; MDR-PA, multidrug-resistant Pseudomonas aeruginosa; OR, odds ratio.
- ∗G1: DTR-PA group, G2: GR-PA group, and G3: non-MDR-PA group. Puncture procedures include thoracentesis, abdominocentesis, bone marrow aspiration, lumbar puncture, lung biopsy, and others.
3.5. Clinical Characteristics of Patients With CR-PA Infection
A total of 79 patients with CR-PA infection were identified, representing 28.9% of all PA patients. Among these cases, two patients were resistant to meropenem but sensitive to imipenem, while two showed the opposite pattern. The remaining 74 cases were non-sensitive to both meropenem and imipenem, including resistance and intermediate susceptibility. Of the CR-PA patients, 79.7% were classified as MDR-PA, while 20.3% were non-MDR-PA. The cases were distributed across departments as follows: 31 in the ICU, 20 in respiratory medicine, 17 in neurosurgery, and 11 in other departments.
3.6. Drug Susceptibility Profiles of Patients With CR-PA and DTR-PA Infection
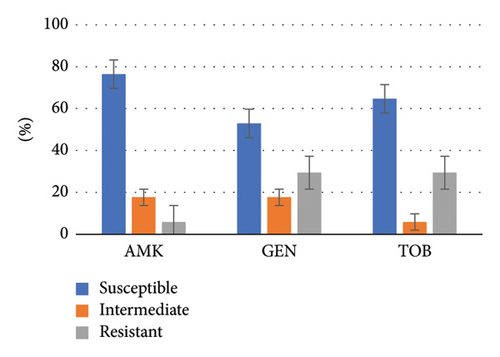
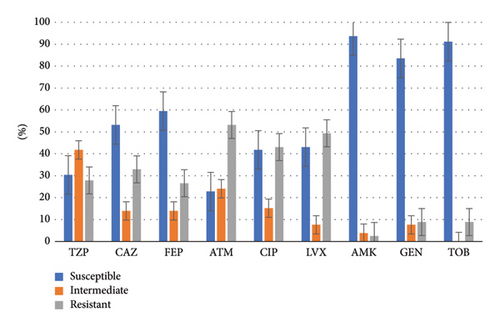
Both CR-PA and DTR-PA groups exhibited high susceptibility to aminoglycosides (exceeding 80% for CR-PA and over 50% for DTR-PA). However, susceptibility to other antibiotics was generally low, with piperacillin–tazobactam and aztreonam showing particularly low susceptibility rates (30.4% and 22.8% for CR-PA, respectively). A detailed breakdown of the drug susceptibility profiles for both groups is illustrated in Figures 3 and 4.
4. Discussion
Antibiotic resistance is now the third leading cause of death globally, after stroke and heart disease. In 2019, bacterial drug resistance was responsible for an estimated 4.95 million deaths, with 1.27 million directly linked to bacterial drug resistance. Lower respiratory infections alone accounted for over 1.5 million deaths. PA is one of the top six pathogens contributing to resistance-related mortality [17].
According to bacterial drug resistance surveillance in China from 2020 to 2021, PA was the most common non-fermentative Gram-negative bacterium, representing 8.4% of all isolates and the third most prevalent Gram-negative pathogen overall after Escherichia coli and Klebsiella pneumoniae. PA is a ubiquitous opportunistic pathogen that is commonly isolated from respiratory specimens, with the highest proportion of isolates originating from ICU, ranging from 11.5% to 39.8% [6, 18]. Studies conducted both domestically and internationally have consistently shown a trend of increasing PA infections followed by a subsequent decline [18, 19]. The percentage of MDR-PA strains has followed a similar pattern, initially increasing and later decreasing [18]. Recent years have witnessed a slowing or even a decline in the prevalence of MDR-PA infections, which may be attributed to enhanced infection control measures implemented in healthcare settings following the coronavirus disease 2019 pandemic [6].
The detection rate of PA in ICU patients was significantly higher compared to that of other hospital wards [20]. ICU admission was identified as a major risk factor for acquiring resistant PA [8, 21–23]. Our study confirmed these findings, showing the highest incidence of PA and MDR-PA in the ICU, with ICU admission independently emerging as a risk factor for MDR-PA infections. Our study found that, in addition to the ICU, the detection rate of PA was higher in the respiratory and neurosurgical departments than in other departments, mainly because of the higher prevalence of chronic lung disease in the respiratory department (where patients are more likely to receive invasive or noninvasive mechanical ventilation) and the greater risk of tracheostomy and mechanical ventilation in the neurosurgical department, and the prolonged bedridden status further increases the risk of infections caused by antibiotic-resistant pathogens.
Our study aimed to identify factors associated with MDR-PA infections in hospitalized patients. Consistent with previous research [24], we found no significant difference in comorbidity burden between the MDR-PA and non-MDR-PA groups. Previous studies have shown that chronic respiratory diseases are a risk factor for CAP caused by PA, while frequent prior use of antibiotics is a known risk factor for MDR [25]. China antimicrobial resistance surveillance system similarly found that chronic obstructive pulmonary disease was associated with PA CAP, but without MDR-PA CAP [20]. Similarly, our study did not identify a significant correlation between chronic pulmonary disease and the risk of MDR-PA infections. In contrast, mechanical ventilation and hemiplegia were more closely associated with MDR infections. Chronic pulmonary disease may be a risk factor for PA infection but not a decisive one. Of course, this hypothesis requires confirmation through larger cohort studies.
Consistent with previous studies, we found that the incidence of PA infections was significantly higher in male patients compared to that in female patients [18]. Male gender was also identified as an independent risk factor for MDR infections, and reports have indicated that the incidence of MDR infections increases significantly with age [22, 26–28]. In addition to gender, we identified hemiplegia as an independent risk factor for MDR-PA infections. The stratification of MDR-PA into DTR-PA and GR-PA groups represents significant practical implications for clinical management and infection control. This stratification also aids in identifying high-risk patient populations, such as those in ICUs or on mechanical ventilation, who may benefit from enhanced infection control measures, including stricter hand hygiene protocols, environmental decontamination, and antimicrobial stewardship programs.
Subgroup analyses revealed that both male gender and hemiplegia predominantly affected patients with GR-PA infections. PA was recognized as one of the major pathogens responsible for aspiration pneumonia in the respiratory tract [29]. Hemiplegia may increase the risk of MDR-PA infection due to factors such as reduced mobility, prolonged periods of bedriddenness, and impaired sputum clearance. Hemiplegic patients often exhibit poor oral health, and swallowing dysfunction along with impaired cough reflex further elevates the risk of aspiration and subsequent pulmonary infections [30]. Therefore, continuous oral care for hospitalized patients is crucial to prevent bacterial proliferation in the oral cavity, which can help reduce the incidence of aspiration-related pulmonary infections [31]. Furthermore, the study has also reported that the oral administration of probiotics can delay the colonization and infection of the respiratory tract by PA [32].
Medical exposure risks, such as ventilator use, are well-documented factors in MDR-PA infections, particularly in settings like ventilator-associated pneumonia [33, 34]. Our study confirmed ventilator dependence as an independent risk factor for MDR-PA infections and found that 58.8% of patients with DTR-PA infections were in ICUs. We found that medical costs for the DTR-PA group were significantly higher, nearly double those of non-resistant strains, creating substantial financial burdens for patients. Ventilator use notably exacerbates MDR, particularly among DTR-PA cases, which are often associated with severe conditions and frequent medical procedures. Patients requiring ventilator support often have severe illness and extended bed rest, which raises their susceptibility to MDR-PA colonization and infection. To effectively control infections caused by MDR, especially DTR-PA, we recommend implementing a series of enhanced comprehensive measures for ventilated patients: (1) adopting preemptive contact isolation precautions to minimize cross-transmission; (2) performing daily oral disinfection in high-risk groups (e.g., hemiplegic patients) to reduce oropharyngeal colonization; (3) conducting regular sputum and environmental cultures for the early detection of P. aeruginosa colonization or contamination; and (4) strengthening healthcare workers’ hand hygiene compliance and optimizing airway management protocols, including maintaining airway patency, promptly clearing sputum accumulation, and facilitating early extubation.
MDR-PA poses a significant clinical challenge due to its complex resistance mechanisms, which include both intrinsic and acquired factors. These mechanisms involve the upregulation of efflux systems, enzyme-mediated drug inactivation, reduced membrane permeability, and biofilm formation. Most PA strains have efflux systems, with MexB, MexE, and MexF overexpressions being particularly associated with MDR phenotypes [35]. Resistance to imipenem often involves carbapenemases, AmpC cephalosporinase, or efflux pumps [36, 37], in addition to mutations in the oprD porin gene. In our study, a significant proportion of patients displayed resistance to both meropenem and imipenem, with minimal cross-susceptibility between these two agents. Furthermore, 79.7% of CR-PA strains also exhibited MDR. This finding indicates a strong correlation between carbapenem resistance and MDR-PA infections. Notably, half of the CR-PA isolates retained susceptibility to cephalosporins, and one-third remained sensitive to piperacillin/tazobactam. These observations imply that mechanisms such as altered outer membrane porins or efflux pump overexpression may be involved in resistance in these cases.
Doripenem is a novel carbapenem antibiotic, which has been reported to exhibit superior efficacy against MDR-PA compared to other carbapenems [38, 39]. Furthermore, doripenem has been shown to have a limited ability to select for carbapenem-resistant mutants in vitro [40]. However, there are reports suggesting that the resistance rates of clinical PA isolates to meropenem and doripenem are similar [41]. Currently, doripenem is not approved as a routine therapeutic agent in China, and the lack of related antimicrobial susceptibility data does not significantly impact our clinical treatment decisions for MDR-PA patients and has limited effect on the interpretation of our results.
Unlike the commonly used agar dilution method for fosfomycin, commercial antimicrobial susceptibility testing kits predominantly employ broth microdilution methods. Due to the limitations of the susceptibility testing kits, our results did not include fosfomycin data. Nevertheless, it is undeniable that fosfomycin exerts a unique mechanism of action against PA, and cross-resistance with other antibiotics is not commonly observed [42]. Fosfomycin is often used in combination with other antibiotics for the treatment of PA infections, and combination therapy appears to be both effective and safe [43, 44].
Our findings highlight a high susceptibility of MDR-PA to aminoglycosides. Similarly, similar patterns were observed in DTR-PA and CR-PA. Despite this, aminoglycosides remain effective against resistant PA strains, including DTR-PA. This finding is consistent with previous studies [6, 45]. Notably, aminoglycosides are effective against mature PA biofilms [46], further enhancing their therapeutic value. However, the potential side effects of aminoglycosides, such as ototoxicity and nephrotoxicity (especially in cases of long-term use or high cumulative doses), may limit their clinical application. Therefore, in clinical practice, a careful balance between the efficacy and toxicity risks of these drugs is necessary. Current guidelines, including those from the Infectious Diseases Society of America (IDSA), recommend aminoglycosides primarily as combination therapy for severe PA infections, rather than monotherapy, to enhance efficacy and minimize resistance selection [47, 48]. Our findings further support guideline-concordant use by demonstrating retained susceptibility (> 80%) of MDR-PA to amikacin in this cohort, reinforcing aminoglycosides’ role as backbone agents in empirical regimens when local resistance rates permit.
In contrast, novel β-lactam/β-lactamase inhibitor combinations such as ceftazidime/avibactam (CAZ-AVI) and ceftolozane/tazobactam (C/T) demonstrate superior safety profiles and targeted activity against MDR-PA [48] However, these agents are not universally accessible in all regions, and their high cost may limit routine use in resource-constrained settings. Therefore, our assertion that “aminoglycosides presents a promising empirical treatment option” should thus be contextualized within these limitations. While aminoglycosides retain a role in combination therapy, their utility as monotherapy for LRTIs is constrained by toxicity and pharmacokinetic challenges. However, strategies such as high-dose extended-interval dosing can optimize bactericidal activity while mitigating nephrotoxicity [49]. Therapeutic drug monitoring (TDM)-guided individualized dosing regimens may optimize medication safety. In addition, adjunctive therapies like inhaled aminoglycosides may enhance lung-specific drug delivery without systemic toxicity, though evidence remains limited to specific patient subgroups [50].
Early identification of drug-resistant PA infections and a comprehensive understanding of local antibiotic resistance patterns are crucial for optimizing clinical outcomes. However, as a single-center study, differences in patient demographic characteristics and institutional factors may limit the generalizability of our findings. In terms of local factors, hospital practices likely influenced our findings. The hospital’s antimicrobial stewardship program could have shaped antibiotic resistance patterns, potentially reducing selective pressure for MDR-PA strains. Infection control measures, varying across departments like the ICU, might have affected PA spread and our observed prevalence and risk factors. Although excluding cases with incomplete drug susceptibility data may have introduced selection bias, these exclusions were necessary to ensure the accuracy and reliability of the research results. Additionally, prior antibiotic exposure, as a potential confounding factor, may affect the interpretation of antibiotic resistance and treatment outcomes.
Despite these limitations, this research provides in-depth insights into the local epidemiology of PA resistance. The data obtained can directly inform clinical decision-making, such as guiding the selection of the most appropriate antibiotics for treatment. Furthermore, this study contributes to the development and refinement of infection control strategies, which are essential for preventing the spread of drug-resistant strains. Strengthening antibiotic stewardship and reinforcing hospital infection control measures—such as mask use, hand hygiene, and patient isolation—represent key strategies in mitigating the spread of MDR-PA infections [6, 51].
5. Conclusion
Our study has identified male gender, hemiplegia, and mechanical ventilation as key risk factors for MDR-PA infections. These findings advocate for (1) targeted surveillance of high-risk populations, (2) aminoglycoside-based combination therapy guided by local susceptibility patterns, and (3) implementing stricter infection control measures for populations at risk of MDR-PA infection. Future multicenter studies are warranted to validate these risk stratification strategies and evaluate their impact on antimicrobial resistance trends.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Yuxia Du conceptualized and designed the article. Mingxia Cai and Jiaming Huang were responsible for data collection. Zhibin Zhou was responsible for data analysis and interpretation. Mingxia Cai drafted the article. Yuxia Du and Zhibin Zhou performed critical revision of the article. All the authors contributed to the critical revision of the article for important intellectual content. All the authors have read and approved the final manuscript. Mingxia Cai and Zhibin Zhou have equal contributions and are both first authors.
Funding
This study was supported by the Natural Science Foundation of Fujian Province, China (grant number: 2021J01262). There is no other funding.
Acknowledgments
This study was supported by the Natural Science Foundation of Fujian Province, China (grant number: 2021J01262).
Supporting Information
A statistical analysis was performed to compare the clinical characteristics of the excluded subjects and the recruited subjects. We found no statistically significant differences between the two. The specific content is included in the Supporting Information.
Open Research
Data Availability Statement
The data analyzed during our study are available from the corresponding author on reasonable request.




