Nomogram for Prediction of Isolated Calf Deep Vein Thrombosis in Internal Medicine Patients
Abstract
This study aims to construct a nomogram anticipating the occurrence of isolated calf deep vein thrombosis (ICDVT), specifically in patients receiving internal medicine care. This case-control study enrolled 610 internal medicine patients admitted to the Jinan Municipal Hospital of Traditional Chinese Medicine between December 2019 and July 2022. Of the total number of patients who participated in the study, 147 were assigned to the ICDVT group, whereas 463 were assigned to the non-ICDVT group. The least absolute shrinkage and selection operator (LASSO) regression was employed to perform feature selection. A prediction model was constructed using a multivariable logistic regression analysis. The validity and practicality of the nomogram were assessed using several statistical measures, including concordance index (C-index), receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA). Gender, age, D-dimer, and diabetes mellitus were correlated with the presence of ICDVT in the considered internal medicine patients. The predictive nomogram revealed acceptable performance with a C-index of 0.883 (95% confidence interval [CI]: 0.822–0.914) in the internal verification. The area under the ROC curve was 0.883 (95% CI: 0.853–0.914), and the Hosmer–Lemeshow goodness-of-fit p value was 0.888. DCA findings indicated that the ICDVT nomogram exhibited a favorable clinical net benefit. The created nomogram has the potential to help clinicians identify high-risk groups of ICDVT among patients in the field of internal medicine, thus enabling early intervention strategies.
1. Introduction
Isolated calf deep vein thrombosis (ICDVT) is distinguished by the occurrence of thrombus development inside the deep veins of the calf without the involvement of the popliteal or more proximal veins, such as the anterior tibial, posterior tibial, fibular, and muscular veins of the soleus and gastrocnemius [1]. Although the ICDVT thrombus has a small and restricted region, there is a risk of thrombus dislodging that results in pulmonary embolism (PE) and thrombus spreading that causes proximal DVT, which could induce severe conditions such as post-thrombotic syndrome, venous ulcer, and chronic pulmonary artery hypertension.
ICDVT has a high incidence, which is in the range of 30%–50% of all DVTs and 60%–70% of outpatients, according to Robert-Ebadi and Righini’s report [2]. Statistically, the incidence of ICDVT-related PE ranges from 0% to 6.2% [3] and is as high as 33.3%, based on the research of Kim [4]. The existing guidelines suggest that anticoagulant therapy should be administered to patients with ICDVT [5]. However, most ICDVT commonly has an insidious onset and nonspecific symptoms, which may consequently lead to delayed diagnosis and treatment.
Hence, it is crucial to ascertain the risk factors associated with ICDVT. Liu et al. corroborated that advanced age, Brunnstrom stage, D-dimer (DD) level, and antiplatelet therapy are linked to ICDVT in stroke patients [6]. Zhao et al. demonstrated that smoking, time delay from injury to surgery, and activated partial thromboplastin time lead to ICDVT after hip fractures [7]. The abovementioned studies investigated ICDVT-related factors in specific populations; however, the risk factors for ICDVT remain poorly defined.
This retrospective study of cases and controls was conducted to elucidate the ICDVT risk factors in patients hospitalized in our hospital between January 2016 and August 2022. We aimed to help physicians with ICDVT’s early diagnosis and prevention, reduce the occurrence of proximal DVT, PE, and other disorders, and enhance the overall well-being and standard of living of the patient.
2. Methods
2.1. Study Design and Patients
The present investigation was conducted as a retrospective case-control study. The research protocol was approved by the Ethics Committee of the Jinan Municipal Hospital of Traditional Chinese Medicine. The data gathering period spanned from December 2019 to July 2022. All patients were obtained from the Jinan Municipal Hospital of Traditional Chinese Medicine, China. All patients who were admitted to our hospital and did not receive surgical treatment were recruited. Patients were excluded if they had (1) malignant tumors, (2) a history of lower limb thrombosis or PE, (3) lower limb fracture within 3 months, (4) incomplete medical records, (5) severe liver or kidney insufficiency, or (6) anticoagulant or antiplatelet drug use.
All patients underwent color Doppler ultrasound examination after admission by experienced sonographers to ascertain the presence of ICDVT. The diagnostic criteria for ICDVT using color Doppler ultrasonography included the following parameters: (1) thrombus formation in the anterior tibial, posterior tibial, peroneal, and muscular calf veins and their combinations; (2) intraluminal low or mixed echoes; (3) the closure of the venous lumen was not achieved with the application of pressure using the ultrasonic probe; (4) the venous width increased, and the blood flow signal was unobvious in the venous thrombus segment [8].
The participants were categorized into two groups: the ICDVT group and the non-ICDVT group, according to the presence of ICDVT by color Doppler ultrasound.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendation guidelines and was conducted according to the Declaration of Helsinki. This study was approved by the Hospital Ethics Committee Review Board (No. 2022-03). Written informed consent was received from patients. Ethical and legal practices were followed, and all information was handled in confidence.
2.2. Data Collection
Characteristics such as demographics, clinical manifestations, medical histories, and laboratory parameters were obtained from the hospital information system (HIS), including age, gender, lower limb swelling, stroke, hypertension, diabetes mellitus (DM), coronary artery disease (CAD), chronic kidney disease (CKD), history of smoking and alcohol, DD, white blood cell (WBC), blood platelet (PLT), activated partial thromboplastin time (APTT), prothrombin time (PT), fibrinogen (FIB), and hypersensitive C-reactive protein (HsCRP).
2.3. Statistical Analysis
All continuous variables had non-normal distributions and were represented as medians and quartiles. Categorical variables were presented as numerical values and percentages (%). Using R (version 4.1.2), data analysis was performed using several R packages, including Rms, ROCR, glmnet, and rms.
This study used the least absolute shrinkage and selection operator (LASSO) binary logistic regression method to determine the most effective predictive features in assessing the risk variables associated with ICDVT. Subsequently, a multivariable logistic regression analysis was conducted using the features that had nonzero coefficients in the LASSO regression model to ascertain the statistically significant predictors. The authors reported the predictors in the form of odds ratio (OR), accompanied by a 95% confidence interval (CI) and p-value. The construction of the predictive model included predictors with a p value < 0.05. Based on the results of multivariable logistic regression analysis, a nomogram was developed to assess the likelihood of ICDVT.
The model underwent internal validation using the bootstrap approach, 1000 resamples, and Harrell’s concordance index (C-index). The area under the receiver operating characteristic (ROC) curve (AUC) was employed as a metric to assess the discriminatory ability of the nomogram. The accuracy of the nomogram was assessed by plotting a calibration curve, whereas the advantages of various threshold probabilities in internal medicine patients were evaluated using decision curve analysis (DCA).
3. Results
3.1. Baseline Characteristics
Data from 610 patients were included in this study. According to ultrasound evaluation, the patients were divided into ICDVT and non-ICDVT groups. Of these, 24.1% (147/610) were ICDVT patients. All patients’ demographics, clinical manifestations, medical histories, and laboratory parameters of the two groups are listed in Table 1. Six of the 17 features obtained from the patients were selected based on nonzero coefficients computed using the LASSO technique (Figures 1(a) and 1(b)). The selected features included gender, age, DM, APTT, DD, and WBC. The aforementioned features were then included in the multivariate logistic regression analysis.
| Variables | Non-ICDVT (N = 463) | ICDVT (N = 147) | Total (N = 610) |
|---|---|---|---|
| Gender (case, %) | |||
| Female | 156 (33.7%) | 96 (65.3%) | 252 (41.3%) |
| Male | 307 (66.3%) | 51 (34.7%) | 358 (58.7%) |
| Age (years) | 59.0 [27.0, 82.0] | 73.0 [40.0, 92.0] | 62.0 [27.0, 92.0] |
| Hypertension (case, %) | |||
| Yes | 210 (45.4%) | 78 (53.1%) | 288 (47.2%) |
| No | 253 (54.6%) | 69 (46.9%) | 322 (52.8%) |
| DM (case, %) | |||
| Yes | 50 (10.8%) | 44 (29.9%) | 94 (15.4%) |
| No | 413 (89.2%) | 103 (70.1%) | 516 (84.6%) |
| CAD (case, %) | |||
| Yes | 66 (14.3%) | 27 (18.4%) | 93 (15.2%) |
| No | 397 (85.7%) | 120 (81.6%) | 517 (84.8%) |
| Stroke (case, %) | |||
| Yes | 88 (19.0%) | 33 (22.4%) | 121 (19.8%) |
| No | 375 (81.0%) | 114 (77.6%) | 489 (80.2%) |
| Limb swelling (case, %) | |||
| Yes | 97 (21.0%) | 40 (27.2%) | 137 (22.5%) |
| No | 366 (79.0%) | 107 (72.8%) | 473 (77.5%) |
| Smoke (case, %) | |||
| Yes | 76 (16.4%) | 21 (14.3%) | 97 (15.9%) |
| No | 387 (83.6%) | 126 (85.7%) | 513 (84.1%) |
| Alcohol (case, %) | |||
| Yes | 73 (15.8%) | 16 (10.9%) | 89 (14.6%) |
| No | 390 (84.2%) | 131 (89.1%) | 521 (85.4%) |
| CKD (case, %) | |||
| Yes | 91 (19.7%) | 24 (16.3%) | 115 (18.9%) |
| No | 372 (80.3%) | 123 (83.7%) | 495 (81.1%) |
| PT (s) | 11.2 [6.51, 15.7] | 10.8 [9.20, 18.7] | 11.0 [6.51, 18.7] |
| APTT (s) | 26.1 [19.4, 42.3] | 25.6 [17.6, 57.0] | 25.7 [17.6, 57.0] |
| FIB (g/L) | 2.93 [1.51, 4.48] | 2.70 [0.92, 5.60] | 2.84 [0.92, 5.60] |
| DD (mg/mL) | 0.580 [0.300, 15.5] | 0.920 [0.150, 18.10] | 0.600 [0.15, 18.10] |
| HsCRP (mg/L) | 6.80 [0.05, 52.20] | 5.79 [2.50, 160] | 6.46 [0.05, 160] |
| WBC (109/L) | 6.66 [2.51, 24.40] | 6.02 [2.42, 15.30] | 6.41 [2.42, 24.40] |
| PLT (109/L) | 218 [100, 494] | 211 [59, 518] | 218 [59, 518] |
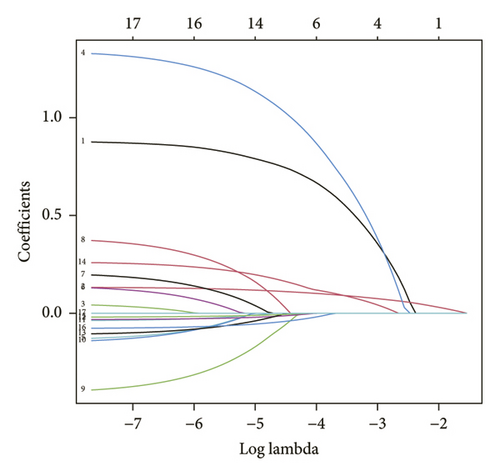
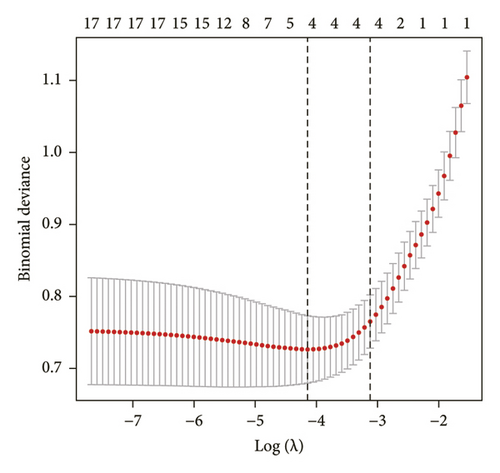
3.2. Development of an Individualized Prediction Model
Multivariate logistic regression analysis revealed that gender, age, DM, and DD were independent predictive variables influencing ICDVT, as demonstrated in Table 2. The independent predictors described earlier were used to develop a prediction nomogram, as displayed in Figure 2.
| Variables | β | Odds ratio | 95% CI | p value | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Gender (female) | 0.899 | 2.458 | 1.509 | 4.004 | < 0.001 |
| Age | 0.130 | 1.139 | 1.107 | 1.171 | < 0.001 |
| DM (yes) | 1.331 | 3.786 | 2.053 | 6.983 | < 0.001 |
| APTT | −0.038 | 0.963 | 0.914 | 1.015 | 0.161 |
| DD | 0.266 | 1.305 | 1.123 | 1.517 | 0.001 |
| HSCRP | −0.021 | 0.980 | 0.959 | 1.001 | 0.064 |
| WBC | −0.083 | 0.920 | 0.836 | 1.013 | 0.090 |
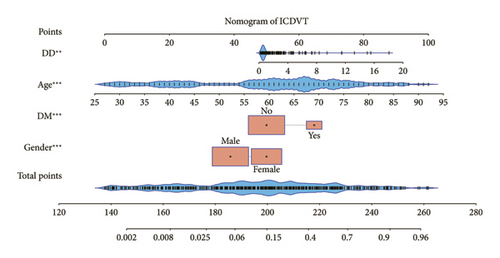
3.3. Performance and Validation of a Nomogram
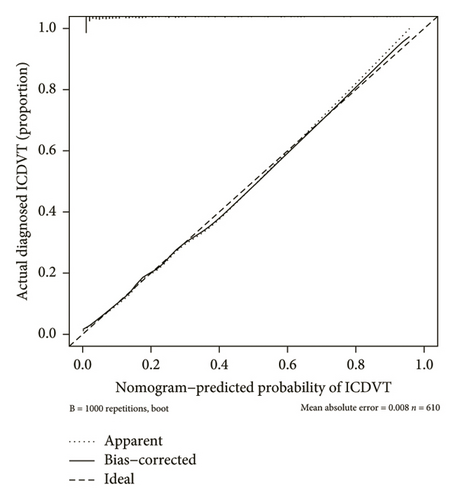
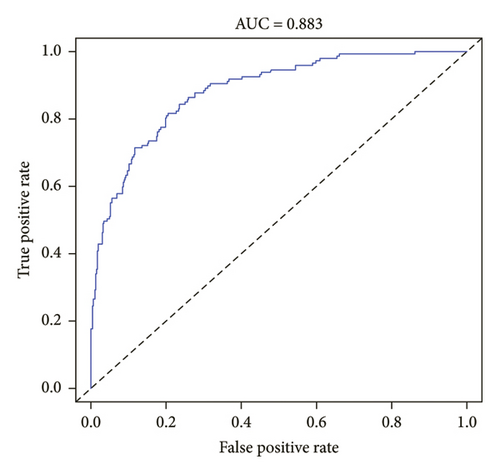
Strong concordance was observed in the calibration curve of the ICDVT risk nomogram for patients in the field of internal medicine (Figure 3). The ICDVT prediction nomogram demonstrated a C-index of 0.883 (95% CI: 0.822–0.914), which was then validated using bootstrapping and verified to be 0.880. The area under the AUC-ROC was determined to be 0.883 (95% CI: 0.853–0.914), as illustrated in Figure 4. Furthermore, the Hosmer–Lemeshow test indicated that the model exhibited a good match (X2 = 3.646, p = 0.888).
3.4. Clinical Use
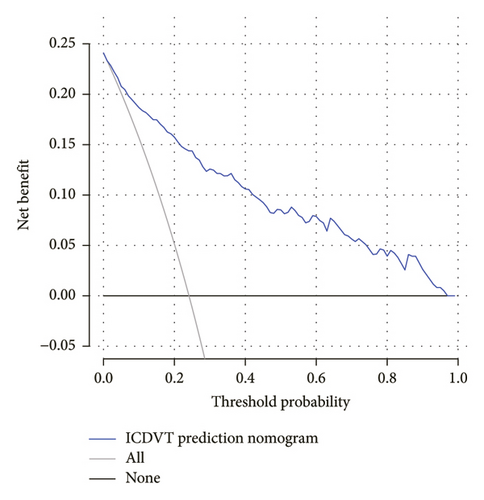
Figure 5 illustrates the DCA of the ICDVT nomogram. Analysis of the decision curve revealed a threshold probability range between 2% and 97%, and the use of this nomogram for predicting ICDVT yielded more advantages than both the approach of predicting ICDVT for all patients and the approach of predicting ICDVT for no patients.
4. Discussion
Calf veins are susceptible to thrombosis because of their unique anatomic structures, including more branches, smaller diameters, and slower flow than large veins in the lower extremities. Recent studies have depicted that ICDVT accounts for 30%–50% of all DVTs in the lower extremities diagnosed using color Doppler ultrasound [9, 10]. According to a study performed by Shimabukuro et al. in Japan, ICDVTs were responsible for half of the cases, many of which were asymptomatic [11]. The adverse consequences of ICDVT include thrombus spread to the proximal veins and PE. Palareti observed that the untreated proximal extension rate of ICDVT ranges from 10% to 15% [12]. A systematic review conducted by Garry, Duke, and Labropoulos summarized that the incidence of ICDVT extending to the proximal vein was approximately 9%, whereas the incidence of PE was approximately 1.5%. Nonetheless, data are scarce on the risk factors for ICDVT in hospitalized patients [13].
Using LASSO and multivariable logistic regression analytic techniques, we identified four risk factors for ICDVT in hospitalized patients in this study, namely gender, age, DD level, and DM. A predictive model was developed and subjected to internal validation using the aforementioned criteria. Internal validation yielded a strong C-index, demonstrating that our prediction model indicated good discrimination and calibration capability, which could be used extensively and reliably for a sizable sample. DCA revealed that the model was clinically significant for decision-making across various probability thresholds.
In this study, the female gender was an independent risk factor for ICDVT. Sun’s study in China reported the same results as our investigation [14]. The higher prevalence of VTE among the female population is not only in Asia but also worldwide [15]. The potential mechanism for the higher DVT incidence in females might be the action of estrogen. A meta-analysis conducted by Mohammed et al. revealed that oral estrogen therapy could increase the occurrence of first-episode VTE and DVT [16]. According to several previous studies, von Willebrand factor (vWF) is regulated by several hormones, including estrogen, and plays a pivotal role in the pathogenesis of venous thrombosis. Estrogen leads to an increase in vWF levels by activating endothelial exocytosis [17–19]. Moreover, pregnancy has been shown to be related to increased vWF production and prolonged vWF half-life [20].
The present study demonstrated that elderly individuals were more susceptible to ICDVT. Prior research has indicated that age is a risk factor for calf vein thrombosis. Its positive association with an increased incidence of ICDVT has been confirmed in stroke patients [21, 22]. Liu et al. demonstrated that perioperative thrombosis after coronary artery bypass grafting is more likely in elderly patients [23]. The risk of developing DVT increases with age by approximately 5% per year in patients with thoracolumbar fractures [24]. The conditions, including infirmity, immobilization, calf muscle pump malfunction, and venous insufficiency, occur more frequently in the elderly and can cause blood flow stasis, which is one of the most important triggers of venous thrombosis [25].
Following previous results, our study has demonstrated that an elevated DD level serves as an autonomous risk factor for ICDVT [22, 26]. DD is the most precise and minute fibrin breakdown product of venous thrombosis. It is an extremely sensitive measure of thrombosis and dissolution and is excessively raised in cases of blood hypercoagulability or secondary hyperfibrinolysis [27]. Elevated DD levels suggest that fibrin formation is increased by fibrinolytic enzymes cleaving cross-linked fibrin [28]. However, DD levels exhibit elevation not just in cases of venous thrombosis but also in other diseases. Consequently, it is unsuitable for exclusive use in the precise detection of DVT [29].
Our study revealed that DM was closely related to the occurrence of ICDVT. The hyperglycemic state is also associated with low-grade chronic inflammation, a potential determinant that contributes to the occurrence of venous thrombosis [30]. The research conducted by Liu depicts that an elevated glucose level before surgery is a distinct risk factor for preoperative DVT [31]. A previous study found that DM is strongly correlated with endothelial dysfunction and platelet hyperactivity, which usually refers to thrombosis [32]. Piazza et al. indicated that DM is an independent predictor of recurrent DVT [33]. A population-based cohort study in Taiwan demonstrated that the incidence of VTE is higher in type 2 DM patients. Type 2 DM remained an independent risk factor for developing DVT and PE after adjusting for age, sex, and comorbidities [34].
A nomogram is a practical and visual prediction tool developed based on the multivariate regression analysis. Based on our research, a nomogram was developed to enhance the prediction of the ICDVT occurrence in patients admitted to the hospital. The nomogram model had favorable predictive properties and revealed relatively good predictive discrimination after internal validation. Nomogram models are available to predict DVT in stroke and chronic obstructive pulmonary disease (COPD) patients. Hu et al. established a risk prediction model for muscular calf vein thrombosis in patients with acute exacerbation of COPD [35]. Liu et al. developed a nomogram for predicting calf muscle vein thrombosis in patients with stroke [6]. Compared to the above studies, our prediction object was ICDVT, which contains muscular calf vein thrombosis, and our study population is internal patients, which include patients with stroke and COPD. These results are more universal. According to previous studies, DM and female gender are independent risk factors for VTE. We further clarified that these two factors were independent risk factors for ICDVT.
To the best of our knowledge, only a few models have been developed to predict ICDVT. This nomogram is the first model developed to predict the risk of ICDVT in internal patients. The present nomogram model exhibited good predictive accuracy for ICDVT occurrence (AUC-ROC = 0.883). Additionally, the nomogram was validated using a larger cohort for ICDVT prediction in internal patients.
This study has numerous limitations. First, the patients in this investigation were from a single center. Second, we did not conduct external validation, which might demonstrate the applicability of the model. A sizable number of studies should be conducted to evaluate the applicability of this model and make possible modifications.
In conclusion, we identified risk factors for ICDVT in hospitalized patients and developed a nomogram to predict the presence of ICDVT. This prediction model had good predictive accuracy and stability, which assists clinicians in identifying higher risk individuals with ICDVT.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82104860) and Haiyou Health High-Caliber Talent Project (grant number 202412).
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




