Controlled Growth Temperatures of High Dielectric Constant Gallium Cerium Oxide Layer on 4H-Silicon Carbide Substrate
Abstract
The potential of using gallium cerium oxide (GaxCeyOz) as a passivation layer (PL) on 4H-silicon carbide (SiC) substrates were thoroughly assessed after annealing in nitrogen–oxygen–nitrogen (N2–O2–N2) ambient at varying temperatures of 600, 700, 800, and 900°C. It was observed that nitrogen ions introduced during the annealing process were predominantly attached to oxygen vacancies (Vo) within the oxide layer at lower temperatures (600 and 700°C), whereas at elevated temperatures (800 and 900°C), there was a substantial increase in the migration of nitrogen ions toward the interface of GaxCeyOz/4H-SiC. Analysis employing X-ray photoelectron spectroscopy (XPS) corroborated the transformation of Ce3+ to Ce4+ at 900°C due to enhanced reoxidation. As a result, the passivation of Vo at 800 and 900°C led to a significantly higher dielectric constant, improved breakdown field, and favorable values for slow trap density (STD), interface trap density, interface state density, as well as effective oxide charge, highlighting the potential of GaxCeyOz PL on 4H-SiC for use in metal–oxide–semiconductor (MOS) applications.
1. Introduction
In the pursuit of energy-efficient technologies to achieve sustainable solutions for the next generation of power electronic devices, various approaches utilizing wide bandgap semiconductor materials have been successfully adopted in the development of these power electronic devices. It was anticipated that wide bandgap semiconductors, especially 4H-silicon carbide (SiC), has emerged as the choice of semiconductor substrate for metal–oxide–semiconductor (MOS) devices that would provide excellent performance in meeting the future market demands of power electronic devices requiring high energy efficient, effective in heat dissipation, high switching frequency, and ability to operate at high temperatures [1]. The 4H-SiC was experiencing a profound exploration for high temperature as well as high power device applications owing to the possession of wide energy bandgap (3.26 eV), high saturation drift velocity (2.7 × 107 cm/s), high thermal conductivity (~4.9 W cm−1 K−1), as well as high critical electrical field (~3 MV/cm) [2]. The possession of both wide energy bandgaps coupled with high thermal conductivity by 4H-SiC would allow the 4H-SiC-based MOS devices to operate reliably at high temperatures (>250°C) due to effective heat dissipation while Si-based MOS devices have displayed degradation in performance at this range of operating temperature [3–5]. Additionally, 4H-SiC-based MOS devices were able to achieve high-frequency switching capability due to the lower impurity concentration of 4H-SiC substrate that allowed this semiconductor material to attain high saturation velocity and mobility [6, 7]. Another potential appeal of using 4H-SiC as the semiconductor substrate was the ability to adopt the matured fabrication and packaging technologies developed for Si-based MOS devices to be employed in the development of 4H-SiC-based MOS devices [8].
Another captivating advantage of 4H-SiC, when compared with other compound semiconductors, is the ability to thermally grow high-quality native silicon dioxide (SiO2) or nitrided SiO2 as a passivation layer (PL), of which 4H-SiC-based MOS devices with nitrided SiO2 has demonstrated low leakage current, as well as leveraging on the well-established fabrication process from Si-based MOS devices [9, 10]. Nevertheless, a challenge arose when using low dielectric constant (k) SiO2 for the passivation for 4H-SiC-based MOS devices due to the breakdown of the device being caused by the low k value of SiO2, hindering the complete exploitation of the high breakdown field strength possessed by 4H-SiC substrate [11]. Therefore, the alternative method of introducing high k material to be employed as a PL for 4H-SiC-based MOS devices has been carried out in full swing to address this issue, of which these 4H-SiC-based MOS devices would be able to sustain a high breakdown electric field as well as low leakage current density [7]. Currently, there exists a diverse range of advanced high k materials, including cerium oxide (CeO2) [12], aluminum (Al) oxide (Al2O3) [13], zirconium oxide (ZrO2) [14], and lanthanum oxide (La2O3) [15], which have been under consideration as potential substitutes for SiO2 in the role of PL for MOS devices based on 4H-SiC [16]. Among the high k materials under scrutiny, CeO2 stands out as a promising contender for supplanting SiO2 as a PL, thanks to its appealing attributes of values of high k spanning from 10 to 16 [17, 18], wide bandgap spanning from 3.0 to 3.6 eV [17, 19], as well as large conduction band offset with respect to 4H-SiC substrate (1.60 eV) [20]. Likewise, the primary utilization of CeO2 as a PL on Si-based MOS devices has shown a leakage current density reduction, leading to an enhancement in the electric breakdown field [21]. In order to emulate the success of Si-based MOS device with CeO2 as PL, CeO2 has been deposited as PL on 4H-SiC as well as AlGaN/GaN heterostructure, wherein CeO2/4H-SiC MOS test structure has demonstrated a low leakage current density of 1.5 × 10−6 A/cm2 at 3.5 MV/cm as well as low interface trap density (1 × 1012 eV−1 cm−2) [20] while CeO2/AlGaN/GaN MOS test structure has revealed several magnitude reduction of leakage current density when compared with AlGaN/GaN test structure without CeO2 PL [22].
Although CeO2 PL has displayed superior passivating properties, the generation of Vo, in coupled with the reduction of Ce4+ to Ce3+ states, remains as a challenging issue to be tackled [23], wherein the generated Vo has induced gap states triggering the reduction of energy bandgap as well as acting as leakage current paths [24]. Moreover, the generation of oxygen vacancy sites in CeO2 PL would act as a hopping site for the diffusion of oxygen ions to the interface [16], provoking the formation of low k SiO2 and a low passivating quality CeO2 for 4H-SiC [25] and GaN [26] substrates, respectively. In order to tackle these problems and enhance the passivating properties of CeO2, an approach involving the doping of tetravalent cations, which include titanium (IV) (Ti4+) [27], hafnium (IV) (Hf4+), silicon (IV) (Si4+), and zirconium (IV) (Zr4+) [28] into CeO2 crystal lattice has been explored. Unfortunately, this exploration has led to the generation of additional active Vo in CeO2 PL as the doping of these tetravalent cations has stimulated the reducibility of Ce4+ to Ce3+ in which the detrimental effects of Vo have been explained in an earlier section [29]. Therefore, the attention was diverted toward the doping of trivalent cation (yttrium (III) (Y3+) [30], lanthanum (III) (La3+) [31], gadolinium (III) (Gd3+) [32], manganese (III) (Mn3+) [33]) into CeO2 crystal lattice, wherein the reducibility to Ce3+ state was less likely to happen as a lower energy is required for the trivalent cation introduction into CeO2 crystal lattice than the energy required to form oxygen vacancy [34, 35]. While the generation of Vo during the modification CeO2 with trivalent dopant was unavoidable, studies have indicated that these vacancies tend to aggregate into defect clusters along with the trivalent cations, ultimately resulting in the inactivation of the Vo [36] that would retard the oxygen ions from using the Vo as a path to diffuse to interface [37].
Considering these literature reviews, a trivalent Ga3+ cation possessing an ionic radius (0.062 nm), which is smaller in comparison to the Ce4+ cation (0.097 nm), has been introduced into CeO2 crystal lattice with the purpose of enhancing the inactivation of Vo, wherein the probability of forming inactive Vo with trivalent cations was dependent on the ionic radius of trivalent cations [38]. Hence, the introduction of trivalent Ga3+ cation in CeO2 crystal lattice would assist in enhancing the passivating properties of ternary gallium cerium oxide (GaxCeyOz) by taking advantage on the excellent properties of Ga2O3, such as large bandgap (~4.9 eV) and moderate k value (~9) [39, 40]. It is important to note that the prior studies on GaxCeyOz PL were conducted exclusively by the same research group associated with the present work [16, 24, 29]. While earlier research provided a preliminary investigation of Ga3+ doped CeO2 PL on 4 H-SiC, which is the only study on the GaxCeyOz PL deposited on 4H-SiC substrates at 800°C [40], this study represents the first comprehensive analysis of the structural, chemical, optical, and MOS characteristics of GaxCeyOz PL deposited on 4H-SiC substrates. Specifically, examination on the effects of postdeposition annealing at varying temperatures (600, 700, 800, and 900°C) in a nitrogen-oxygen–nitrogen (N2–O2–N2) ambient, offering novel insights into the material’s behavior under these conditions, which has not been reported previously.
2. Experimental
The n-type 4H-SiC (0001) substrate was divided into 1 cm2 sections, cleaned via the RCA process, and immersed in a diluted hydrofluoric acid solution to eliminate the native oxide layer, followed by a rinse with deionized water and drying with nitrogen gas. In the preparation of a gallium precursor with a concentration of 0.25 M, a precise procedure was executed wherein 0.319 g of gallium nitrate hydrate was meticulously dissolved in a volume of 5 ml of methanol, followed by an extensive stirring process conducted at a controlled temperature of 30°C for a duration of 15 min, ensuring the complete dissolution of the compound. Similarly, a cerium precursor of the same molarity, 0.25 M, was synthesized through the dissolution of 1.0936 g of cerium acetylacetonate hydrate in a carefully measured mixture of 3 ml of methanol and 6 ml of acetic acid, which was subsequently subjected to stirring at an elevated temperature of 60°C for an equivalent period of 15 min, thereby facilitating the formation of a homogenous solution. Following the successful preparation of both precursor solutions, the next step involved combining both solutions, which was accompanied by a vigorous stirring process maintained at 60°C for a total of 30 min, thereby ensuring thorough mixing and interaction of the components. Ultimately, the resulting mixture was applied onto a 4H-SiC substrate utilizing a spin-coating technique executed at a speed of 3000 rpm for a precise duration of 30 s, thereby achieving a uniform coating necessary for subsequent experimental procedures. Subsequently, postdeposition annealing was carried out at temperatures between 600 and 900°C in an N2–O2–N2 ambient in a horizontal tube furnace. A gas flow rate of 100 ml/min was chosen in this work, while the heating-up rate was set to 10°C/min.
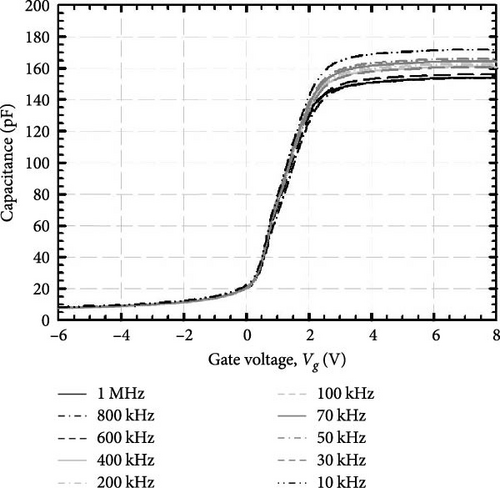
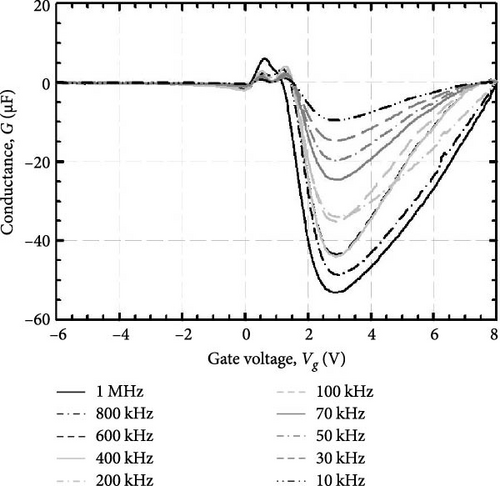
The crystal structure of the investigated GaxCeyOz PLs was analyzed using grazing incident X-ray diffraction (Bruker D8 Discover employing Cu kα radiation (λ = 1.5406 Å)) operated at 40 kV and 40 mA with a scan range of 25°–62°, a step size of 0.05°, and a step time of 1.5 s. The chemical states of the investigated GaxCeyOz PL were characterized by X-ray photoelectron spectroscopy (XPS) (Axis Ultra DLD Kratos spectrometer) employing a monochromatic Al kα X-ray source (hv = 1486.6 eV) at 9 mA for emission and 10 kV for anode HV (high voltage). Additionally, an analysis area of 300 × 700 µm was utilized to characterize the investigated PL at a base pressure of 2.7 × 10−9 torr under an ultra-vacuum environment. The pass energy of the hemispherical analyzer was set at 160 eV for the wide scan and 20 eV for the high-resolution narrow scans at a take-off angle of 0°. It is noteworthy that all scans were carried out under charge neutralization conditions, utilizing a low-energy electron gun within the field magnetic lens to ascertain the elemental chemical states. The cross-sectional images and surface morphology were acquired using field-emission scanning electron microscopy (FESEM) (FEI Nova Nano SEM 450), while energy dispersive X-ray (EDX) spectra of the investigated samples were acquired using EDX spectroscopy (JSM-6460 LV). The acquired X-ray reflectivity (XRR; Bruker D8 Discover) measurements were used to estimate the thickness of the investigated GaxCeyOz PL as well as the SiO2 interfacial layer (IL) through fitting the attained XRR curves adopting Bruker DIFFRAC Leptos (7.10.0.12) software. The atomic force microscopy (AFM; Dimension Edge, Bruker) in tapping mode was used to extract surface topographies and root–mean–square (RMS) roughness of the investigated GaxCeyOz PLs. The direct (ED) and indirect (EID) bandgaps for the investigated GaxCeyOz PLs were extracted by analyzing ultraviolet–visible-near-infrared (UV–VIS-NIR) spectra acquired in the 190–1000 nm wavelength range via a UV–VIS-NIR spectrophotometer (Cary 5000) set up in diffused reflectance mode. The thermal evaporation (AUTO 306) technique was used to deposit Al contact with a diameter of 0.2 mm on the investigated GaxCeyOz PLs through a shadow mask while a blanket of Al was evaporated on the backside of 4H-SiC substrate. The capacitance–voltage (C–V) and conductance–voltage (G–V) characteristics for the Al/GaxCeyOz/4H-SiC/Al MOS test structure were measured using the Keithley 4200-SCS semiconductor parameter analyzer in which an applied voltage was swept from −6 to 8 V with a sweep rate of 30 mV/s while a variation of frequency (1 MHz–10 KHz) was employed. Subsequently, the current–voltage (I–V) measurements were carried out using a similar system.
3. Results and Discussion
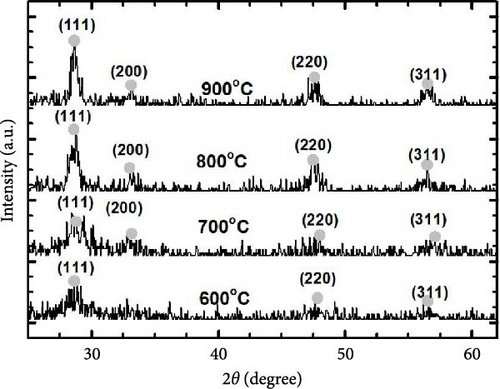
Figure 1 presents GIXRD patterns of GaxCeyOz PL deposited on 4H-SiC substrates and annealed at 600–900°C in a controlled N2–O2–N2 ambient. All GaxCeyOz PL exhibited a polycrystalline phase, with peaks corresponding to the (111), (200), (220), and (311) planes of the cubic fluorite CeO2 structure (ICDD 00-034-0394). Notably, the (200) plane was absent in the 600°C annealed sample. Phase separation was ruled out, as no peaks corresponding to monoclinic (ICDD 00-041-1103) or cubic Ga2O3 (ICDD 00-020-0426) were observed. The absence of phase separation in the GIXRD analysis confirms the successful incorporation of Ga3+ into the CeO2 lattice, forming a homogeneous solid solution. In the case of phase separation, random grain orientation leads to non-uniformity in the electrical characterization of the PL, which is undesirable [41]. Therefore, no phase separation in the present study is a significant advantage, as it ensures uniform dielectric properties, minimizes defect sites, and enhances interfacial quality with the 4H-SiC substrate. The single-phase structure contributes to improved electrical performance, including a higher effective dielectric constant, reduced leakage current, and better breakdown voltage, which are critical for high k PL applications. This homogeneity also underscores the thermodynamic stability of Ga3+ doped CeO2 under the optimized processing conditions, further validating the robustness of the present approach. Further investigation has disclosed that all of the diffraction peaks were moved to a higher diffraction angle in comparison to the ICDD file no. 00-034-0394 denoting the incorporation of the trivalent Ga3+ cation with a smaller ionic radius (0.62 Å) than Ce4+ (0.97 Å) into the CeO2 crystal lattice has been successful, aiding the formation of ternary GaxCeyOz phase.
| Annealing temperatures (°C) | O (at. %) | Ce (at. %) | Ga (at. %) | N (at. %) |
|---|---|---|---|---|
| 600 | 60.57 | 20.43 | 12.96 | 6.04 |
| 700 | 59.39 | 21.02 | 10.28 | 9.30 |
| 800 | 61.72 | 20.15 | 8.78 | 9.35 |
| 900 | 65.59 | 18.02 | 8.70 | 7.69 |
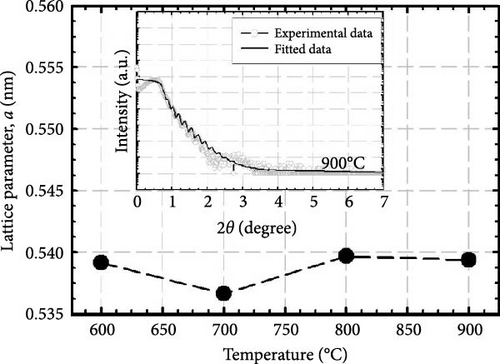
Upon elevating the postdeposition annealing temperature to 800°C, a marginal increase in the nitrogen content was recorded, reaching a level of 9.35 at%, which indicates a heightened diffusion phenomenon of nitrogen ions into the GaxCeyOz PL. The application of a significantly elevated annealing temperature of 800°C has provided an augmented amount of thermal energy, thereby facilitating a more pronounced influx of nitrogen ions that are capable of migrating toward the critical interface region that exists between the GaxCeyOz PL and the underlying 4H-SiC substrate. Consequently, this scenario has led to a diminished probability of nitrogen ions persisting within the GaxCeyOz crystalline lattice structure when compared to the interface. Therefore, the nitrogen ions that have gathered in proximity to the interface region have established bonds with the dangling bonds of silicon and/or carbon [43], which in turn has effectively hindered the reactive interactions between oxygen ions and the surface of 4H-SiC, thereby serving to reduce the formation of SiO2 IL. Therefore, the oxygen ions have diffused outward, consequently increasing the probability of oxygen ions being attached to the Vo site in the GaxCeyOz crystal lattice while undergoing annealing at 800°C, resulting in lattice expansion. However, a reduction in a was attained at a maximum annealing temperature of 900°C. The abrupt decrease in a value observed at 900°C may be attributed to the oxygen ions acquiring adequate energy to disrupt the bonding of nitrogen ions with the Si and/or C dangling bonds [16], wherein these nitrogen ions have diffused outward from the GaxCeyOz PL contributing to the reduction in at% of nitrogen (7.69 at%). The disruption of the attachment between nitrogen ions and the dangling bonds of Si and/or C while annealing to 900°C could also be reinforced by reaching the maximum at% of oxygen, allowing oxygen ions with adequate energy to diffuse into the GaxCeyOz PL to disrupt these attachments.
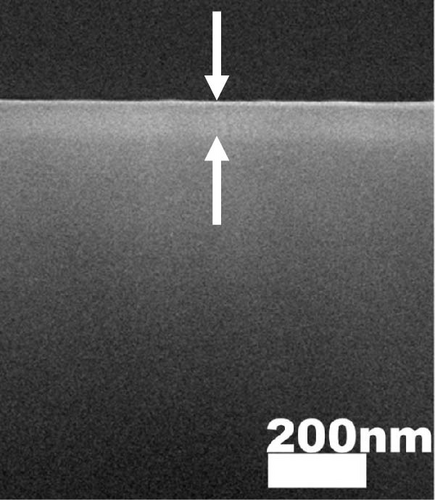
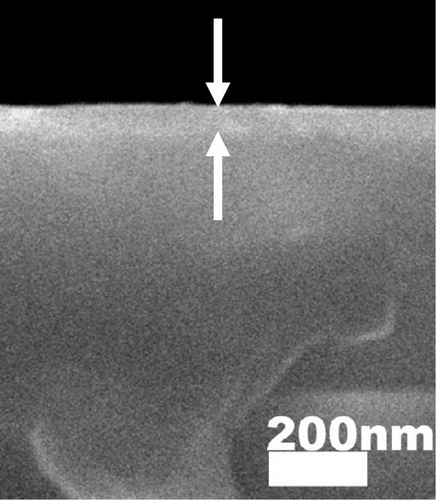
The cross-sectional FESEM images of the investigated GaxCeyOz PLs were presented in Figure 3, as the information related to the average total oxide thickness of the investigated PLs was extracted from 10 different locations using ImageJ software (Table 2). It was noticed that the GaxCeyOz PLs were undergoing a densification process when postdeposition annealing was carried out from 600 to 800°C as the measured average total oxide thickness ranging from 71.0 to 45.7 nm was lower than the average total oxide thickness of the as-deposited GaxCeyOz PL (186.2 nm) [40]. This observation has suggested that during postdeposition annealing from 600 to 800°C, oxygen ions could be filling the Vo in the GaxCeyOz crystal lattice as well as the removal of defects that would aid in the densification of the GaxCeyOz PL [44]. At 700°C, the GaxCeyOz PL exhibited a higher concentration of newly formed oxygen vacancies (Vo), resulting in less pronounced densification compared to the GaxCeyOz PL at 800°C. However, at 900°C, the average total oxide thickness showed a slight increase to 47.4 nm, compared to 45.7 nm for the GaxCeyOz PL annealed at 800°C. The increase in oxide thickness during postdeposition annealing at 900°C indicates that the formation of a SiO2 IL was more significant at this temperature, while it is suggested that SiO2 IL formation may be less prominent at or below 800°C.
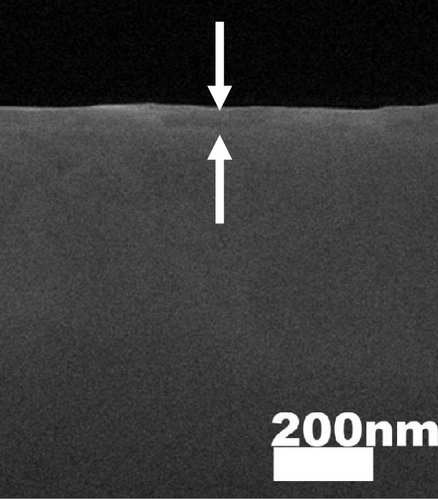
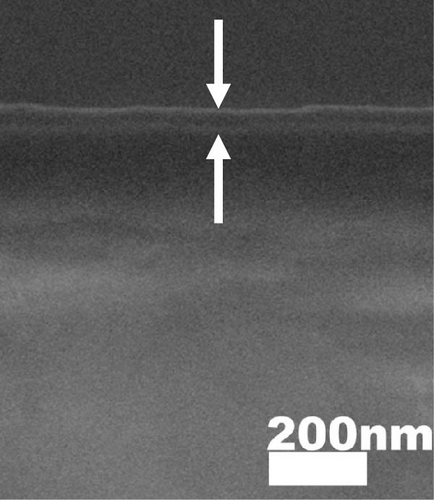
XRR measurements were employed to assist in evaluating trends in film density and the presence of a SiO2 IL in GaxCeyOz PL at various postdeposition annealing temperatures. The experimental and fitted XRR curve for the GaxCeyOz PL annealed at 900°C is shown in the inset of Figure 2. Table 2 summarizes the thickness of the GaxCeyOz PL and SiO2 IL across different annealing temperatures. The XRR results provided a clearer view, revealing that as the postdeposition annealing temperature increased from 600°C to 900°C, the GaxCeyOz PL thickness reduced from 74.128 to 43.921 nm, indicating densification. Additionally, the thickness of the SiO2 IL increased from 1.466 to 4.723 nm with increasing annealing temperature, suggesting that higher oxygen ion diffusivity to the interface was temperature-dependent. Table 2 shows a significant variation in SiO2 IL thickness (1.510 nm) as the temperature switched to 700°C from 600°C, consistent with earlier explanations that nitrogen ions accumulate in the GaxCeyOz PL region, forming bonds with Vo [16]. This leads to oxygen ions released during nitrogen attachment with Vo, along with inward-diffusing oxygen ions during the dwelling stage, diffusing to the interface and reacting with the 4H-SiC surface, promoting SiO2 IL formation. In contrast, SiO2 IL formation slowed down at 800°C, likely due to nitrogen ion migration to the interface. The nitrogen ions at the interface act as a barrier, preventing oxygen ions from reacting with the 4H-SiC surface, resulting in a smaller SiO2 IL thickness variation (0.282 nm) when the temperature increased from 700 to 800°C. The nitrogen ions’ effectiveness in limiting SiO2 IL formation diminished at 900°C, where a larger variation in SiO2 IL thickness (1.465 nm) was observed between the samples annealed at 800 and 900°C. This increase in SiO2 IL thickness suggested that oxygen ions had enough energy to break nitrogen bonds with the Si and/or C dangling bonds, allowing oxygen ions to react with the 4H-SiC surface. While SiO2 IL formation intensified at 900°C, the use of the GaxCeyOz PL on the 4H-SiC substrate effectively controlled SiO2 IL thickness (4.723 nm) compared to GaxCeyOz (24.239 nm) [29] and CeO2 (40.194 nm) [17] PLs deposited on Si substrates subjected to similar annealing conditions.
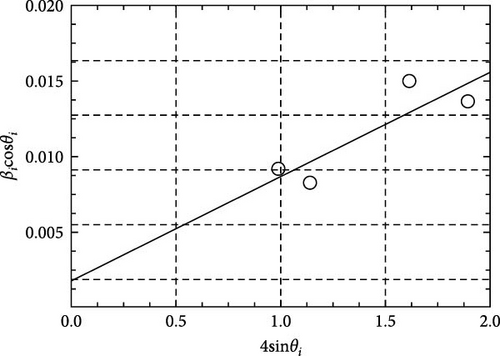
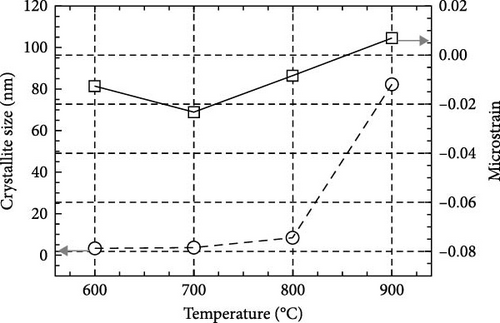
Figure 6 delineates the overarching mechanism that transpires within the GaxCeyOz/4H-SiC system. Initially, Ga3+ cations are posited to occupy the Ce4+ sites within the CeO2 lattice, thereby facilitating the formation of GaxCeyOz PL material while concomitantly generating Vo to achieve charge neutrality (Figure 6a). During the annealing process, with the infusion of nitrogen ions (N3−), a portion of these nitrogen ions is hypothesized to interact with the vacancies, while others may migrate to the interface to form bonds with silicon and/or carbon dangling bonds (Figure 6b). At the comparatively lower temperature of 600°C, it was noted that the N3− ions predominantly occupied the available Vo sites within the GaxCeyOz PL. As the temperature was elevated to 700°C, the propensity for N3− ions to diffuse toward the interface increased. Simultaneously, the binding of N3− ions to the Vo resulted in the liberation of additional O2− ions, thereby generating more Vo to maintain charge equilibrium (Figure 6c). The liberated oxygen ions may either migrate to the interface to form a SiO2 IL or diffuse out of the PL. The application of a higher thermal treatment at 800°C has imparted a heightened amount of thermal energy, consequently promoting a more significant influx of nitrogen ions capable of traversing toward the critical interface region that exists between the GaxCeyOz PL and the underlying 4H-SiC substrate. This phenomenon has consequently resulted in a reduced likelihood of nitrogen ions remaining within the GaxCeyOz crystalline lattice structure in contrast to their presence at the interface. Hence, the nitrogen ions that have concentrated near the interface region have formed bonds with the dangling bonds of silicon and/or carbon, thereby effectively obstructing the reactive interactions between oxygen ions and the surface of 4H-SiC, which in turn has mitigated the formation of the SiO2 IL. Consequently, the oxygen ions have diffused outward, thereby augmenting the likelihood of oxygen ions attaching to Vo sites (Figure 6d) in the GaxCeyOz crystal lattice during the annealing process at 800°C. However, at 900°C, the oxygen ions acquired sufficient energy to disrupt the bonding interactions between nitrogen ions and the silicon and/or carbon dangling bonds (Figure 6e), resulting in the outward diffusion of these nitrogen ions from the GaxCeyOz PL. Furthermore, these oxygen ions have also accelerated the passivation of vacancies, facilitating the reoxidation of Ce3+ to Ce4+ at the temperature of 900°C.
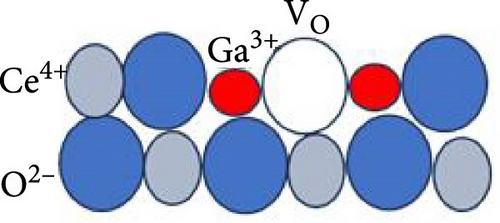
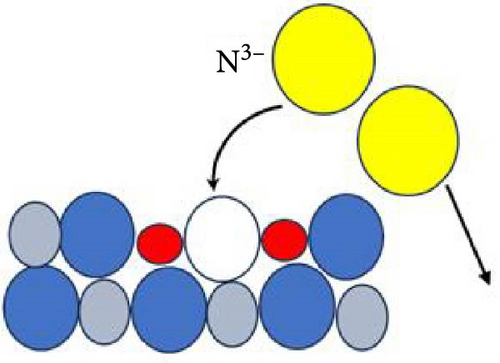
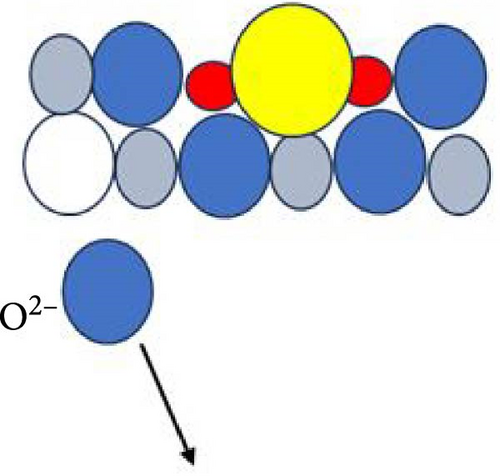
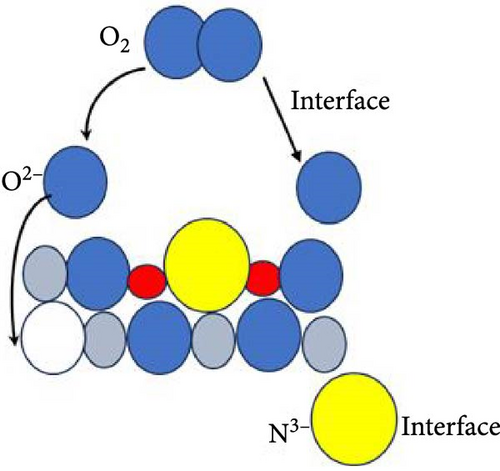
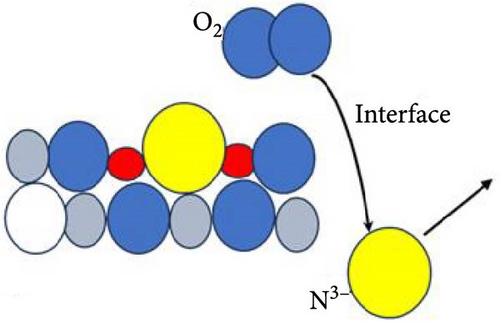
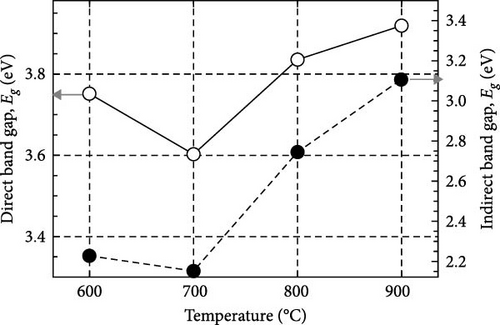
Figure 8e shows the typical FESEM surface morphology of the investigated GaxCeyOz PL that was annealed at 900°C. It was noticed that the surface of all GaxCeyOz PLs was smooth without observable cracks or voids. AFM characterization was employed to obtain the 3-dimensional surface topographies of the investigated PLs, as presented in Figure 8. The 3-dimensional surface topography (Figure 8a) for the GaxCeyOz PL annealed at 600°C has disclosed the formation of smaller dimension protrusions with random distribution throughout the surface of GaxCeyOz PL. As the annealing temperature was increased to 700 (Figure 8b) and 800°C (Figure 8c), the distribution of protrusions was more uniform with a vertical growth of protrusions on the surface of this GaxCeyOz PL. With the employment of a maximum temperature of 900°C, the migration of more oxygen ions toward the region of GaxCeyOz PL has further stimulated the growth of protrusions, wherein the formation of the larger dimension of protrusions was observed in Figure 8d. As a whole, the RMS roughness values were enhanced from 0.94 to 2.53 nm with respect to annealing temperatures (Figure 8).
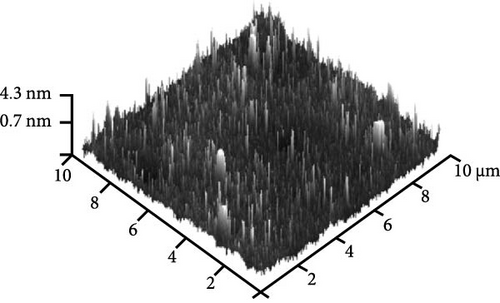
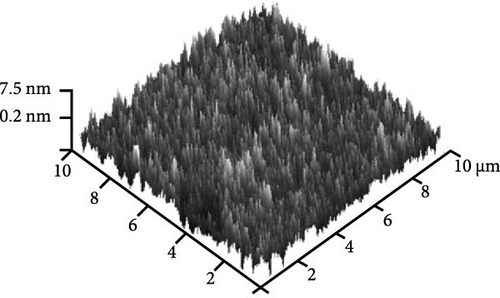
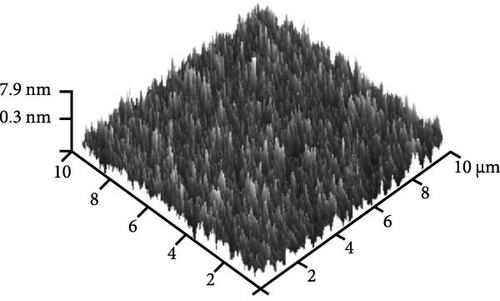
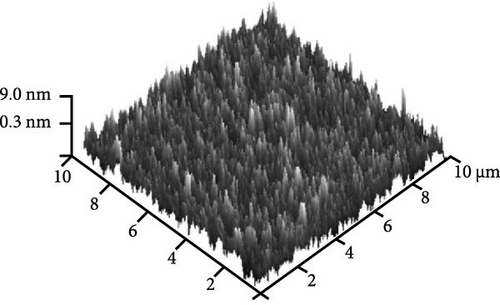
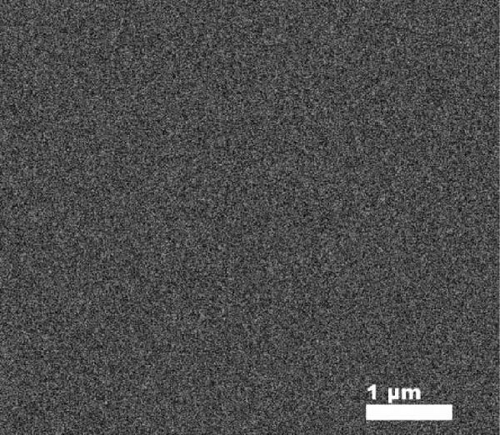
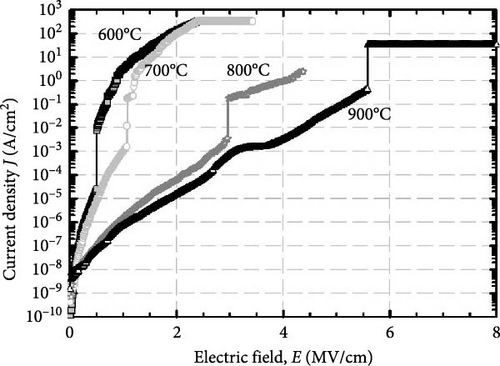
Figure 9 presents the leakage current density-electric field (J–E) characteristics of the GaxCeyOz PLs annealed at different temperatures in N2–O2–N2 ambient. As a whole, it was noticed that the GaxCeyOz PLs annealed at 800 and 900°C have demonstrated higher electric breakdown field (EB) of 2.95 and 5.58 MV/cm, respectively, when compared with the GaxCeyOz PLs annealed at 600 (0.51 MV/cm) and 700°C (1.06 MV/cm). A substantial improvement in the EB for GaxCeyOz PLs annealed at 800 and 900°C could be related to the existence of a lower density of defects with regards to Vo, wherein the attainment of larger ED and EID when compared with GaxCeyOz PLs annealed at 600 and 700°C has suggested that a higher concentration of oxygen ions have occupied the Vo within the GaxCeyOz lattice, aiding the reduction of the density of defects. Moreover, the employment of annealing at 700°C has offered an adequate amount of energy for more nitrogen ions to diffuse to the region of GaxCeyOz PL in which these nitrogen ions would either attach to the Vo, contributing to the formation of new Vo to achieve charge neutrality or the unbonded nitrogen ions lingering at the region of GaxCeyOz PL. As a consequence, the GaxCeyOz PL subjected to annealing at 700°C has demonstrated a poorer J–E characteristic than GaxCeyOz PL annealed at/beyond 800°C due to the narrowing of ED and EID as a result of nitrogen ions attached to the Vo in GaxCeyOz crystal lattice as well as the presence of negatively charged defects, namely, unbonded nitrogen ions in the region of GaxCeyOz PL that would act as scattering center for the injected electrons to break the bonding of GaxCeyOz when E was being applied. Of these investigated samples, the GaxCeyOz PL annealed at 600°C has demonstrated the poorest J–E characteristic due to the acquisition of the lower at% nitrogen as disclosed by EDX (Table 1), wherein the probability of incorporated nitrogen attaching to Vo and migrating toward the interface to passivate Si and/or C dangling bonds [16] was the lowest amongst the investigated GaxCeyOz PL.
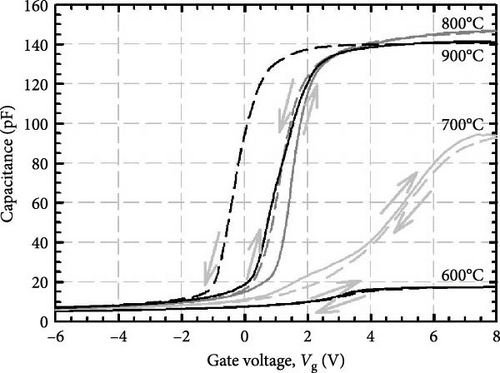
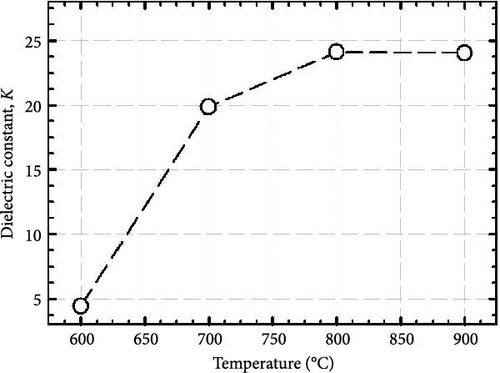
It is worth mentioning the GaxCeyOz PLs annealed at 800 and 900°C have demonstrated superior J–E characteristics as well as k values, and of which further investigation on the chemical composition present in these PLs was conducted using XPS characterization. Figure 12 presents the survey spectrum obtained for the GaxCeyOz PLs annealed at 800 and 900°C. Based on this, the XPS peaks corresponding to Ce 3d, O 1s, Ga 3d, and N 1s were identified and are shown in Figure 13. The XPS peaks were calibrated based on the C 1s peak located at 284.6 eV for the GaxCeyOz PL annealed at 800 and 900°C. Deconvolution of a well-resolved spectrum of the Ce 3d core level at an energy range of 870–930 eV resulted in the identification of six distinct peaks, as depicted in Figure 13a. It was noted that the GaxCeyOz PL, subjected to annealing temperatures of 800 and 900°C, yielded Ce4+−related peaks at energies of 887.6 (v″), 897.7 (v‴), 900.3 (u), 905.6 (u″), and 916.1 eV (u‴), as well as 887.7, 897.8, 900.5, 906.8, and 916.3 eV, respectively. The Ce4+ states have been discerned by examining the emission originating from the Ce 3d5/2 (v″ and v‴) as well as the emission originating from the Ce 3d3/2 core levels (u, u″, and u‴) [51]. Moreover, the Ce3+-related peak was detected at 882.2 (Vo) and 882.3 (Vo) originating from s annealed at 800 and 900°C, respectively. Although the detection of Ce3+ states suggested the formation of Vo was accompanied by the reduction of Ce4+ to Ce3+, the detection of only one peak for the investigated samples suggested that the concentration of Vo was minimal at these temperatures. Moreover, shifting toward the lower binding energy for all of the peaks as compared to the reported peak position related to the Ce 3d core spectra suggested the successful doping of a trivalent Ga3+ cation into the CeO2 crystal lattice to form the GaxCeyOz PL [52, 53]. In addition, shifting in the position of identified peaks toward higher binding energy was witnessed as the annealing temperature was increased from 800 to 900°C. This observation suggested the reoxidation of the Ce3+ to the Ce4+ state has taken place at 900°C by releasing electrons from the Ce state [54]. The aforementioned observation aligned with the preceding explanation that a higher concentration of oxygen ions was incorporated into the GaxCeyOz PL at 900°C. In this context, the incorporated oxygen ions could not only diffuse to the interface but also passivate Vo, consequently leading to the possibility of reoxidation from the Ce3+ to Ce4+ state.
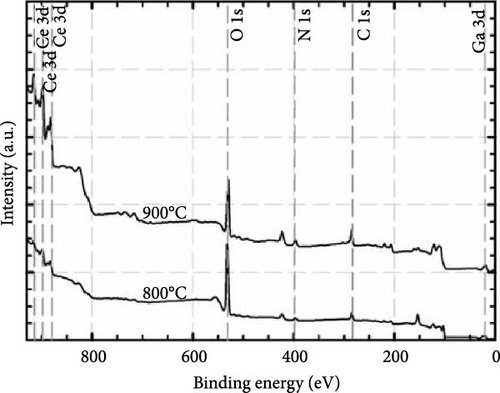
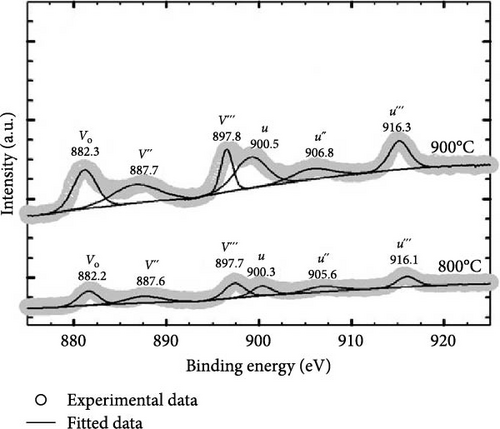
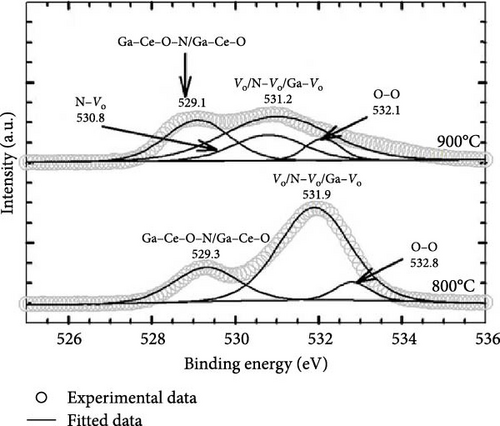
Further clarity was obtained by deconvoluting O 1s spectra for the GaxCeyOz PL annealed at 800 and 900°C, and the resulting spectra are shown in Figure 13b. It is well known that the O 1s spectra peak is related to low energy (OL), mid energy (OM), and high energy (OH) ascribed to crystal lattice oxygen, Vo, and adsorbed oxygen, respectively [55]. Consequently, the OL peaks of 529.3 and 529.1 for the GaxCeyOz PL at 800 and 900°C, respectively, were ascribed to Ga–Ce–O–N bonding [56]. Moreover, the OM peak for the GaxCeyOz PL at 800 (531.9 eV) and 900°C (530.8 and 531.2 eV) was related to the Vo induced by the formation of Ga–Ce–O bonding or N–Vo or Ga–Vo defect cluster. The presence of an additional OM peak for the GaxCeyOz PL at 900°C suggested that the incoming oxygen ions have gained sufficient energy to break the nitrogen attachment with the Si and/or C dangling bonds, wherein some of the released nitrogen ions could have diffused inwards toward the region of the GaxCeyOz PL to form an attachment with the Vo. The shifting in binding energies to lower values that were observed with the increase in annealing temperature from 800 to 900°C provides additional verification for the preceding assertion that the conversion of Ce3+ to Ce4+ through reoxidation has been accomplished at 900°C. Consequently, the liberated electron from the Ce state would be captured by the O state, resulting in a decrease in binding energy. In addition, the OH peaks at 532.8 and 532.1 eV for the GaxCeyOz PL annealed at 800 and 900°C, respectively, were related to the additional oxygen ions in the interstitial positions [57].
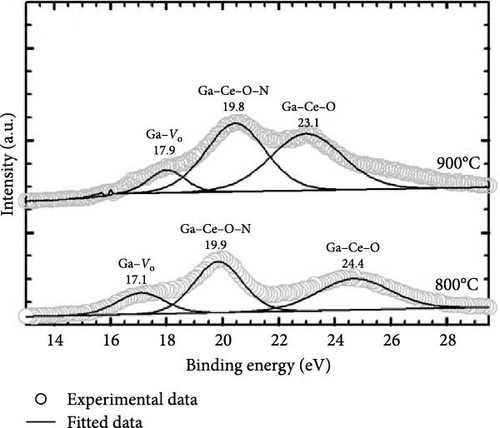
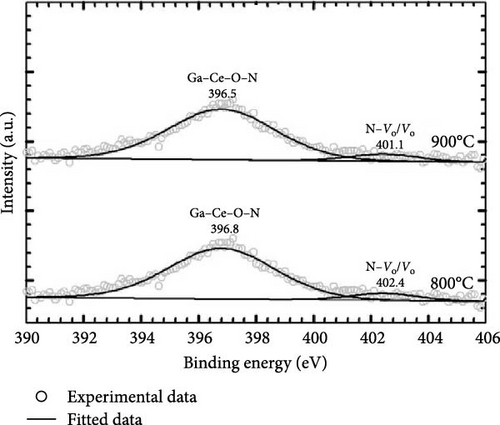
Additional information on the formation of the GaxCeyOz phase was attained through the acquisition of Ga 3d (Figure 13c) spectra, wherein sharp peaks related to Ga–Ce–O–N bonding were observed for the GaxCeyOz PL annealed at 800°C (19.9 eV) and 900°C (19.8 eV). The peaks at 19.9 (800°C) and 19.8 eV (900°C) could be arising from the Ga 3d5/2 core level, while the peaks at 24.4 and 23.1 eV were due to Ga 3d3/2 core level emission suggesting the presence of Ga3+ states in the PL [58]. The peaks at 19.9 and 19.8 eV binding energies for the GaxCeyOz PL at 800 and 900°C were ascribed to Ga–Ce–O–N bonding as it was within the range of reported Ga–N (19.6 eV) [59] and Ga–O (21.9 eV) [60] bonding positions. Moreover, the peaks of 24.4 and 23.1 eV for the GaxCeyOz PL at 800 and 900°C, respectively, were attributed to the formation of Ga–Ce–O bonding, wherein the lower binding energy observed for the sample at 900°C further supported that reoxidation of Ce3+ to Ce4+ occurred at 900°C, improving the passivating characteristics of the GaxCeyOz PL (Table 1). In addition, it should be noted that, as no phase separation was detected by the GIXRD (Figure 1), the XPS analysis further supported the idea that Ga3+ could be mainly residing at GaCe sites at the CeO2 crystal lattice, leading to the formation of the GaxCeyOz phase. The presence of a low-intensity peak at 17.1 and 17.9 eV in the GaxCeyOz PL provided evidence for the existence of the Ga–Vo defect cluster [61]. The N 1 s spectra were deconvoluted, revealing the emergence of two distinct peaks, as illustrated in Figure 13d, where one prominent peak was observed at energy levels of 396.8 eV and 396.5 eV for the GaxCeyOz PL annealed at a temperature of 800 and 900°C, respectively, thereby providing further corroborative evidence for the incorporation of nitrogen within the crystalline lattice structure of CeO2. It has been previously documented in the literature that the specific bonding positions associated with the N 1s spectra for N–O, Ce–N, and Ga–N, which have been identified at binding energies of 393.7 [62], 396.1 [63], and 397.3 eV [63], respectively, according to prior studies. Hence, 396.8 and 396.5 can be ascribed to the Ga–Ce–O–N bonding. Moreover, the relatively low-intensity peak observed at 402.4 and 401.1 at 800 and 900°C, respectively, could be due to the attachment of nitrogen to the oxygen vacancy site, leading to the formation of N–Vo bonding or Vo states resulted from the breaking of the attachment [64].
4. Conclusion
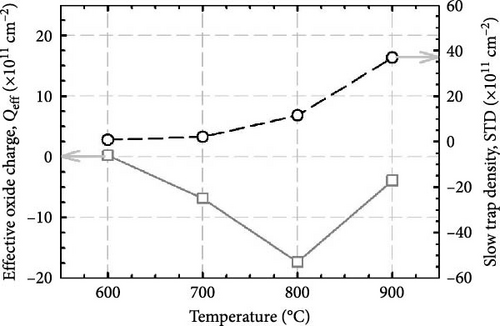
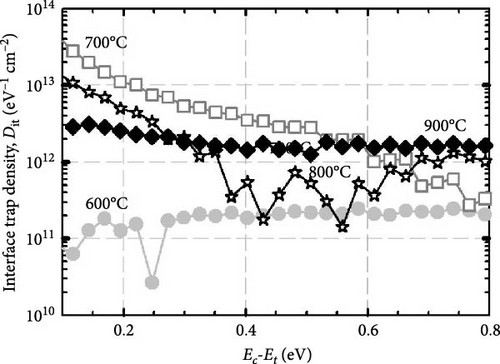
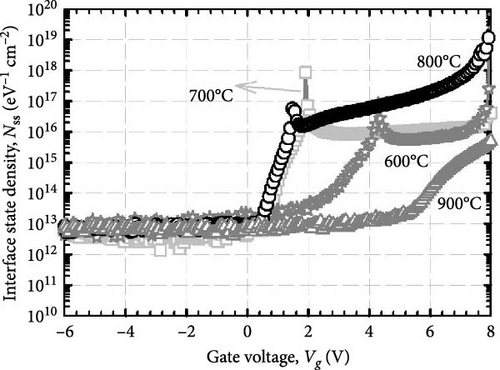
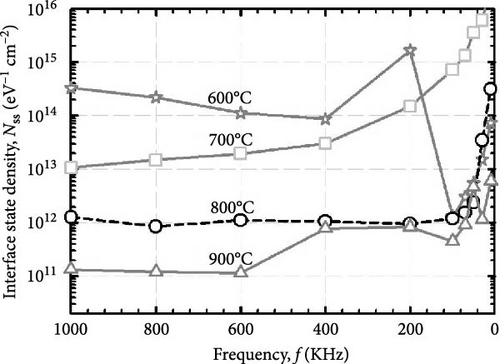
The formation of GaxCeyOz PL on 4H-SiC substrate following postdeposition annealing at various temperatures of 600, 700, 800, and 900°C in an ambient of N2–O2–N2 was presented. The successful doping of Ga3+ cation into the CeO2 crystal lattice to form the ternary GaxCeyOz PL has been confirmed by GIXRD, UV–VIS–NIR, as well as XPS measurements. It was found that nitrogen ions from the annealing ambient would plausibly attach to the Vo, generating additional Vo and/or diffused to the interface to passivate Si and/or C dangling bonds. It has been noted that at lower temperatures of 600 and 700°C, nitrogen ions primarily resided in the region of the GaxCeyOz PL to attach with the Vo. However, at higher temperatures of 800 and 900°C, the likelihood of nitrogen ions acquiring sufficient energy to diffuse to the GaxCeyOz/4H-SiC interface and formed attachments with Si and/or C dangling bonds increased. Consequently, the formation of the SiO2 IL was minimized at higher temperatures. Due to the increased doping of oxygen ions at higher temperatures of 800 and 900°C, the passivation of Vo was enhanced at these temperatures. Therefore, a superior J–E was observed at these temperatures, with an EB of 2.95 and 5.58 Mv/cm observed for the GaxCeyOz PL annealed at 800 and 900°C, respectively. Additionally, the higher ED and EID observed at higher temperatures of 800 and 900°C further confirmed that these PLs were composed of lower concentrations of Vo. XPS analysis confirmed that the Ce3+ state, in conjunction with the Vo, was minimal at the higher temperatures, with reoxidation of Ce3+ to Ce4+ being observed as the annealing temperature increased from 800 to 900°C. These observations were made in conjunction with the obtained k values of 24.11 and 24.01 for the GaxCeyOz PL at 800 and 900°C, respectively, with a minimal reduction in the k value at 900°C attributed to the slight increase in SiO2 thickness. Nevertheless, this study demonstrated that the impact of SiO2 thickness on the reduction of the k value was minimal. A further analysis of STD, Qeff, Dit, and Nss suggested that the GaxCeyOz PLs annealed at 800 and 900°C outperformed other samples in terms of performance, with the GaxCeyOz PL annealed at 900°C exhibiting optimum performance.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors wish to express their gratitude for the financial assistance by the Ministry of Higher Education Malaysia through the Fundamental Research Grant Scheme (FRGS), referenced by Project Code: FRGS/1/2023/STG05/USM/02/8.
Acknowledgments
The authors wish to express their gratitude for the financial assistance by the Ministry of Higher Education Malaysia through the Fundamental Research Grant Scheme (FRGS), referenced by Project Code: FRGS/1/2023/STG05/USM/02/8.
Open Research
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.




