Critical Heat Flux Dependence on Surface Orientation and Bubble Dynamics in Pool Boiling Over Silicon and Silicon Dioxide Surfaces
Abstract
In the evolving energy landscape, there is an increasing demand for efficient and reliable heat transfer methods to prevent overheating in renewable energy systems. Pool boiling presents viable solutions, and the surface orientation of the heated surface is a key parameter which affects its performance. This research investigates the effect of surface orientation on critical heat flux (CHF) in pool boiling using silicon (Si) and silicon dioxide (SiO2) surfaces. Experiments were conducted across seven preset orientation angles ranging from 0° to 180°. The experimental results indicated that these conditions had a notable effect on heat transfer performance, with the highest CHF observed at a 60° orientation for both types of surfaces. At 180°, a significant reduction in CHF was exhibited at the SiO2 surface, with CHF values less than 5% of those at 0°. Si surfaces exhibited larger bubble departure angles and smaller bubble sizes at higher orientation angles compared to SiO2 surfaces. These findings, in which CHF peaks at 60°, challenge the predictions of many existing models that predict a steady decrease of CHF as the surface orientation increases. This research involves a detailed analysis of vapor bubble dynamics, and the interactions between bubbles and the heating surface across different surface orientations. Through the examination of bubble detachment, coalescence, and liquid-vapor interactions, this study aims to provide a clearer understanding of the mechanisms driving CHF variations.
Summary
- •
The effect of surface orientation on critical heat flux (CHF) was investigated.
- •
Bubble departure behaviors were classified based on orientation angles.
- •
Enhanced CHF is observed with complex bubble departure modes.
1. Introduction
Nucleate boiling heat transfer, also known as natural convective heat transfer with liquid–vapor phase change, is a highly efficient mechanism for energy conversion and heat transfer [1, 2]. This process involves the formation of vapor bubbles at nucleation sites on the heated surface, which substantially increases the effective heat exchange area. As a result, heat transfer to the surrounding environment is significantly enhanced, leading to elevated heat transfer coefficients (HTCs) [3, 4]. Given its inherent involvement of liquid–vapor phase change, nucleate boiling heat transfer is characterized by relatively minor temperature differentials throughout the process. This mitigates thermal resistance and enables proficient heat transfer [5]. The continuous vapor bubble movement promotes turbulent flow within the cooling liquid, disrupting its thermal boundary layers, and influencing the onset of the critical heat flux (CHF) [6].
Nucleate boiling heat transfer has been used in various applications over the past years and remains a subject of significant interest within the evolving energy landscape, where it is specifically pertinent for preventing overheating in thermally sensitive components of renewable energy systems [7]. The sustained interest in this field is due to its potential benefits in passive thermal management applications requiring high heat flux, enabled by its high HTC [8]. The applications of nucleate boiling heat transfer span across various renewable energy systems [9], such as photovoltaic systems [10], solar thermal [11], geothermal [12], and biomass energy systems [13]. Moreover, it is also utilized in innovative thermal management solutions in other fields [9], such as electronics cooling [14], fuel cells [15], and batteries for electric vehicles [16].
Compared to conventional single-phase cooling systems, nucleate pool boiling heat transfer leverages the latent heat of vaporization of the working fluid to dissipate high heat fluxes at low-temperature gradients [17, 18]. This results in a simplified and compact system design, which offers exceptional operational reliability and stability due to the reduced need for complex mechanical components. Owing to these advantages, and the limitations of conventional single-phase cooling systems in high-performance devices, nucleate pool boiling has garnered significant interest in various industrial and scientific applications in the recent years, including the cooling of electronic and power devices and heat treatment of metal components [19, 20].
The boiling model facilitates the prediction of boiling mechanisms to improve the optimization of nucleate pool boiling heat transfer in practical applications [21]. An in-depth comprehension of the parameters, properties, and mechanisms of the boiling model is a crucial step in refining its design [22, 23]. One key parameter of this boiling model is the orientation of the heated surface. The surface orientation during pool boiling affects the wetting dynamics, nucleation site density, and the dynamics of vapor bubbles, thereby, influencing the overall heat transfer performance [24]. Understanding the impact of surface orientation is crucial for designing and refining the boiling model. Drawing upon previous research, this section summarizes key findings regarding the influence of surface orientation on boiling heat transfer characteristics.
1.1. Previous Studies on Surface Orientation
Marcus and Dropkin [25] and Kaneyasu et al. [26] both explored the effects of surface orientation on pool boiling heat transfer using copper surfaces. Marcus and Dropkin [25] observed a 20% increase in HTC when changing the surface orientation from 0° to 90°, while Kaneyasu et al. [26] found that HTC gradually increased with orientation angle at low heat fluxes (<100 kW/m2), but had limited influence at higher heat fluxes (≥100 kW/m2). Guo and El-Genk [27] extended these studies to orientations beyond 90°, showing a decrease in CHF as the orientation angle increases, especially in downward-facing positions. Yang, Baek, and Chang [28] identified two distinct CHF behaviors: a gradual change region for moderate orientation angles and a steep change region near 170°. Kim et al. [29] found that CHF generally decreased with increasing orientation and decreasing gap size, but enhanced vapor movement in downward-facing positions (180°) led to different results. Despite these findings, surface properties like roughness and wettability were largely overlooked in these early studies.
In the 2000s and 2010s, researchers shifted focus to surface properties and their influence on boiling characteristics. Liao, Bao, and Liu [30] explored the effects of surface orientation and contact angle on CHF, finding minimal CHF changes for upward-facing surfaces, but sharp decreases for downward-facing ones. Reducing contact angle enhanced CHF at small orientation angles. Kwark et al. [31] studied hydrophilic nano-coated surfaces, observing similar CHF trends across all angles, but the coated surfaces showed higher CHF due to rapid rewetting. Jung and Kim [32] employed advanced visualization techniques to analyze various boiling parameters, integrating the results into a comprehensive model. Aznam et al. [33] demonstrated that surfaces modified with nanoparticles and honeycomb structures significantly enhanced CHF, especially at high orientation angles (170°), due to improved wettability and capillary action. Jun et al. [34] found that for porous copper structures, wettability had little impact on CHF across all orientation angles. Kim et al. [35] reported improvements in both HTC and CHF for graphene-coated surfaces at all angles, with slug bubbles becoming thinner and wider as the orientation increased, triggering CHF earlier. Mei et al. [36] observed minimal CHF impact for angles less than 120°, but a sharp decrease beyond that, reaching a minimum at 180°. Tanjung and Jo [37] identified distinct bubble behavior regions based on visualized vapor studies, classifying CHF-triggering mechanisms into three regions based on surface orientation.
Recent studies have focused on using different materials and surface modifications to propose new CHF prediction correlations. Kam et al. [38] analyzed CHF trends for carbon steel (SA508), a material used in disaster mitigation strategies for downward-facing surfaces. Xie et al. [24] studied copper surfaces enhanced with nano-grass structures, showing that CHF decreased with increasing orientation angles for both regular and nano-enhanced surfaces, and proposed a new CHF correlation for downward-facing surfaces based on horizontal surface CHF. Wang et al. [39] conducted experiments on surfaces with varying roughness, finding that orientation angle had little impact on CHF for angles below 90°, but a sharp decrease occurred for angles above 90°. Lee et al. [40] explored the influence of heater size, surface roughness, and orientation on CHF, suggesting that a minimum heater size is necessary for CHF data to be relevant in industrial applications, highlighting the need to account for surface dimensions and orientation in CHF correlations.
1.2. Previous Studies on Wettability
Previous studies have highlighted the significant role of surface wettability in determining the efficiency of heat transfer mechanisms during the pool boiling process [41–43]. Previous studies have reported on how surface wettability affects key phenomena in the boiling process such as the density of nucleation sites for bubble formation, the diameter and frequency of bubble departure from the surface, the overall shape and structure of the formed vapor, and the dynamics of vapor bubble growth and coalescence. The changes in these complex dynamics are crucial as they directly affect parameters such as CHF and HTC, thereby, impacting the overall heat transfer performance.
Kirichenko and Chernyakov [44] derived a CHF correlation based on the contact angle, but found limitations for angles near 0°. Kandlikar [45] developed a model that incorporates dynamic receding contact angle and heater orientation, which accurately predicts CHF for various fluids. Chu, Enright, and Wang [46] enhanced CHF by fabricating microstructures that amplified capillary forces, achieving a maximum CHF of 208 W/cm2. O’Hanley et al. [47] used dimensional analysis to introduce new dimensionless parameters.
Previous studies have identified surface orientation and surface wettability as critical factors which influence boiling characteristics, arranged in Table 1. By understanding the interplay between these surface properties and the dynamics of bubble behavior, deeper insights into the underlying mechanisms of boiling heat transfer are provided. This research aims to elucidate the combined effects of these factors on boiling heat transfer characteristics. Specifically, the experiments utilize bare silicon (Si) and thermally treated silicon dioxide (SiO2) surfaces to explore how modifications in surface wettability affect the boiling dynamics.
| References | Working fluid | Orientation angle (˚) | CHF ratio | Boiling surface (width×length) | Contact angle (˚) | Roughness (μm) |
|---|---|---|---|---|---|---|
| Marcus and Dropkin [25] | DI | 0, 22.5, 45, 67.5, 90 | — | Copper (50.8 mm × 50.8 mm) | — | — |
| Nishikawa et al. [26] | DI | 0, 90, 120, 150, 165, 175 | 0.7275–1.17275 | Copper (42 mm × 175 mm) | — | — |
| Guo and El-Genk et al. [27] | DI | 90, 135, 150, 165, 170, 175, 180 | — | Copper (50.8 mm in diameter) | — | — |
| Yang et al. [28] | DI | 0, 30, 90, 120, 150, 180 | 0.52768–1.05446 | Stainless steel (30 mm × 150 mm) | — | — |
| Kim et al. [29] | DI | 90, 95, 105, 115, 125, 135, 145, 155, 165, 170, 175, 180 | — | Copper (15 mm × 35 mm) | — | — |
| Liao et al. [30] | DI | 0, 45, 90, 135, 180 | 0.22193–1 | Copper (20 mm in diameter) | 0 | 0.105 |
| 0.23652–1 | 30 | 0.105 | ||||
| 0.22192–1 | 55 | 0.197 | ||||
| Kwark et al. [31] | DI | 0, 45, 90, 135, 180 | 0.26577–1.09928 | Copper (10 mm × 10 mm) | — | — |
| Jung and Kim et al. [32] | DI | 0, 30, 60, 90 | 1–1.05392 | Indium-tin-oxide (ITO) film (not specified) | 67 | — |
| Aznam et al. [33] | DI | 0, 90, 135, 150, 160, 170 | 0.12908–1 | Ceramic (12 mm × 12 mm) | 50 | — |
| Jun et al. [34] | DI | 0, 45, 90, 135, 180 | 0.30151–1.14774 | Copper (10 mm × 10 mm) | — | — |
| Kim et al. [35] | DI | 0, 45, 90, 120, 135, 150, 160, 180 | 0.53744–1.02203 | Silicon (Si) wafer (20 mm × 25 mm) | 45.6 | — |
| Mei et al. [36] | DI | 0, 45, 90, 120, 150, 160, 165, 170, 175, 180 | 0.41788–1.03611 | Copper (22 mm × 28 mm) | — | — |
| Tanjung and Jo et al. [37] | DI | 0, 45, 90, 135, 150, 165, 175, 180 | 0.13879–1.07359 | Copper (1.5 mm × 100 mm) | 81.79 ±0.34 | 0.17 ± 0.03 |
| Kam et al. [38] | DI | 90, 105, 120, 135, 150, 165, 180 | — | SS304 (40 mm × 100 mm) | 73 | — |
| SA508 (40 mm × 100 mm) | 77 | — | ||||
| Xie et al. [24] | DI | 0, 30, 60, 90, 120, 150, 165, 180 | 0.50649–1.07359 | Copper (10 mm × 10 mm) | 76.5 | 0.254 |
| Wang et al. [39] | DI | 0, 45, 90, 120, 150, 165, 175 | 0.42798–1.05556 | Copper (20 mm × 20 mm) | 84.33 | 0.146 |
| Lee et al. [40] | DI | 0, 45, 90, 135, 170, 180 | 0.20949–1.07115 | Stainless steel (120 mesh) (25 mm × 100 mm) | 60.02 ± 1.8 | 0.169 ± 0.021 |
| 0.41089–1.06436 | Stainless steel (120 mesh) (50 mm × 100 mm) | 60.02 ± 1.8 | 0.169 ± 0.021 | |||
2. Experiments
2.1. Sample Preparation
The experimental samples consist of polished Si wafers measuring 25 mm × 20 mm and oxidized Si wafers of the same dimensions. Si and SiO2 were chosen due to their well-documented material properties, which enable precise thermal characterization. Their high thermal conductivity makes them particularly suitable for boiling studies and their chemical stability under boiling experiment conditions ensures the reliability of the experimental results.
A platinum layer, capable of being heated based on the Joule effect, was deposited on the backside of the samples. Due to the area of the platinum layer, the actual heating area was 15 mm × 10 mm and the heat flux was calculated by dividing the applied power by this area. The temperature of the samples was measured using the linear relationship between the resistance of the platinum layer and its temperature. Contact angle measurements, including static and dynamic contact angles, and surface roughness measurements were conducted for each sample. Before measurements and the experiment, a cleaning process using acetone, ethanol, and water was conducted.
2.2. Pool Boiling Setup
The experimental setup employed in this investigation of pool boiling is outlined in Figure 1. The tank, made of aluminum, possesses dimensions of 350 (W) × 150 (L) × 200 (H) mm. Visual observation and recording of the boiling phenomena are facilitated through two visualization windows constructed from polycarbonate. Deionized water was used as the working fluid, maintained in a saturated state by two immersion heaters installed within the tank. To ensure a consistent fluid level during experimentation, a reflux condenser is positioned on top of the tank. The sample is attached to a jig made of PEEK using an adhesive binary epoxy (Duralco 4460), and the jig is mounted on an aluminum shaft positioned at the center of the visualization windows, allowing for multidirectional rotation. The rotating stage (Standa 183984), mounted on the shaft, was installed using a level to ensure precise alignment. Angle adjustments were made manually with a scale increment of 1°, minimizing potential errors. The precision of the setup and the minimized error in angle adjustment ensured accurate control of surface orientation throughout the experiments. The DC power supplied to the platinum heater affixed to the sample is regulated using a power supply (Ametek XG 60-25). Visual analysis of the nucleate boiling process is conducted throughout the experiment using a high-speed camera (Photron FASTCAM) operating at 20,000 frames per second with a resolution of 512 × 352 pixels. All data, including applied voltage, current, and sample temperature, are obtained at 1 Hz through a data acquisition system (Keysight DAQ970A).
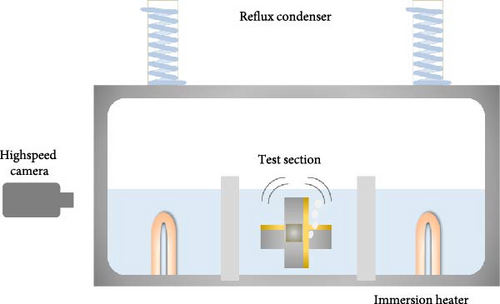
2.3. Experimental Procedures
In this experiment, deionized water was preheated using immersion heaters for an hour to remove noncondensable gases. This process ensures optimal conditions for pool boiling experiments. The experiments meticulously controlled heat flux through precise adjustments in voltage supplied by a dedicated power source. The procedure involved incrementally increasing the voltage every minute and then stabilizing it for 3 min to accurately measure heat flux. For the initial detection of the onset of nucleate boiling (ONB) at lower heat flux levels, the power was carefully increased in increments of 5 kW/m2 starting from 50 kW/m2, and then in larger steps of 50 kW/m2 to approach the CHF threshold. CHF was determined as the point immediately preceding a sharp rise in the system’s temperature, marking the limit of safe operational heat flux.
The experiments were systematically conducted at seven preset surface orientation angles ranging from 0° to 180°, with 30° intervals. This approach facilitated a systematic investigation into the effect of surface orientation on CHF, allowing for a detailed analysis of boiling dynamics across various orientations under controlled laboratory conditions. In this study, 0° inclined surface refers to a surface that is positioned horizontally with the heated surface facing upward, resulting in the vertically upward heat transfer direction. On the other hand, 180° inclined surface represents a surface that is also horizontal but facing downward, resulting in the vertically downward heat transfer direction.
2.4. Uncertainty Analysis
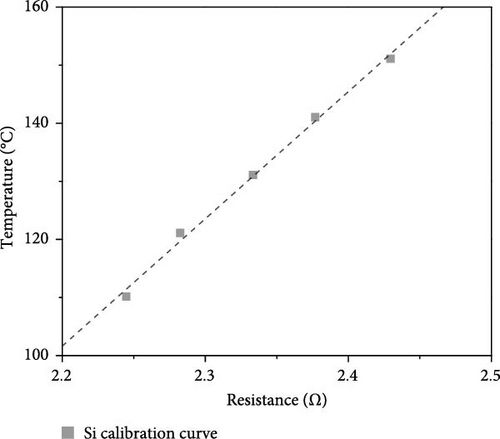
In Equation (1), Vheat, Vref, Aheat, and Rref represent the voltage across the heater, the voltage across the reference resistor, the heated area, and the resistance of the reference resistor, respectively. The wall temperature was determined by using the linear relationship between the resistance of platinum and its temperature, as shown in Figure 2. Heat transfer is assumed to take place through one-dimensional conduction from the surface of the platinum heater to its exposed surface in contact with the working fluid. This assumption allows the precise calculation of the surface temperature distributions, which is a necessary step for evaluating the boiling phenomena.
3. Experimental Results
3.1. Surface Characteristics
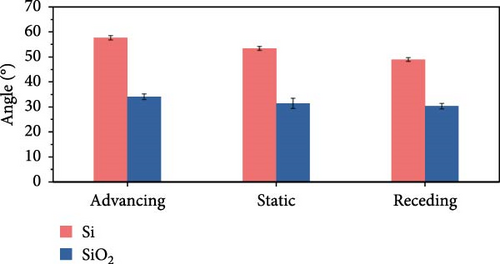
As shown in Figure 3, surface characteristics were quantitatively assessed through measurements of roughness and wettability. Both Si and SiO2 samples exhibited similar roughness values, of approximately 0.03 µm. Static contact angle measurements revealed that Si had an angle of about 53°, while SiO2 displayed an angle of approximately 31°. Dynamic contact angles were determined with the surface inclined at 30°. For Si, the advancing contact angle was about 58°, and the receding contact angle was about 49°. In contrast, SiO2 had advancing and receding angles measured at approximately 34° and 30°, respectively. These results highlight the differences in wettability between the two materials under identical testing conditions.
3.2. Pool Boiling
Before investigating the influence of orientation angle on CHF, a comparative analysis of wettability effects using horizontally oriented Si and SiO2 surfaces was conducted. Experimental outcomes revealed that the surface with a lower contact angle, SiO2, exhibited a higher CHF at approximately 1000 kW/m2 compared to Si, which demonstrated a CHF of about 700 kW/m2. Additionally, the discrepancy in wall superheat near CHF was approximately 15 K higher for the Si surface. This suggests that the differences in rewetting behavior are likely due to variations in wettability. As illustrated in Figure 4, the impact of wettability on thermal transfer efficiency was distinctly more pronounced, offering a clearer differentiation between the coefficients of heat transfer.
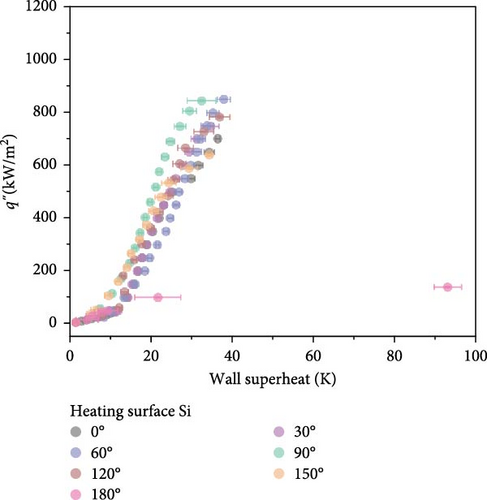
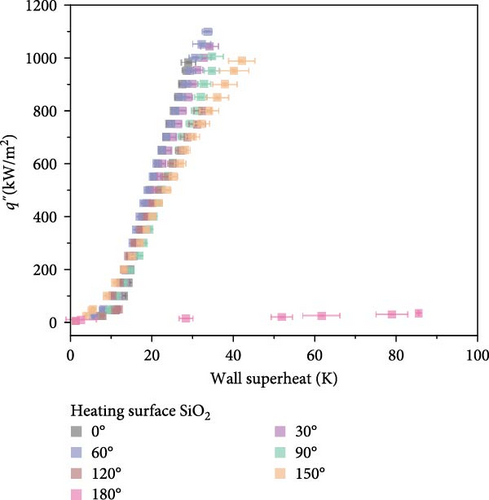
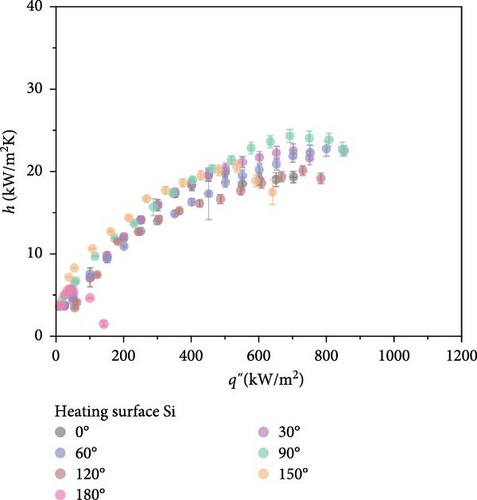
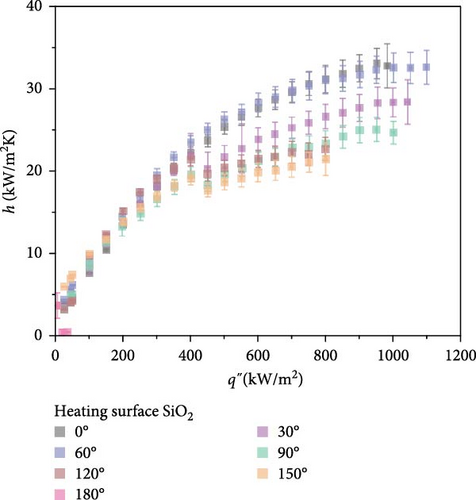
As shown in Figure 4, comparative boiling experiments on Si and SiO2 surfaces with orientation angles ranging from 0° to 180° revealed similar boiling curves at all angles except 180°. The maximum wall superheat observed for Si surfaces was approximately 25 K, whereas for SiO2 surfaces it was around 40 K. The boiling HTCs of both surfaces were comparable at lower heat fluxes below 250 kW/m2. However, at higher heat fluxes, the Si surface demonstrated superior HTCs compared to the SiO2 surface. Improvements in the CHF were also similar across different angles, with the highest enhancement observed at 60°, where the CHF value for Si surfaces reached 850.5 kW/m2, and for SiO2 surfaces, it was 1098.2 kW/m2.
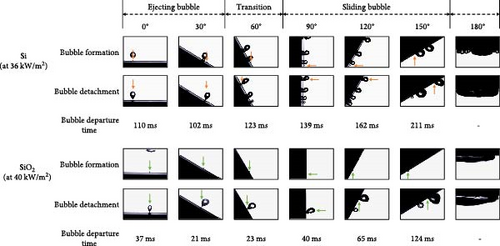
High-speed imaging was employed to analyze bubble dynamics, the results of which are presented in Figure 5. The data were obtained at a heat flux of 40 kW/m2, which corresponds to the point immediately following the ONB. At 180°, a substantial vapor layer underneath the heater prevented visual observations. For each condition, at least 10 bubbles were analyzed to ensure statistical reliability. Figure 5 shows that the standard deviation across all conditions is relatively small, suggesting that the sample size used for quantification was adequate to capture consistent and reliable trends in bubble departure. This leads to the reliable analysis about the effects of surface orientation on bubble departure behavior.
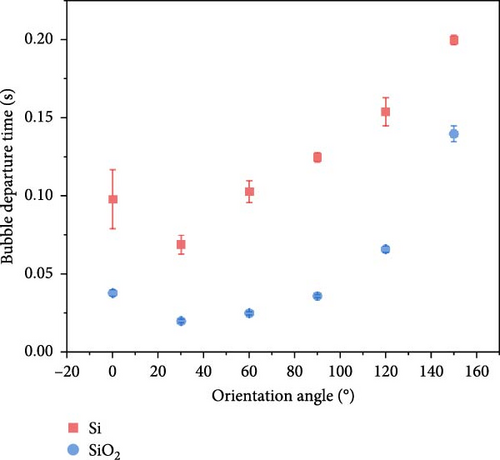
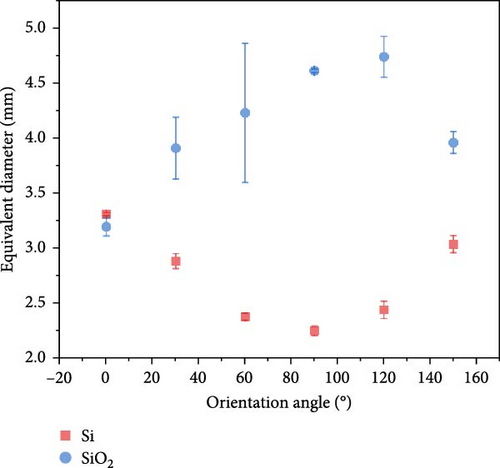
Both Si and SiO2 surfaces exhibited an increasing trend in bubble departure time with rising orientation angles, with the slowest departure time observed at 150°. This trend is somewhat similar to the increase in CHF with orientation angle, suggesting that bubble departure time plays a significant role in enhancing CHF. The analysis of maximum diameters showed consistent values across the entire range, making it challenging to discern the effects of orientation angles. At 0° orientation, the diameter for Si was measured at 3.306 mm and for SiO2 at 3.192 mm, indicating similar values. Throughout the entire range of orientation angles, the SiO2 surfaces exhibited slightly larger bubble diameters compared to Si. The results indicate that the diameter of the bubbles was minimally influenced by the orientation angle, suggesting that factors other than orientation might play a more significant role in determining bubble size during boiling experiments.
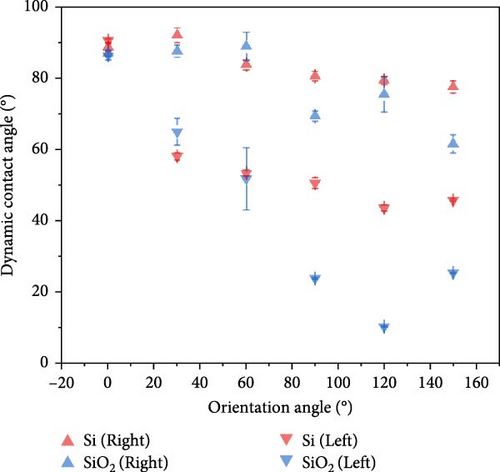
In this study, the departure angle is defined as the angle between the tangent to the bubble at the point of detachment and the wall. Measurements of bubble departure angles on Si and SiO2 surfaces showed a general trend of decreasing angles with increasing surface orientation. For Si, the departure angle was about 90° at a 0° orientation, decreasing gradually to stabilize at around 60° from a 120° orientation onwards. Similarly, for SiO2, the departure angle gradually decreased with increasing orientation angle, stabilizing at about 40° from 120° onwards.
These observations suggest that surfaces with similar roughness may exhibit comparable bubble behavior in the horizontal direction, but bubble dynamics can vary significantly with orientation angle. This indicates the importance of considering surface orientation in applications involving boiling heat transfer to better predict and control bubble behavior and heat transfer efficiency.
4. Discussion
Previous studies have presented inconsistent observations on the CHF for orientation angles between 0° and 90°, with some indicating a decrease in CHF and others reporting stability or an increase. In this research, CHF exhibited a slight increase in Si and a slight reduction in SiO2. The enhancement ratios of the CHF for each surface can be observed in Figure 6. For both surfaces, CHF increases up to 60° and then decreases, showing the minimum CHF at 180°. Notably, at 180°, the CHF of the Si surface was about 20% of the horizontal direction, while the SiO2 surface exhibited a significantly lower value, less than 5%. Despite similar surface roughness and only a 30° difference in contact angle, this substantial reduction in CHF in the downward orientation highlights the importance of analyzing boiling characteristics in this orientation for boiling surfaces used in various industrial sectors.
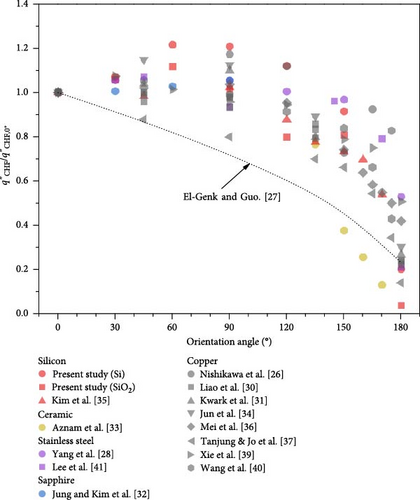
Researchers such as Liao, Bao, and Liu [30] and Guo and El-Genk [27] have developed correlation equations for predicting CHF based on surface orientation angles. Yet, these models struggle to account for data from various studies that demonstrate CHF enhancement ratios greater than 1.0 within the orientation angle range of 30°–90°. This gap underscores the need to investigate the mechanisms driving these increased CHF enhancement ratios and to develop more accurate models. Additionally, there is a noted tendency of these models to underpredict CHF in the orientation angle range of 150°–180° when compared to existing experimental data.
It has been observed that the type of material does not significantly affect the CHF enhancement ratio changes, suggesting the importance of focusing on surface variables such as contact angle and surface roughness rather than the material itself. Figure 7 illustrates the impact of these surface variables on the CHF enhancement ratio at a 90° orientation angle. As the contact angle increases, the CHF ratio slightly rises, whereas it slightly decreases with increasing surface roughness. These variations in the CHF enhancement ratio are believed to stem from changes in bubble behavior due to alterations in surface variables, highlighting the need for an integrated approach that combines control of surface variables with analysis of bubble dynamics.
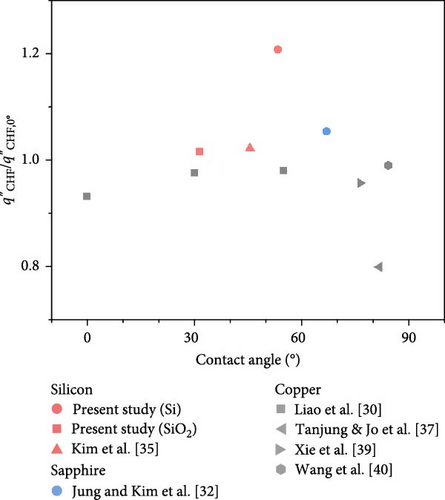
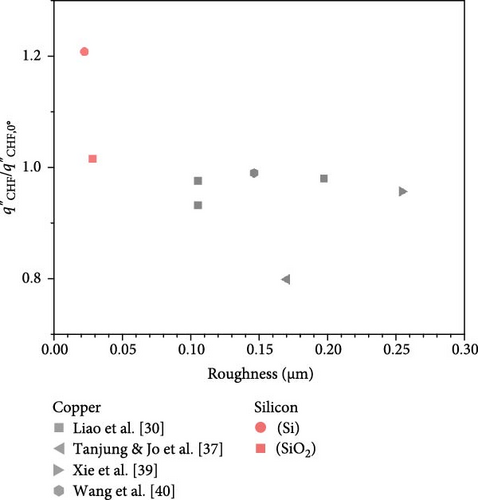
Figure 8 depicts the behavior of steam on surfaces oriented at angles of 0°, 30°, 60°, 90°, 120°, 150°, and 180°. The vapor behavior allows for categorization into three distinct regions based on the angle of orientation. The first region, the upward-facing region, covers angles from 0° to 30°. In this region, bubbles are removed vertically from the surface by buoyancy. The second region, termed the near-vertical region, ranges from 60° to 120°. In this segment, the formation of large-scale steam layers initiated by bubble coalescence begins at heat fluxes around 60% of the CHF. However, despite the appearance of these steam layers, a significant decrease in CHF is not observed, indicating effective bubble removal facilitated by buoyancy. Last, the downward-facing region, spanning 150°–180°, is characterized by the immediate entrapment of bubbles upon formation and the subsequent development of large-scale steam layers with minimal bubble removal. This leads to a pronounced decline in CHF.

To thoroughly examine the impact of bubble behavior on CHF, high-speed imaging analysis was conducted on single bubbles situated on both Si and SiO2 surfaces. In regions with orientation angles of 30° or less, bubbles were observed to depart directly from their nucleation sites, a phenomenon in which the ejecting mode dominates, and thus, termed as the “ejecting bubble region.” Conversely, in areas with angles exceeding 90°, bubbles slide along the surface before escaping, and thus, the area is termed as the “sliding bubble region.” At 60°, both ejection and sliding behaviors were observed, creating a transition regime where both modes exist. This led to the designation of this region as the “transition region.” Notably, at this orientation angle, both surfaces exhibited the highest CHF values. This clear demarcation of bubble detachment modes based on surface orientation contributes to a deeper understanding of how orientation affects boiling dynamics and ultimately, CHF performance.
High-speed imaging results revealed that at high heat fluxes, bubbles in the ejecting bubble region either detached directly from their places of formation or merged with nearby bubbles to escape, whereas in the transition region, sliding bubbles merged with stationary ones to facilitate faster escape. This induced escape appears to reduce the residence time of bubbles on the heating surface, facilitating the supply of the cooling liquid. It also delays the formation of vapor layers which insulate the surface. Therefore, CHF is enhanced when the surface orientation is increased in the ejecting bubble and transition regions, peaking at 60°. This classification correlates with the trends in CHF enhancement or degradation as depicted in Figure 9, suggesting a strong link between the mode of bubble departure and CHF. This observation underscores the need to further investigate how the dynamics of ejecting or sliding bubbles impact heat transfer.
5. Conclusions
This study systematically investigated the effect of surface orientation on CHF in pool boiling for Si and SiO2 surfaces. The highest CHF for both types of surfaces was observed at a 60° orientation. At 180°, a significant reduction in CHF was noted, particularly for the SiO2 surface, which showed CHF values less than 5% of those at 0°. Si surfaces exhibited larger bubble departure angles and smaller bubble sizes at higher orientation angles compared to SiO2 surfaces. These findings, in which CHF peaks at 60°, challenge the predictions of many existing models that predict a steady decrease of CHF as the surface orientation increases.
By categorizing orientation angles into three distinct regions—upward-facing (0°–30°), near-vertical (60°–120°), and downward-facing (150°–180°)—significant variations in CHF and bubble dynamics were observed. These regions were defined based on the behavior of vapor generated by bubble coalescence: in the upward-facing region, bubbles moved directly upward; in the near-vertical region, bubbles moved almost vertically; and in the downward-facing region, bubbles tended to form a vapor layer under the heater, affecting CHF adversely.
In addition, an attempt was made to categorize the regions based on vapor bubble dynamics. The findings demonstrated that in the 0°–30° surface orientation, bubbles primarily departed from their nucleation sites with minimal lateral movement and this region was termed as the “Ejecting bubble region.” Conversely, in the 90°–180° orientation, bubbles slid along the surface before departure and this region was termed as the “Sliding bubble region.” The “Transition region” at 60° exhibited both ejection and sliding behaviors, suggesting a critical shift in bubble dynamics that directly correlates with CHF trends. This categorization is crucial as it directly links the mode of bubble departure to CHF variations, emphasizing the need for targeted optimization of surface orientations to enhance boiling heat transfer.
This research identifies the inaccuracies of the existing CHF prediction models and underscores the need for improvised models. Surface orientation angles are categorized into regions based on vapor behavior and vapor bubble dynamics, linking these behaviors to CHF performance. This categorization highlights the significance of accounting for bubble behaviors, including bubble ejection and sliding, when optimizing surface orientations to enhance heat transfer efficiency. Future investigations should aim to elucidate the detailed mechanisms underlying these observations to develop more accurate predictive models for CHF under varying surface orientation conditions.
Nomenclature
-
- A:
-
- area (m2)
-
- h:
-
- heat transfer coefficient (Wm−2 K−1)
-
- I:
-
- current (A)
-
- P:
-
- power (W)
-
- q:
-
- heat flux (Wm−2)
-
- R:
-
- electrical resistance (Ω)
-
- T:
-
- temperature (K)
-
- t:
-
- thickness (m)
-
- U:
-
- uncertainty
-
- V:
-
- voltage (V)
Subscripts/Superscripts
-
- c:
-
- circuit
-
- heat:
-
- heater
-
- p:
-
- power
-
- ref:
-
- reference resistor
-
- super:
-
- superheat
-
- w:
-
- wall
Abbreviations
-
- CHF:
-
- critical heat flux
-
- HTC:
-
- heat transfer coefficient.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jaehyeok Yang and Hyunjin Yong contributed equally to this study.
Funding
This work was supported by Inha University Research Grant.
Acknowledgments
This work was supported by Inha University Research Grant.
Open Research
Data Availability Statement
The authors confirm that the data supporting the findings of the current study are available within the manuscript. Raw data that support the findings of this study are available from the authors upon request.




